Effect of Microstructure on the Precipitation of β-Mg2Si during Cooling after Homogenisation of Al-Mg-Si Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
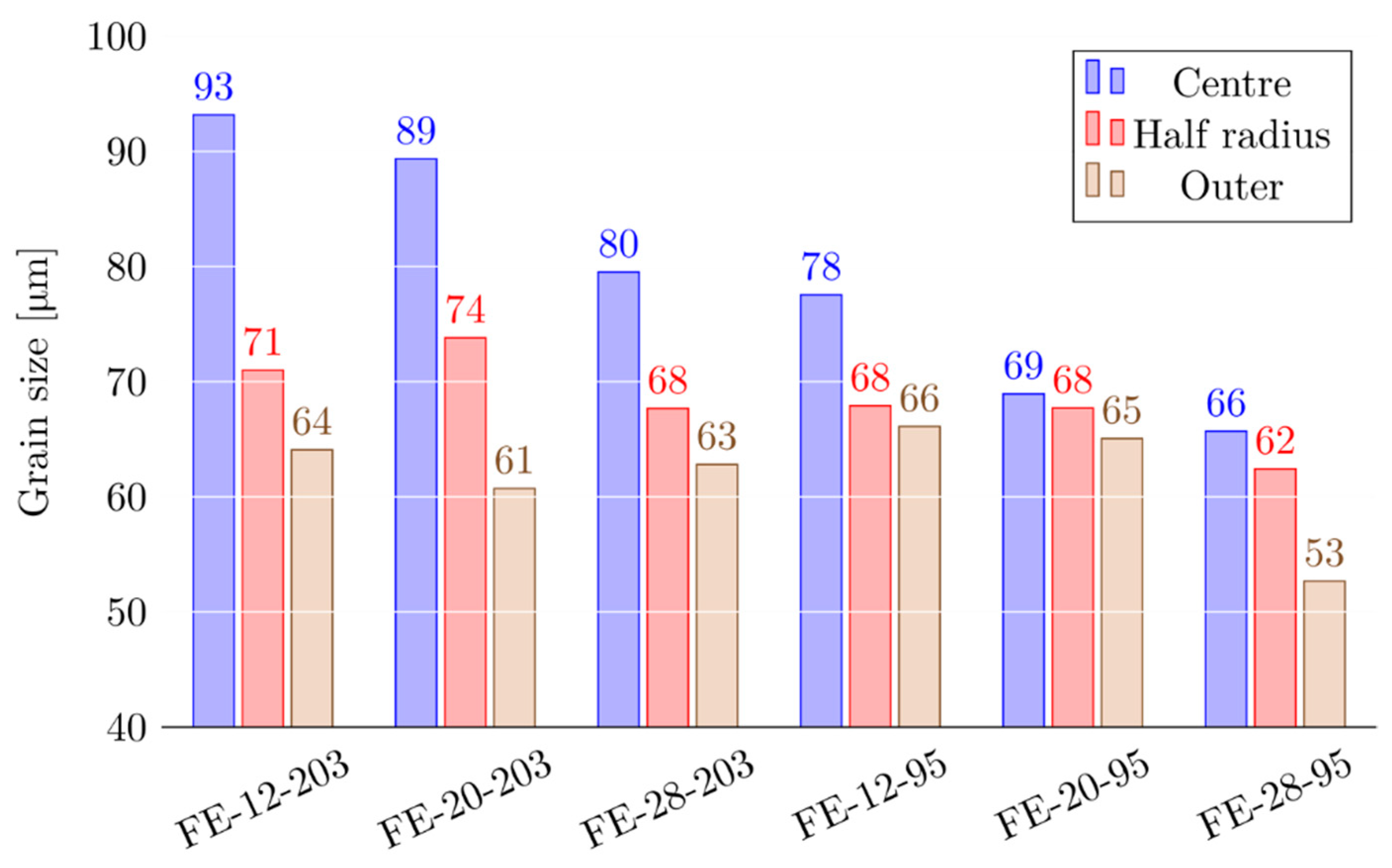
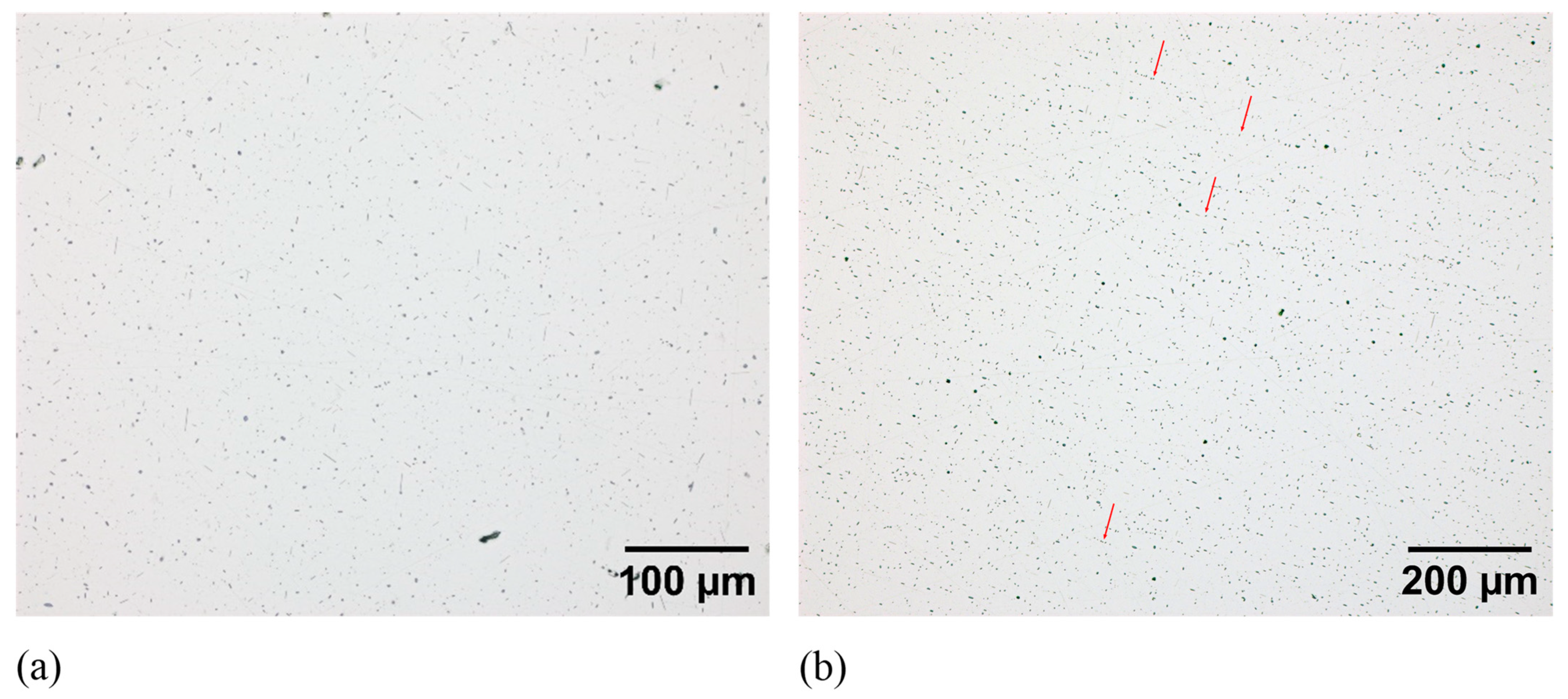
