Effect of Coiling Temperature on Microstructures and Precipitates in High-Strength Low-Alloy Pipeline Steel after Heavy Reduction during a Six-Pass Rolling Thermo-Mechanical Controlled Process
Abstract
:1. Introduction
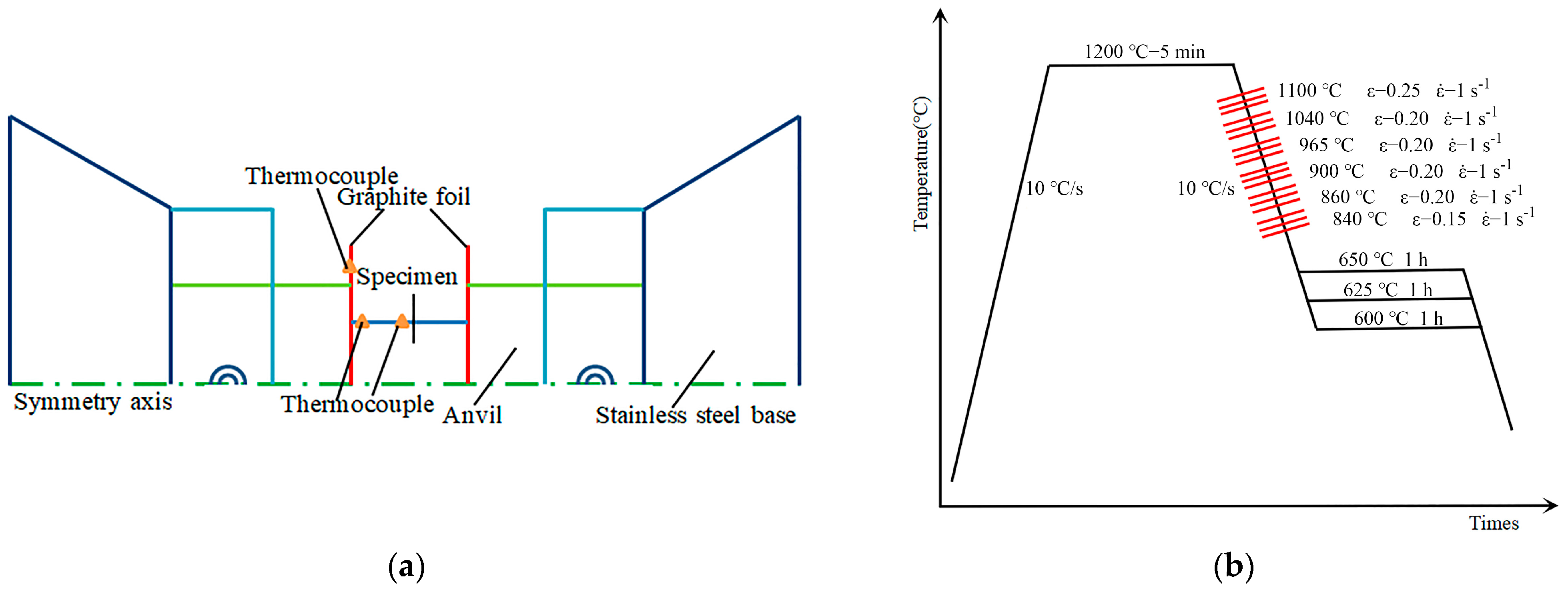
2. Materials and Methods
3. Results
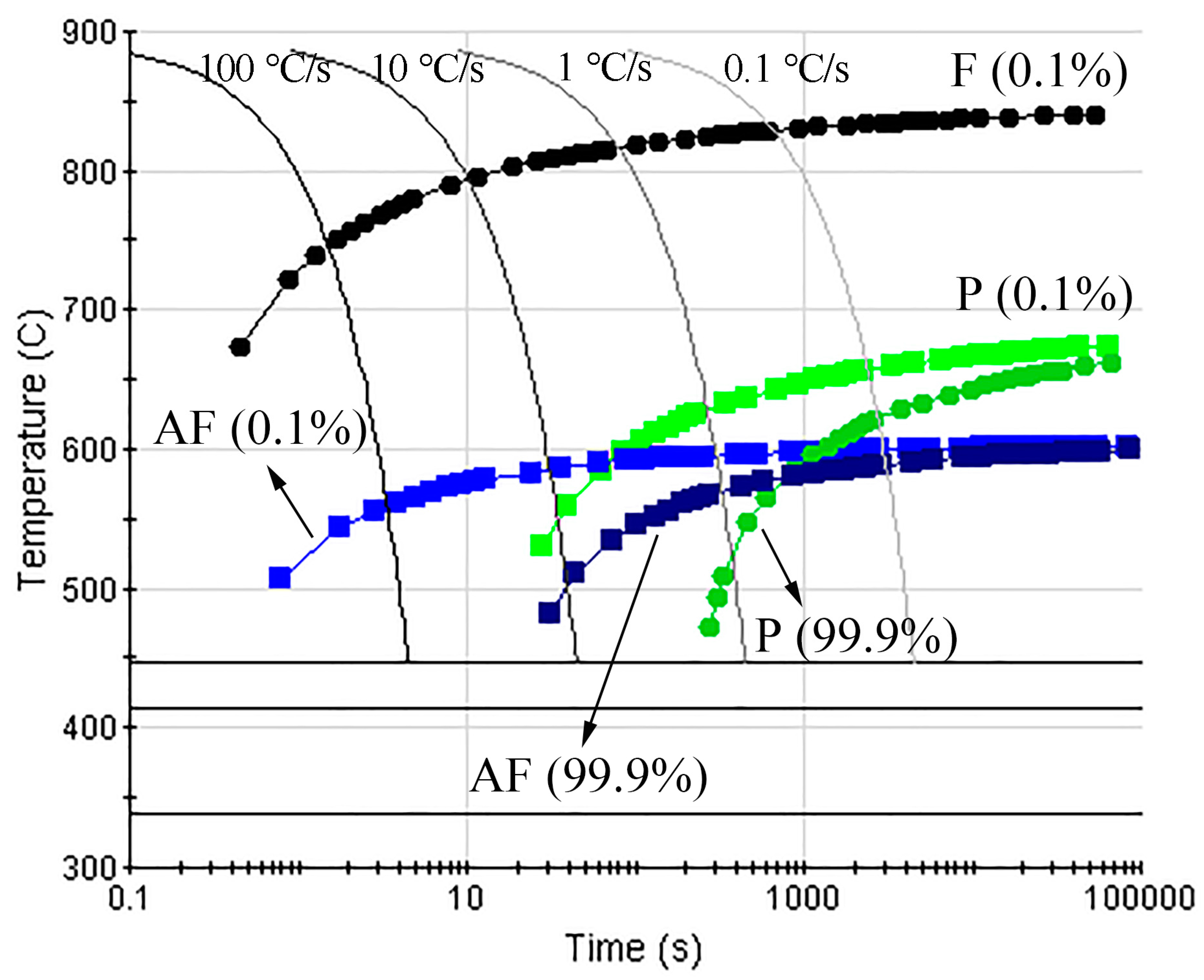
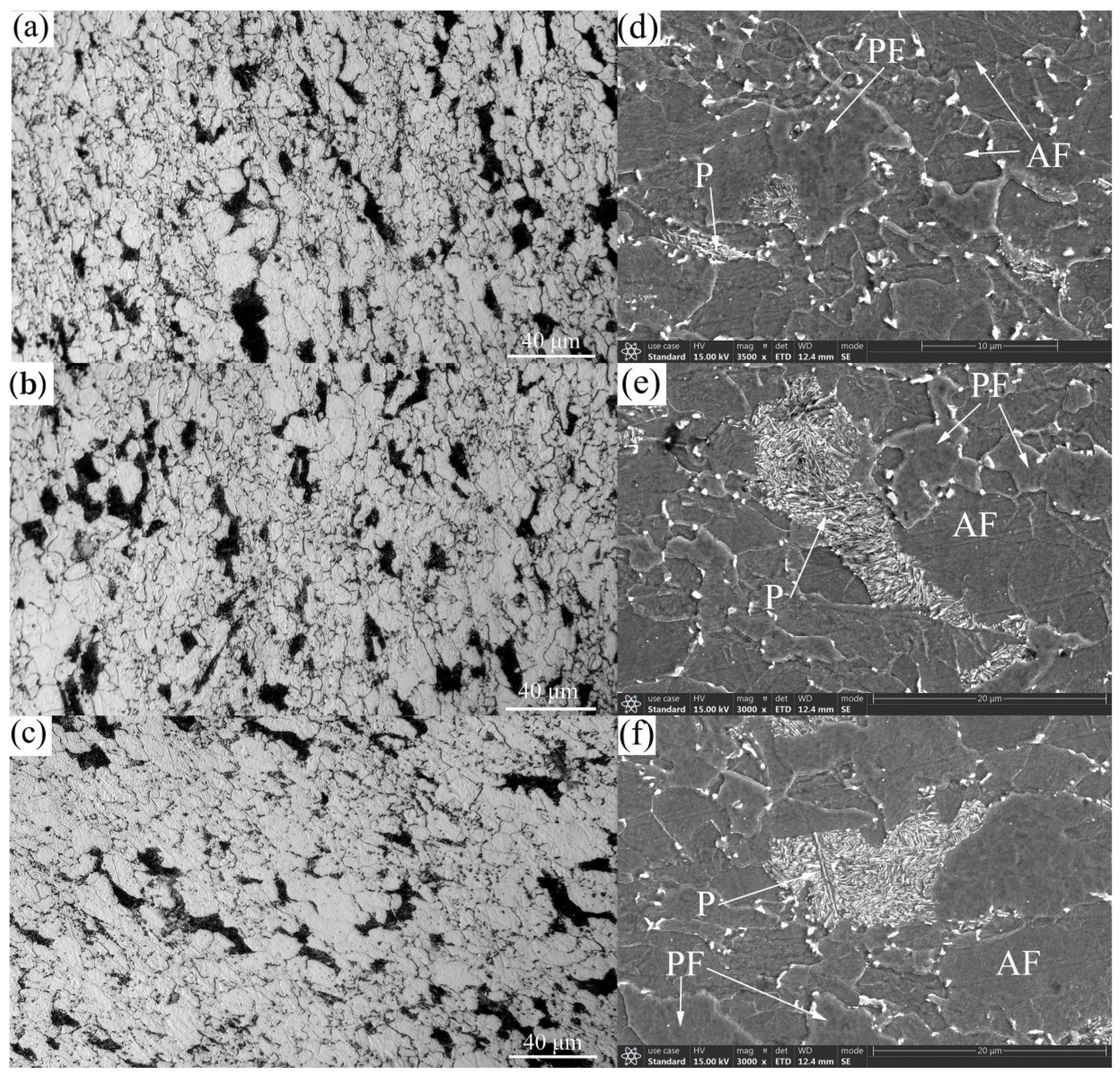
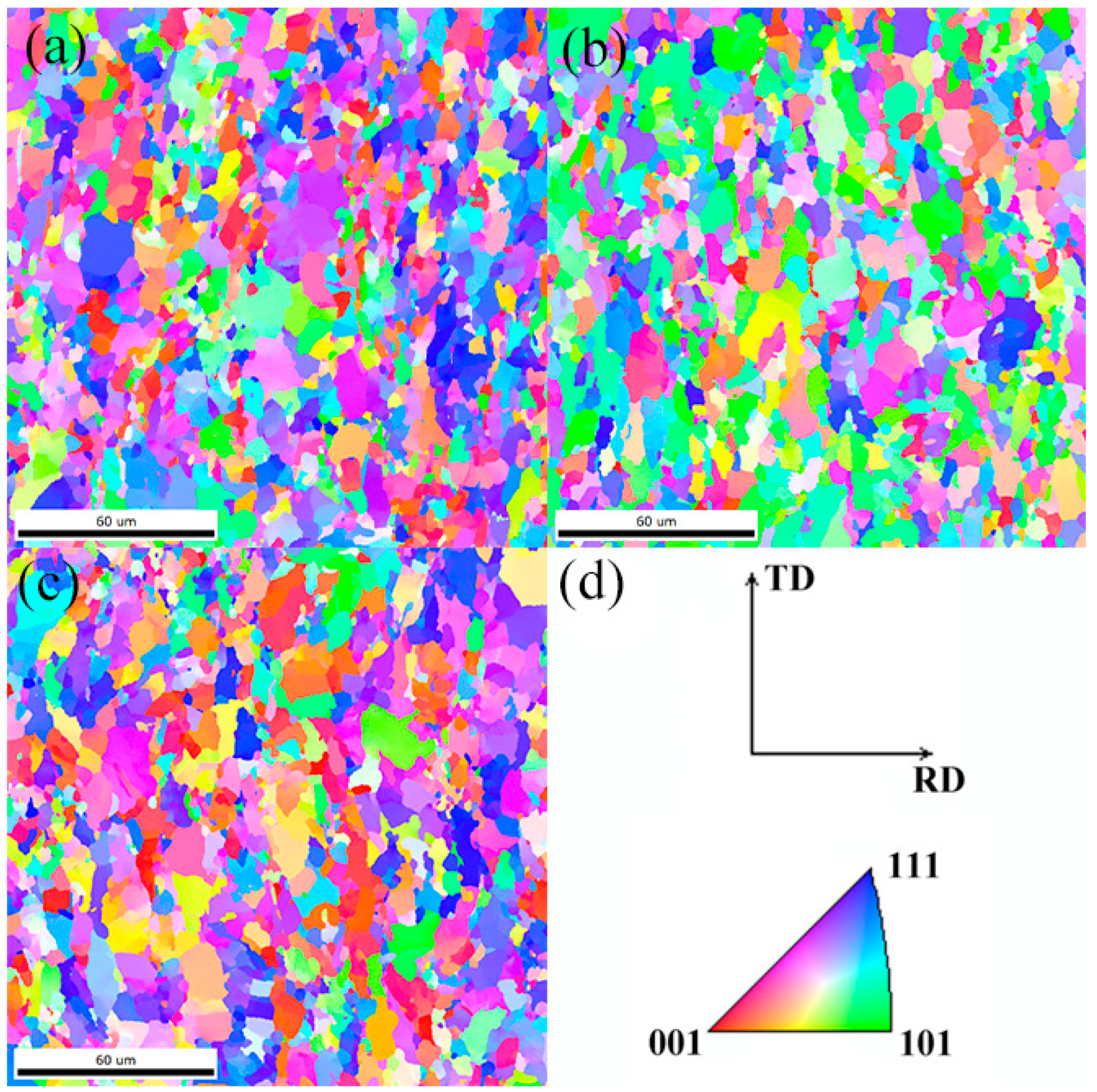
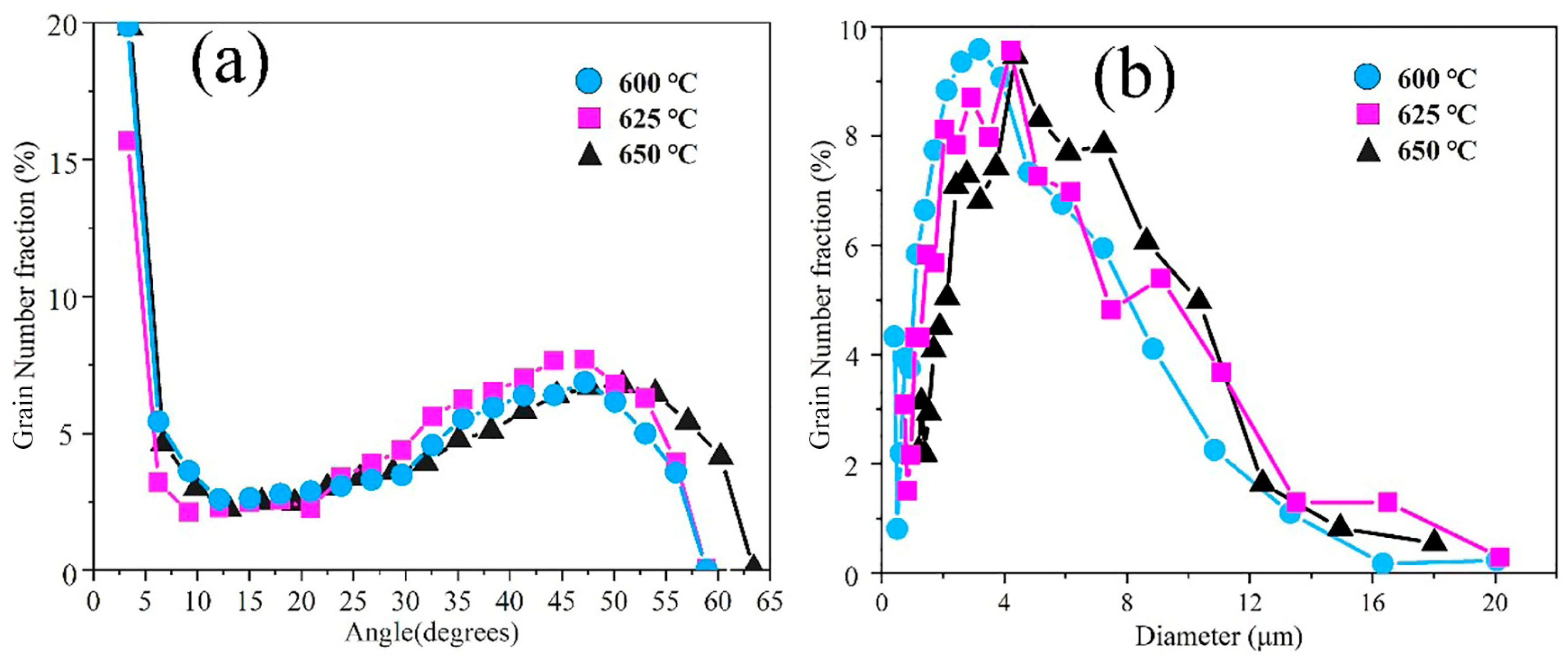
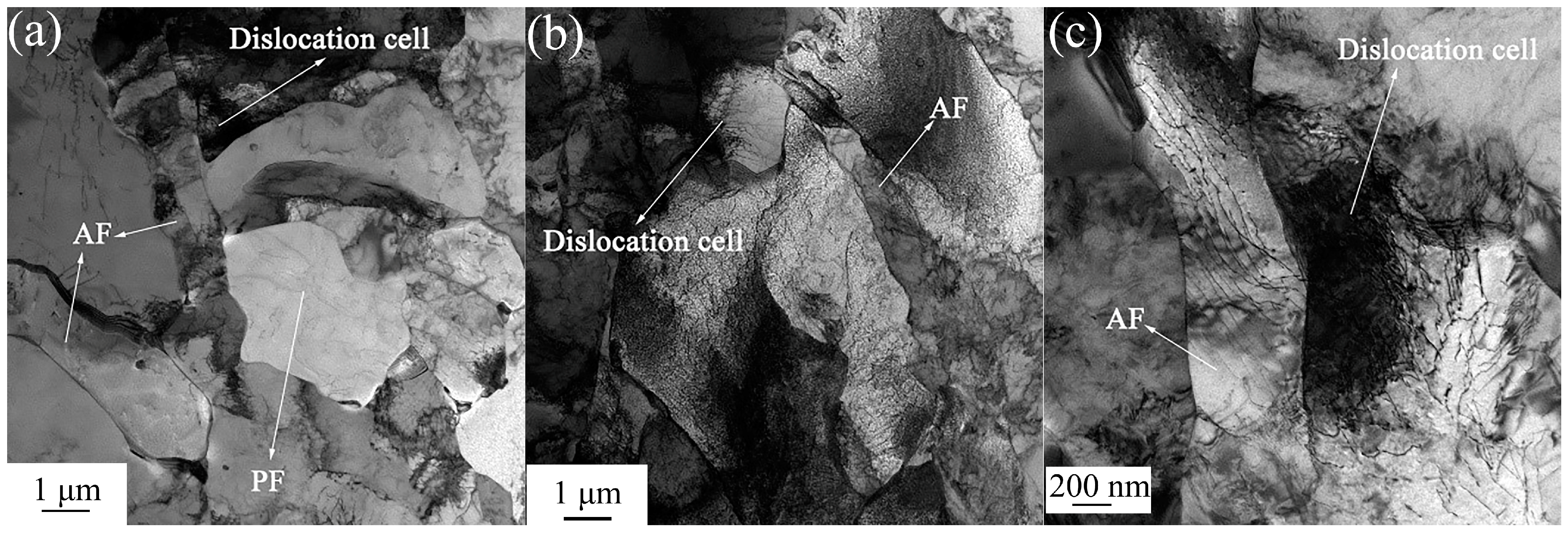
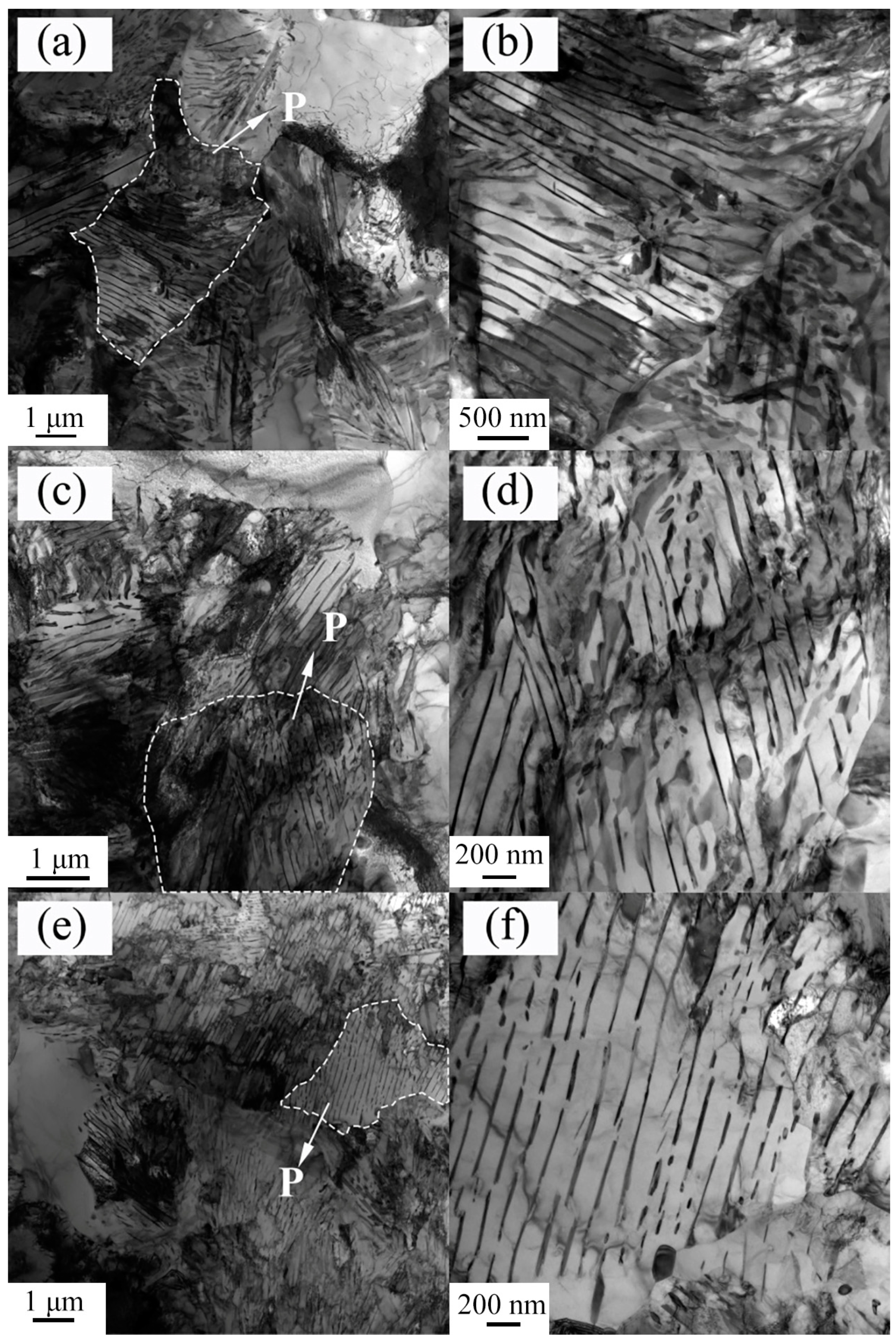
3.1. Microstructures and CCT Diagrams
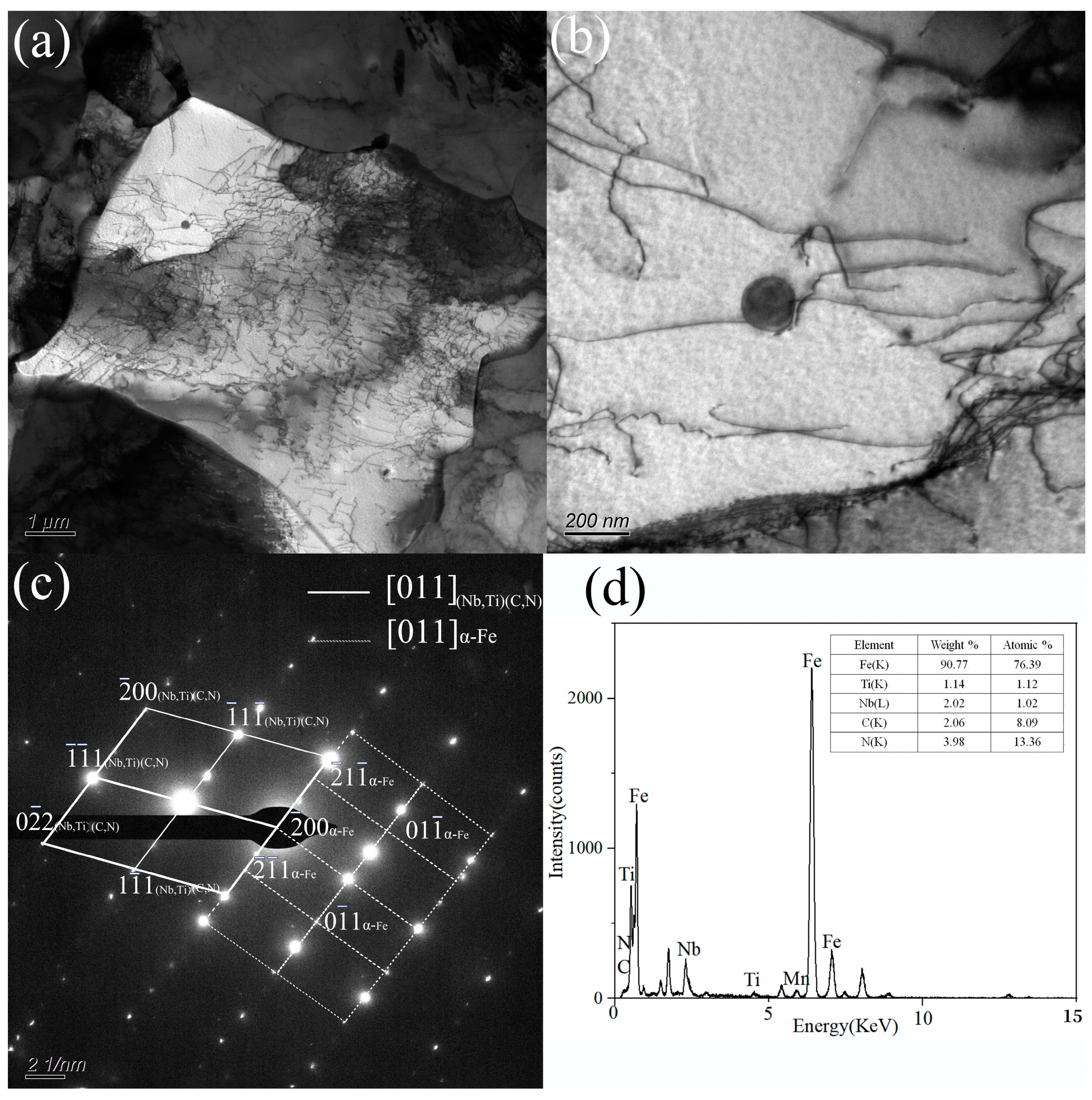
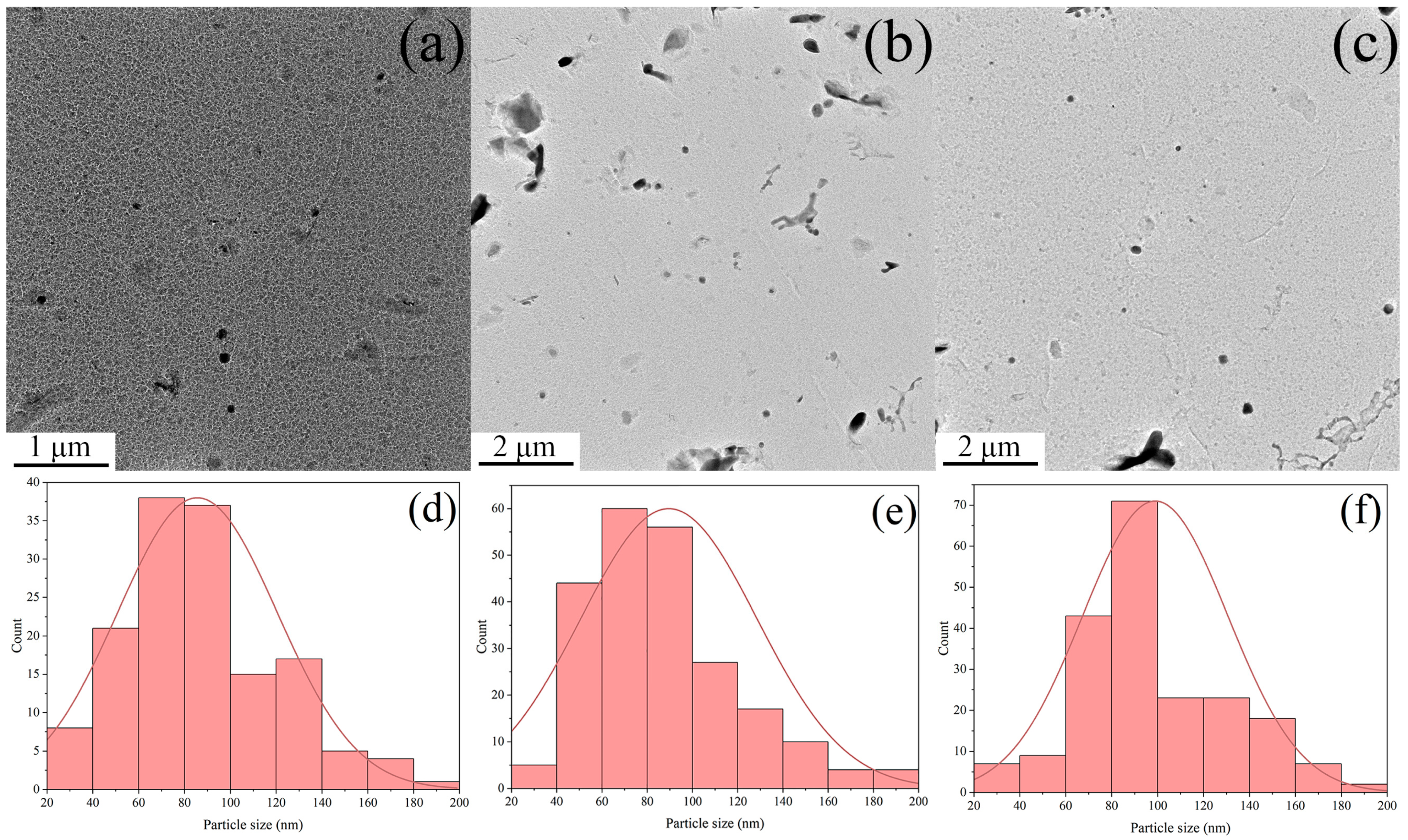
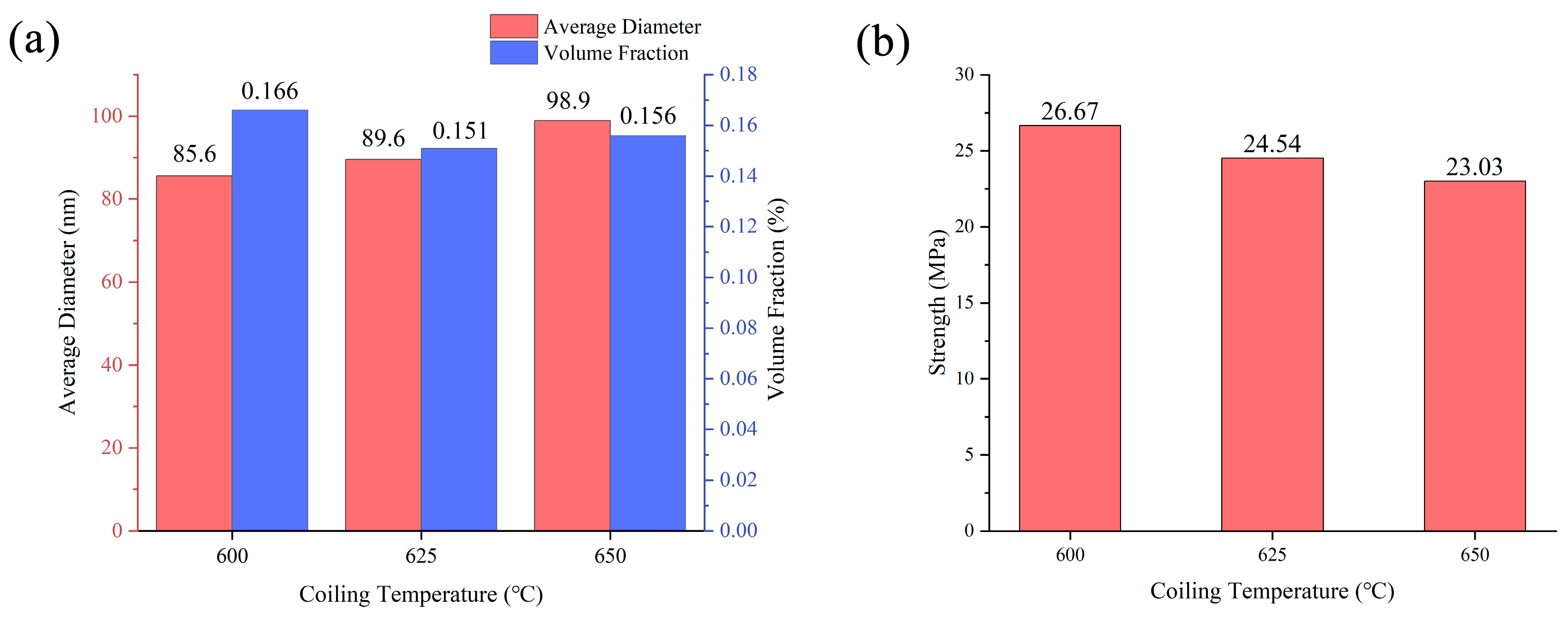
3.2. Morphology and Statistical Analysis of Precipitates
4. Discussion
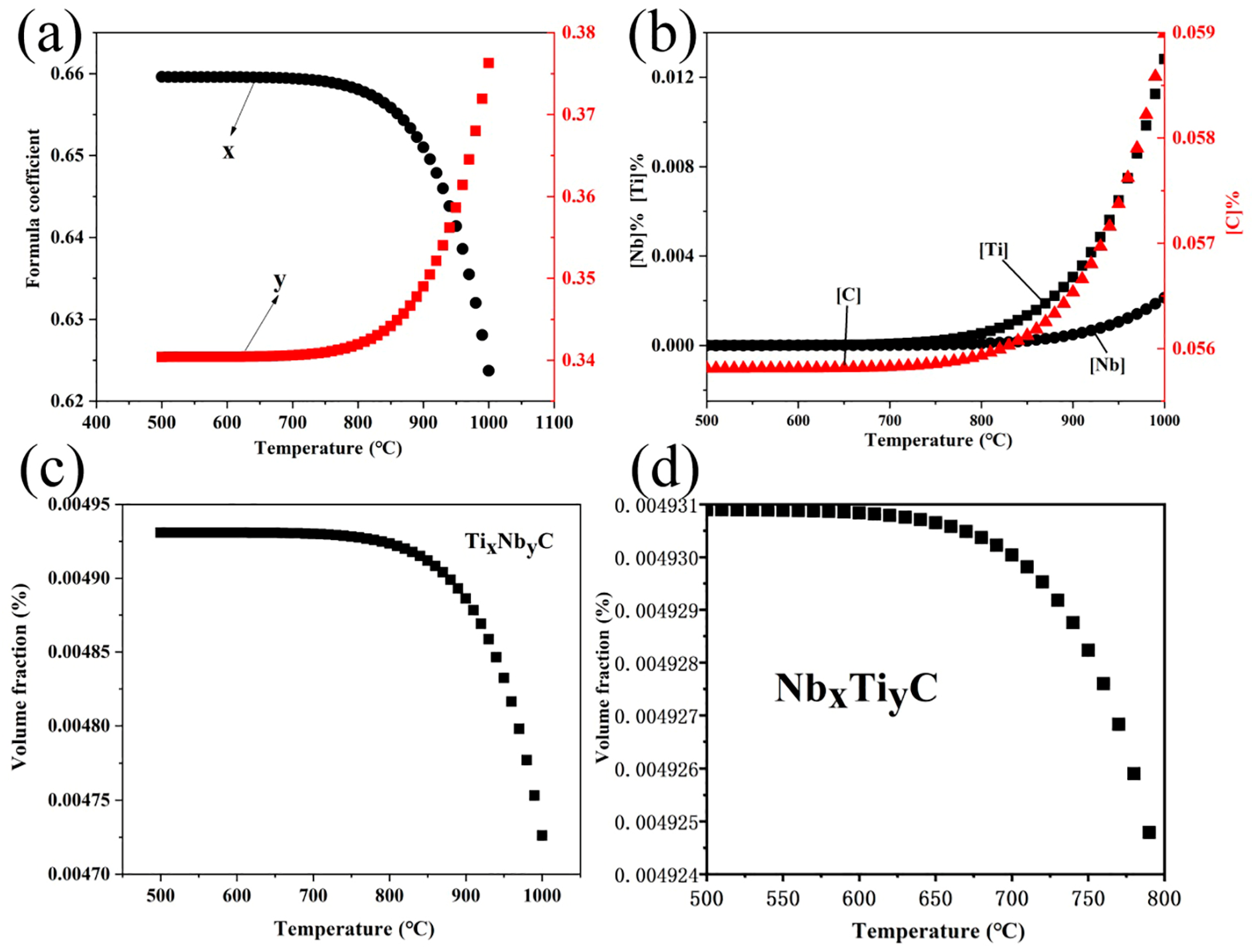
4.1. Solid Solution and Volume Fraction of the Precipitated Particles
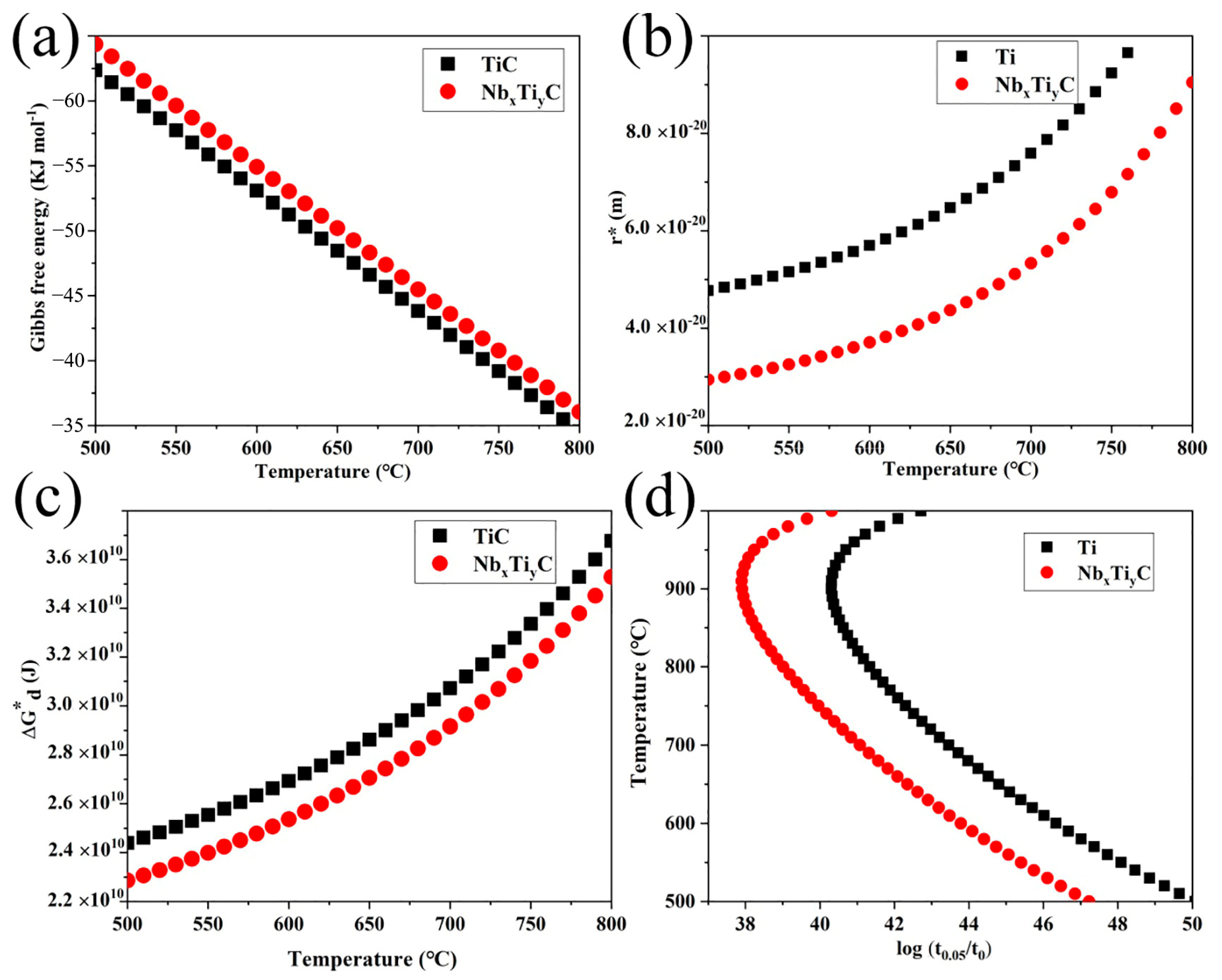
4.2. Nucleation of Precipitate during Strain-Induced Precipitation
4.3. Growth of Precipitate during Strain-Induced Precipitation
4.4. Precipitation–Time–Temperature (PTT) Curves
4.5. Estimation of Precipitation Strengthening
5. Conclusions
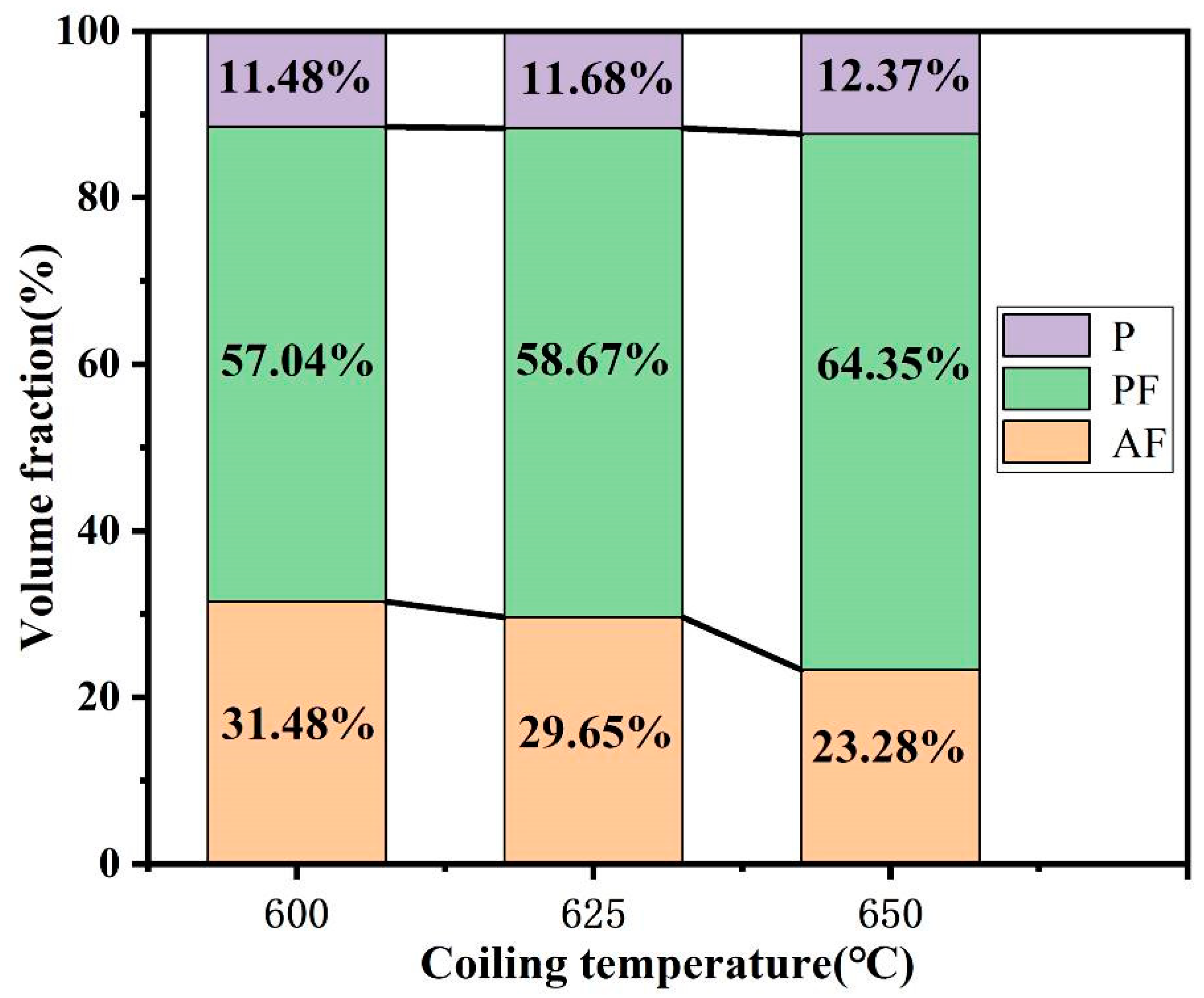
- The microstructures consisted of acicular ferrite with high-density dislocation, polygonal ferrite, and pearlite. The volume fractions of pearlite were found to be 11.48, 11.68, and 12.37%, while the volume fractions of the acicular ferrite were 31.48, 29.65, and 23.28% at coiling temperatures of 600, 625, and 650 °C, respectively. The pearlite increased with the coiling temperature while the acicular ferrite decreased with the coiling temperature;
- The TiC, (Nb,Ti)C, and (Nb,Ti)(C,N) precipitates formed in the matrix, and the orientation relationship with the matrix was [011](Nb,Ti)(C,N)//[011]α-Fe. The average size of precipitates was 96.0, 98.9, and 105.6 nm, while the volume fraction was 0.166%, 0.151%, and 0.156% at coiling temperatures of 600, 625, and 650 °C, respectively. The estimation of incremental yield strength through precipitation strengthening could reach 26.67 MPa at a coiling temperature of 600 °C and decrease with the coiling temperature;
- According to a theoretical model based on classical nucleation and growth theory, (Nb, Ti)C and (Nb, Ti)(C, N) exhibit a high driving force and, therefore, can form before TiC precipitates.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasad, P.S.; Ghosh, K.S. Effect of Heat Treatment and NC Ni Coating on Electrochemical and Hydrogen Embrittlement Behaviour of a High Strength Low Alloy (HSLA) Steel. Electrocatalysis 2022, 13, 551–566. [Google Scholar] [CrossRef]
- Narimani, M.; Hajjari, E.; Eskandari, M.; Szpunar, J.A. Elevated temperature tensile behavior of S900 HSLA steel and its welded joints. J. Constr. Steel Res. 2023, 203, 107782. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, X.; Long, J.; Zheng, K.; Zheng, Z. Effects of Zirconium on the Structure and Mechanical Properties of High-Strength Low-Alloy Steels under Quenched or Tempered Conditions. Steel Res. Int. 2022, 93, 2200352. [Google Scholar] [CrossRef]
- Zhu, W.T.; Cui, J.J.; Feng, Y.; Chen, Z.Y.; Zhao, Y.; Chen, L.Q. Achieving Excellent Strength-Ductility Balance and Lower Yield Ratio in a 690 MPa-Grade Multiphase Construction Steel. Steel Res. Int. 2022, 93, 2100750. [Google Scholar] [CrossRef]