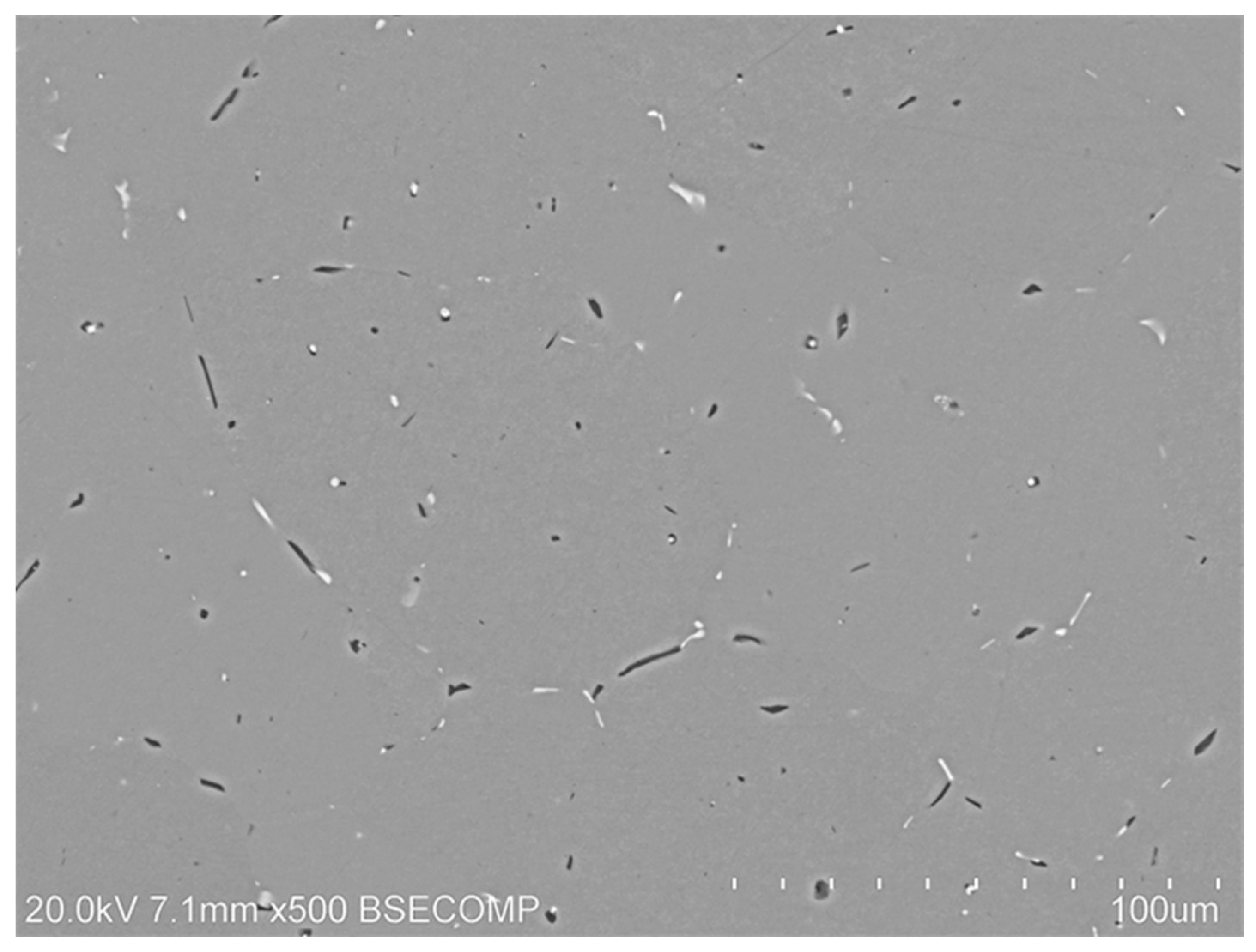
3.1. Characterisation of Initial Structure
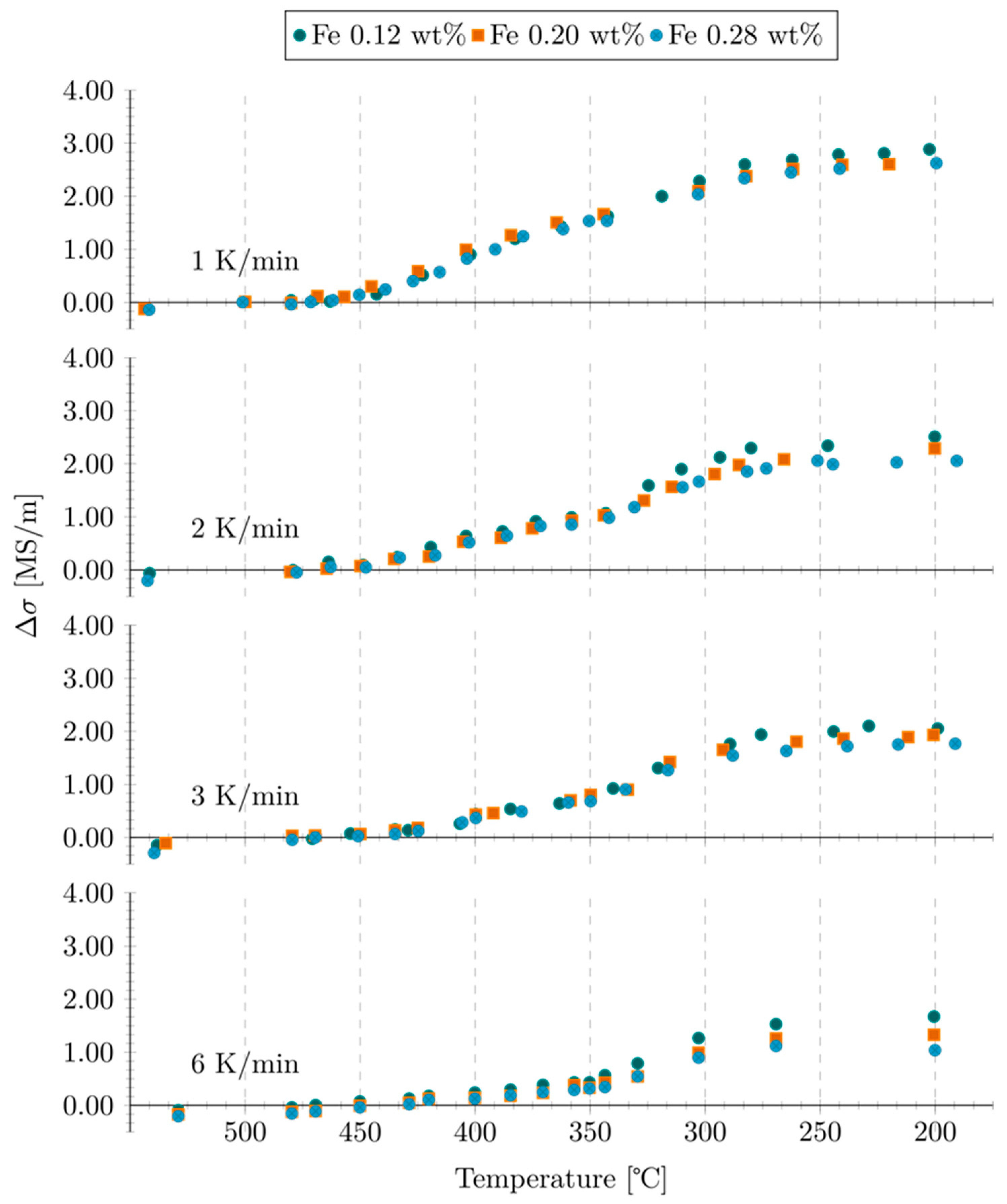
3.2. Iron Containing Alloys
3.3. Iron-Free Material
4. Discussion
5. Conclusions
- In the Ø203mm billets, the precipitation of β-Mg2Si was characterised by precipitation at the grain and dendrite boundaries above 450 °C, followed by precipitation of intragranular β-Mg2Si at a significantly lower temperature.
- With shorter secondary dendrite arm spacing, precipitation of intragranular β-Mg2Si was suppressed to a large degree, leading to an overall coarser β-Mg2Si structure.
- In iron-containing alloys, no effect of increased iron content on β-Mg2Si precipitation was found.
- For iron-free alloys, the precipitation of β-Mg2Si was found to be identical in terms of onset temperature and transformation kinetics to iron-containing alloys. In contrast to iron-containing alloys, no marked precipitation of the metastable phase was observed.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oddvin, R. Extrusion of AlMgSi Alloys. In Proceedings of the 9th International Conference on Aluminium Alloys, Brisbane, Australia, 2–5 August 2004; pp. 32–46. [Google Scholar]
- Zhu, H.L.; Couper, M.J.; Dahle, A.K. Effect of Process Variables on Mg-Si Particles and Extrudability of 6xxx Series Aluminum Extrusions. JOM 2011, 63, 66–71. [Google Scholar] [CrossRef]
- Sheppard, T. Extrusion of Aluminium Alloys; Kluwer Academic Publishers: New York, NY, USA, 1999. [Google Scholar]
- Sun, Y.; Johnson, D.R.; Trumble, K.P.; Priya, P.; Krane, J.M. Effect of Mg2Si Phase on Extrusion of AA6005 Aluminum Alloy. In Light Metals 2014; Springer: Cham, Switzerland, 2016; pp. 429–433. [Google Scholar]
- Birol, Y. Precipitation during homogenization cooling in AlMgSi alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 1875–1881. [Google Scholar] [CrossRef]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Birol, Y. The effect of homogenization practice on the microstructure of AA6063 billets. J. Mater. Process. Technol. 2004, 148, 250–258. [Google Scholar] [CrossRef]
- Zajac, S.; Bengtsson, B.; Johansson, A.; Gullmann, L.O. Optimisation of Mg2Si Phase for Extrudability of AA 6063 and AA 6005 Alloys. Mater. Sci. Forum 1996, 217–222, 397–402. [Google Scholar] [CrossRef]
- Arnoldt, A.R.; Schiffl, A.; Höppel, H.W.; Österreicher, J.A. Influence of different homogenization heat treatments on the microstructure and hot flow stress of the aluminum alloy AA6082. Mater. Charact. 2022, 191, 112129. [Google Scholar] [CrossRef]
- Falkinger, G.; Reisecker, C.; Mitsche, S. Analysis of the evolution of Mg2Si precipitates during continuous cooling and subsequent re-heating of a 6061 aluminum alloy with differential scanning calorimetry and a simple model. Int. J. Mater. Res. 2022, 113, 316–326. [Google Scholar] [CrossRef]
- Westengen, H.; Ryum, N. Precipitation reactions in an Aluminium 1-wt-percent Mg2Si alloy. Z. Fur Met. 1979, 70, 528–535. [Google Scholar]
- Qian, X.; Parson, N.; Chen, X.G. Effect of post-homogenisation cooling rate and Mn addition on Mg2Si precipitation and hot workability of AA6060 alloys. Can. Metall. Q. 2020, 59, 189–200. [Google Scholar] [CrossRef]
- Kahlenberg, R.; Wojcik, T.; Falkinger, G.; Krejci, A.L.; Milkereit, B.; Kozeschnik, E. On the precipitation mechanisms of β-Mg2Si during continuous heating of AA6061. Acta Mater. 2023, 261, 119345. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.G. Effects of Al(MnFe)Si dispersoids with different sizes and number densities on microstructure and ambient/elevated-temperature mechanical properties of extruded Al–Mg–Si AA6082 alloys with varying Mn content. J. Alloys Compd. 2021, 861, 157937. [Google Scholar] [CrossRef]
- Benarieb, I.; Puchkov, Y.A.; Sbitneva, S.V.; Zaitsev, D.V. Study of Decomposition of Supersaturated Solid Solution upon Quenching of Al–Mg–Si Alloy Sheets at Different Cooling Regimes. Phys. Met. Metallogr. 2023, 124, 901–907. [Google Scholar] [CrossRef]
- Bratland, D.H.; Grong, O.; Shercliff, H.; Myhr, O.R.; Tjotta, S. Modelling of precipitation reactions in industrial processing. Acta Mater. 1997, 45, 1–22. [Google Scholar] [CrossRef]
- Strobel, K.; Easton, M.A.; Sweet, L.; Couper, M.J.; Nie, J.F. Relating Quench Sensitivity to Microstructure in 6000 Series Aluminium Alloys. Mater. Trans. 2011, 52, 914–919. [Google Scholar] [CrossRef]
- Milkereit, B.; Starink, M.J. Quench sensitivity of Al-Mg-Si alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 76, 117–129. [Google Scholar] [CrossRef]
- Qin, S.S.; Bendo, A.; Tsuchiya, T.; Lee, S.; Zou, Y.; Matsuda, K. Effect of Cooling Rate on Precipitation during Homogenization Cooling in Balanced Al-Mg2Si Alloy. Mater. Trans. 2020, 61, 2115–2120. [Google Scholar] [CrossRef]
- Du, Q.; Jia, L.; Tang, K.; Holmedal, B. Modelling and experimental validation of microstructure evolution during the cooling stage of homogenization heat treatment of Al–Mg–Si alloys. Materialia 2018, 4, 70–80. [Google Scholar] [CrossRef]
- Miesenberger, B.; Kozeschnik, E.; Milkereit, B.; Warczok, P.; Povoden-Karadeniz, E. Computational analysis of heterogeneous nucleation and precipitation in AA6005 Al-alloy during continuous cooling DSC experiments. Materialia 2022, 25, 101538. [Google Scholar] [CrossRef]
- Van den Eynde, S.; Bracquene, E.; Diaz-Romero, D.; Zaplana, I.; Engelen, B.; Duflou, J.R.; Peeters, J.R. Forecasting global aluminium flows to demonstrate the need for improved sorting and recycling methods. Waste Manag. 2022, 139, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Dantzig, J.A.; Rappaz, M. Solidification; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hatch, J.E. Properties of Physical Metallurgy; Aluminum Association Inc.: Arlington, VA, USA; ASM International: Almere, The Netherlands, 1984. [Google Scholar]
- ASM Hanbook: Materials Characterization, 9th ed.; ASM International: Almere, The Netherlands, 1986; Volume 10.