- Fan, X.; Mi, Z.; Yang, L.; Su, H. Application of DFT Simulation to the Investigation of Hydrogen Embrittlement Mechanism and Design of High Strength Low Alloy Steel. Materials 2023, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Lin, Z.; Fa, Y.; Zhao, X.; Zhu, Z.; Ya, W.; Sun, Z.; Yu, X. Microstructure and Mechanical Properties of High-Strength, Low-Alloy Steel Thin-Wall Fabricated with Wire and Arc Additive Manufacturing. Metals 2023, 13, 764. [Google Scholar] [CrossRef]
- Xiao, J.H.; Yan, L.; Zhang, P.; Li, G.L.; Li, B.Y.; Zhao, T.; Wang, H.; Chen, L.J.; Wang, D. Effects of minor Ce doping on the microstructure and mechanical performances of a EH47 grade HSLA steel for ship and ocean engineering. Mater. Charact. 2023, 201, 112931. [Google Scholar] [CrossRef]
- Cheng, H.L.; Luo, X.C.; Wu, X. Recent research progress on additive manufacturing of high-strength low-alloy steels: Focusing on the processing parameters, microstructures and properties. Mater. Today Commun. 2023, 36, 106616. [Google Scholar] [CrossRef]
- Mičian, M.; Frátrik, M.; Kajánek, D. Influence of Welding Parameters and Filler Material on the Mechanical Properties of HSLA Steel S960MC Welded Joints. Metals 2021, 11, 305. [Google Scholar] [CrossRef]
- Natarajan, V.V.; Liu, S.; Challa, V.S.A.; Misra, R.D.K.; Sidorenko, D.M.; Mulholland, M.D.; Manohar, M.; Hartmann, J.E. Processing-structure-mechanical property relationship in Ti-Nb microalloyed steel: Continuous cooling versus interrupted cooling. Mater. Sci. Eng. A 2016, 671, 254–263. [Google Scholar] [CrossRef]
- Lis, A.K. Mechanical properties and microstructure of ULCB steels affected by thermomechanical rolling, quenching and tempering. J. Mater. Process. Technol. 2000, 106, 212–218. [Google Scholar] [CrossRef]