- Aaronson, H.I.; Masato, E.; Lee, J.K. Mechanisms of Diffusional Phase Transformations in Metals and Alloys; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kelton, K.G.; Lindsey, A. Nucleation in Condensed Matter, Applications in Materials and Biolgy; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Milkereit, B.; Starink, M.J.; Rometsch, P.A.; Schick, C.; Kessler, O. Review of the Quench Sensitivity of Aluminium Alloys: Analysis of the Kinetics and Nature of Quench-Induced Precipitation. Materials 2019, 12, 4083. [Google Scholar] [CrossRef] [PubMed]
- Aveson, J.W.; Reinhart, G.; Nguyen-Thi, H.; Mangelinck-Noël, N.; Tandjaoui, A.; Billia, B.; Goodwin, K.; Lafford, T.A.; Baruchel, J.; Stone, H.J.; et al. Dendrite bending during directional solidification. In Proceedings of the 12th International Symposium on Superalloys, Seven Springs, PA, USA, 9–13 September 2012; pp. 615–624. [Google Scholar]
- Starink, M.J. Analysis of nucleation and growth with the model for diffusion-controlled precipitation reactions based on the extended volume concept. J. Alloys Compd. 2015, 630, 250–255. [Google Scholar] [CrossRef]











| Alloy | Ø [mm] | Fe [wt.%] | Mg [wt.%] | Si [wt.%] | Ti [wt.%] | Al [wt.%] |
|---|---|---|---|---|---|---|
| VIG03 | 95 | 0.00 | 0.62 | 0.36 | 0.00 | 99.00 |
| FE-00-95 | 95 | 0.00 | 0.60 | 0.36 | 0.04 | 98.99 |
| FE-12-95 | 95 | 0.12 | 0.63 | 0.40 | 0.02 | 98.79 |
| FE-20-95 | 95 | 0.19 | 0.63 | 0.41 | 0.01 | 98.71 |
| FE-28-95 | 95 | 0.28 | 0.62 | 0.43 | 0.08 | 98.55 |
| FE-12-203 | 203 | 0.11 | 0.63 | 0.39 | 0.02 | 98.81 |
| FE-20-203 | 203 | 0.19 | 0.63 | 0.40 | 0.02 | 98.73 |
| FE-28-203 | 203 | 0.27 | 0.62 | 0.42 | 0.02 | 98.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennum, E.; Marthinsen, K.; Tundal, U.H. Effect of Microstructure on the Precipitation of β-Mg2Si during Cooling after Homogenisation of Al-Mg-Si Alloys. Metals 2024, 14, 215. https://doi.org/10.3390/met14020215
Hennum E, Marthinsen K, Tundal UH. Effect of Microstructure on the Precipitation of β-Mg2Si during Cooling after Homogenisation of Al-Mg-Si Alloys. Metals. 2024; 14(2):215. https://doi.org/10.3390/met14020215
Chicago/Turabian StyleHennum, Endre, Knut Marthinsen, and Ulf H. Tundal. 2024. "Effect of Microstructure on the Precipitation of β-Mg2Si during Cooling after Homogenisation of Al-Mg-Si Alloys" Metals 14, no. 2: 215. https://doi.org/10.3390/met14020215