- Jacques, P.J.; Furnémont, Q.; Lani, F.; Pardoen, T.; Delannay, F. Multiscale mechanics of TRIP-assisted multiphase steels: I. Characterization and mechanical testing. Acta Mater. 2007, 55, 3681–3693. [Google Scholar] [CrossRef]
- Lobanov, M.L.; Zorina, M.A.; Karabanalov, M.S.; Urtsev, N.V.; Redikultsev, A.A. Phase Transformation Crystallography in Pipeline HSLA Steel after TMCP. Metals 2023, 13, 1121. [Google Scholar] [CrossRef]
- Zavdoveev, A.; Poznyakov, V.; Baudin, T.; Rogante, M.; Kim, H.S.; Heaton, M.; Demchenko, Y.; Zhukov, V.; Skoryk, M. Effect of heat treatment on the mechanical properties and microstructure of HSLA steels processed by various technologies. Mater. Today Commun. 2021, 28, 102598. [Google Scholar] [CrossRef]
- Jia, Y.; Deng, X.T.; Wang, Q.; Li, C.R.; Wang, Z.D. Improvement of Mechanical Properties and Wear Resistance of Direct-Quenched Wear-Resistant Steel by Deformed Austenite. Steel Res. Int. 2023, 94, 2200903. [Google Scholar] [CrossRef]
- Kim, Y.W.; Song, S.W.; Seo, S.J.; Hong, S.G.; Lee, C.S. Development of Ti and Mo micro-alloyed hot-rolled high strength sheet steel by controlling thermomechanical controlled processing schedule. Mater. Sci. Eng. A 2013, 565, 430–438. [Google Scholar] [CrossRef]
- Yi, H.L.; Du, L.X.; Wang, G.D.; Liu, X.H. Development of Nb-V-Ti Hot-Rolled High Strength Steel with Fine Ferrite and Precipitation Strengthening. J. Iron Steel Res. Int. 2009, 16, 72–77. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Nathani, H.; Hartmann, J.E.; Siciliano, F. Microstructural evolution in a new 770 MPa hot rolled Nb–Ti microalloyed steel. Mater. Sci. Eng. A 2005, 394, 339–352. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Zhou, W.H.; Misra, R.D.K.; O’Malley, R.; Jansto, S.G. The effect of coiling temperature on the microstructure and mechanical properties of a niobium–titanium microalloyed steel processed via thin slab casting. Mater. Sci. Eng. A 2014, 595, 143–153. [Google Scholar] [CrossRef]
- García-Sesma, L.; López, B.; Pereda, B. Effect of coiling conditions on the strengthening mechanisms of Nb microalloyed steels with high Ti addition levels. Mater. Sci. Eng. A 2019, 748, 386–395. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, C.G.; Lee, T.H.; Lee, S. Effects of Coiling Temperature on Microstructure and Mechanical Properties of High-strength Hot-rolled Steel Plates Containing Cu, Cr and Ni. ISIJ Int. 2000, 40, 692–698. [Google Scholar] [CrossRef]
- Li, Z.; Lv, Z.; Wang, C.; Liu, L.; Cui, K.; Zhao, Z. Effect of Coiling Temperature on Microstructure and Properties of Titanium Strengthened Weathering Building Steel. Metals 2023, 13, 804. [Google Scholar] [CrossRef]
- Wang, Y.D.; Tang, Z.H.; Xiao, S.F.; Siyasiya, C.W.; Wei, T. Effects of final rolling temperature and coiling temperature on precipitates and microstructure of high-strength low-alloy pipeline steel. J. Iron Steel Res. Int. 2022, 29, 1236–1244. [Google Scholar] [CrossRef]
- Monschein, S.; Ragger, K.S.; Zügner, D.; Fasching, J.; Schnitzer, R. Influence of the Ti Content on the Grain Stability and the Recrystallization Behavior of Nb-Alloyed High-Strength Low-Alloyed Steels. Steel Res. Int. 2022, 93, 2200094. [Google Scholar] [CrossRef]
- Martins, C.A.; de Faria, G.L.; Mayo, U.; Isasti, N.; Uranga, P.; Rodríguez-Ibabe, J.M.; de Souza, A.L.; Cohn, J.A.C.; Rebellato, M.A.; Gorni, A.A. Production of a Non-Stoichiometric Nb-Ti HSLA Steel by Thermomechanical Processing on a Steckel Mill. Metals 2023, 13, 405. [Google Scholar] [CrossRef]
- Chen, M.Y.; Gouné, M.; Verdier, M.; Bréchet, Y.; Yang, J.R. Interphase precipitation in vanadium-alloyed steels: Strengthening contribution and morphological variability with austenite to ferrite transformation. Acta Mater. 2014, 64, 78–92. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Gonzalez, B.M. Boron and Thermomechanical Processing Effects on the Metallurgical Properties of a Niobium-Titanium High-Strength Low-Alloy Steel. J. Mater. Eng. Perform. 2022, 31, 9544–9554. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gan, X.L.; Wang, S.Y.; Yang, H.L.; Li, Y.Q.; Xu, G. Effect of Coiling Temperature on Microstructure and Properties of a Ti-Nb Microalloyed High-Speed Guardrail Steel. Steel Res. Int. 2023, 94, 2200840. [Google Scholar] [CrossRef]
- Patra, P.K.; Sam, S.; Singhai, M.; Hazra, S.S.; Ram, G.D.J.; Bakshi, S.R. Effect of Coiling Temperature on the Microstructure and Mechanical Properties of Hot-Rolled Ti-Nb Microalloyed Ultra High Strength Steel. Trans. Indian Inst. Met. 2017, 70, 1773–1781. [Google Scholar] [CrossRef]
- Song, Z.H.; Song, H.Y.; Liu, H.T. Effect of cooling route on microstructure and mechanical properties of twin-roll casting low carbon steels with an application of oxide metallurgy technology. Mater. Sci. Eng. A 2021, 800, 140282. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hui, W.J.; Xie, Z.Q.; Zhang, Y.J.; Zhao, X.L. Enhancing mechanical properties of a V + Ti microalloyed bainitic forging steel via tailoring microstructure through controlled forging. J. Mater. Sci. 2020, 55, 10849–10862. [Google Scholar] [CrossRef]
- Lan, L.Y.; Chang, Z.Y.; Kong, X.W.; Qiu, C.L.; Zhao, D.W. Phase transformation, microstructure, and mechanical properties of X100 pipeline steels based on TMCP and HTP concepts. J. Mater. Sci. 2017, 52, 1661–1678. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, Y.; Zhou, Y.N.; Dong, X.M.; Zhang, C.X.; Gao, G.H.; Schneider, R. Effect of precipitation of (Ti,V)N and V(C,N) secondary phases on mechanical properties of V and N microalloyed 1Cr steels. Mater. Sci. Eng. A 2018, 738, 203–212. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.F.; Li, Y.M.; Xie, Q.; Huang, Z.Y. Complex isothermal precipitation behavior of V(C, N) in V-, N-added low carbon steel. J. Mater. Sci. 2021, 56, 2638–2649. [Google Scholar] [CrossRef]
- Sun, X.J.; Kang, J.Y.; Yong, Q.L. Revealing the difference of precipitation kinetics between TiC and VC in low-carbon tempered martensitic steels. J. Mater. Sci. 2020, 55, 16018–16032. [Google Scholar] [CrossRef]
- Gan, X.L.; Yuan, Q.; Zhao, G.; Ma, H.W.; Liang, W.; Xue, Z.L.; Qiao, W.W.; Xu, G. Quantitative Analysis of Microstructures and Strength of Nb-Ti Microalloyed Steel with Different Ti Additions. Metall. Mater. Trans. A 2020, 51, 2084–2096. [Google Scholar] [CrossRef]
- Chen, W.J.; Gao, P.F.; Wang, S.; Zhao, X.L.; Zhao, Z.Z. Strengthening mechanisms of Nb and V microalloying high strength hot-stamped steel. Mater. Sci. Eng. A 2020, 797, 140115. [Google Scholar] [CrossRef]
- Militzer, M.; Hawbolt, E.B.; Meadowcroft, T.R. Microstructural model for hot strip rolling of high-strength low-alloy steels. Metall. Mater. Trans. A 2000, 31, 1247–1259. [Google Scholar] [CrossRef]
- Huang, H.H.; Yang, G.W.; Zhao, G.; Mao, X.P.; Gan, X.L.; Yin, Q.L.; Yi, H. Effect of Nb on the microstructure and properties of Ti-Mo microalloyed high-strength ferritic steel. Mater. Sci. Eng. A 2018, 736, 148–155. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yong, Q.L.; Sun, X.J.; Yang, Z.G.; Li, Z.D.; Zhang, C.; Weng, Y.Q. An analytical model for the kinetics of strain-induced precipitation in titanium micro-alloyed steels. ISIJ Int. 2012, 52, 1661–1669. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]

| C | Si | Mn | S | P | Ti | Nb | V | N | Al |
|---|---|---|---|---|---|---|---|---|---|
| 0.093 | 0.376 | 1.540 | 0.005 | 0.0084 | 0.094 | 0.078 | 0.000 | 0.005 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Yang, W.; Siyasiya, C.W.; Tang, Z. Effect of Coiling Temperature on Microstructures and Precipitates in High-Strength Low-Alloy Pipeline Steel after Heavy Reduction during a Six-Pass Rolling Thermo-Mechanical Controlled Process. Metals 2024, 14, 249. https://doi.org/10.3390/met14020249
Lei Y, Yang W, Siyasiya CW, Tang Z. Effect of Coiling Temperature on Microstructures and Precipitates in High-Strength Low-Alloy Pipeline Steel after Heavy Reduction during a Six-Pass Rolling Thermo-Mechanical Controlled Process. Metals. 2024; 14(2):249. https://doi.org/10.3390/met14020249
Chicago/Turabian StyleLei, Yicong, Wen Yang, Charles W. Siyasiya, and Zhenghua Tang. 2024. "Effect of Coiling Temperature on Microstructures and Precipitates in High-Strength Low-Alloy Pipeline Steel after Heavy Reduction during a Six-Pass Rolling Thermo-Mechanical Controlled Process" Metals 14, no. 2: 249. https://doi.org/10.3390/met14020249





