Controllable Synthesis of Flower-like Hierarchical CuCo2S4 Nanostructure Arrays for High-Performance Hybrid Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of (Cu+2, Co)2(CO3)(OH)2 Nanosheets
2.3. Synthesis of CuCo2S4
2.4. Characterization
2.5. Electrochemical Measurements
2.6. Fabrication of Asymmetrical Supercapacitor (ASC)
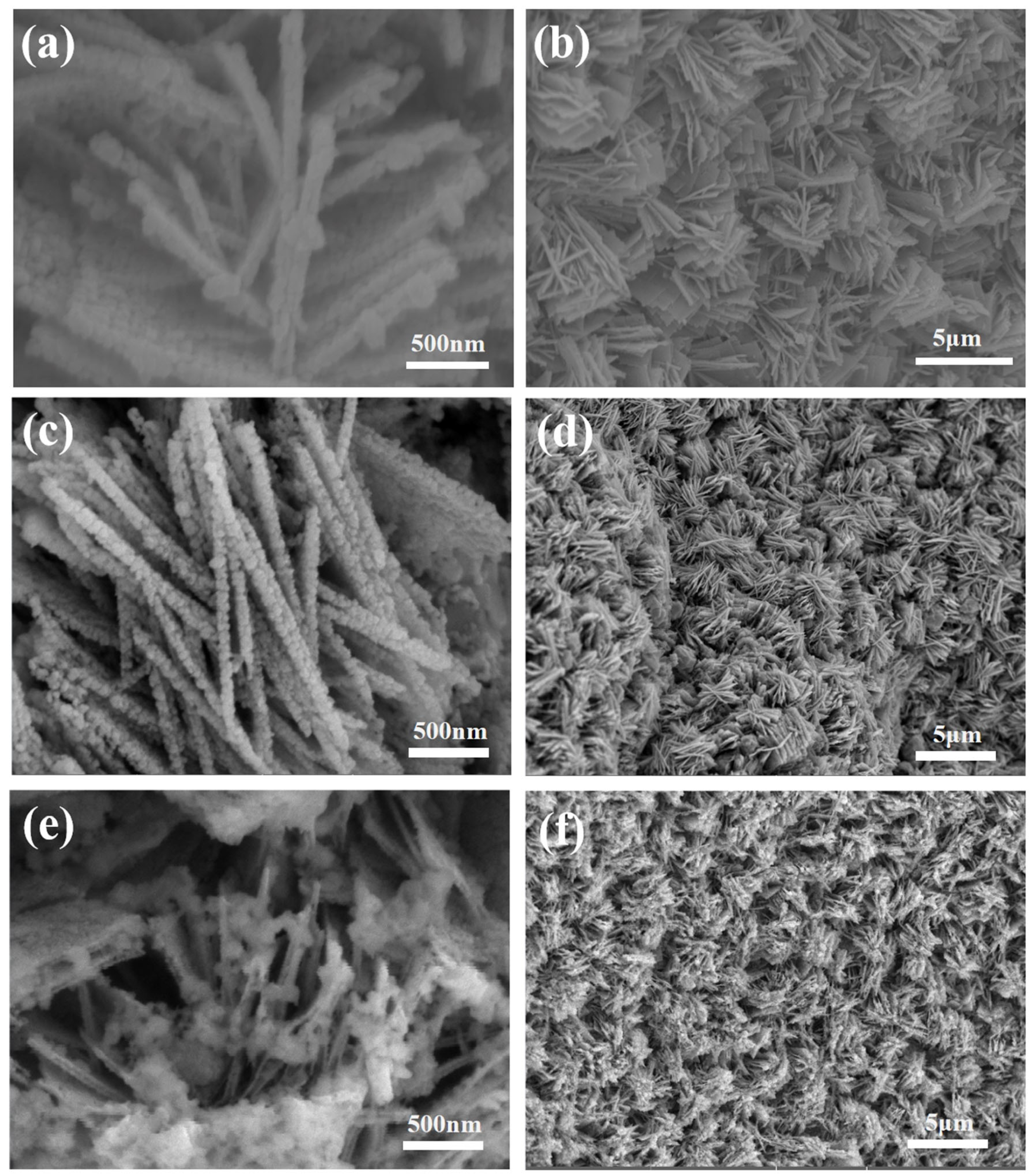
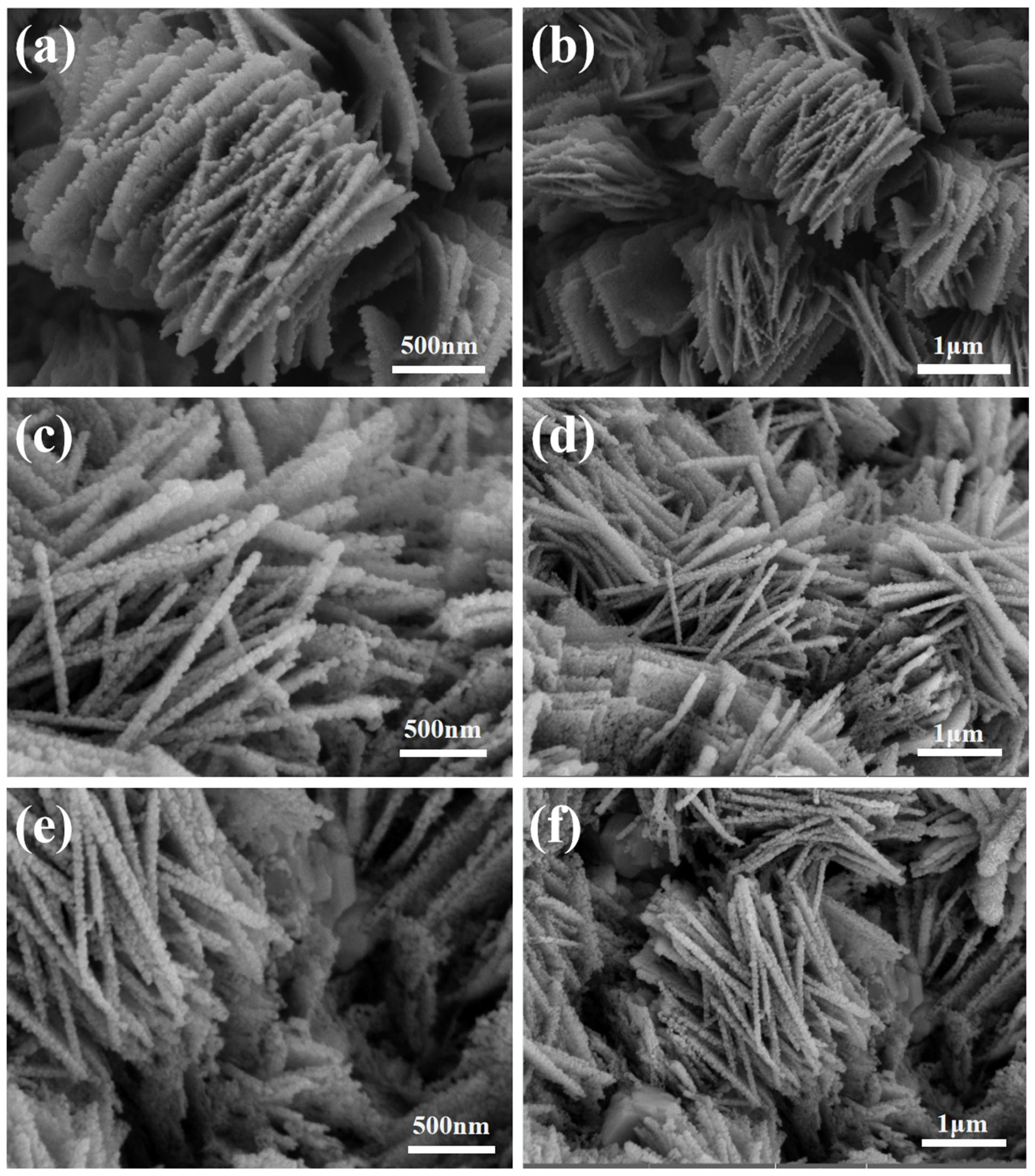
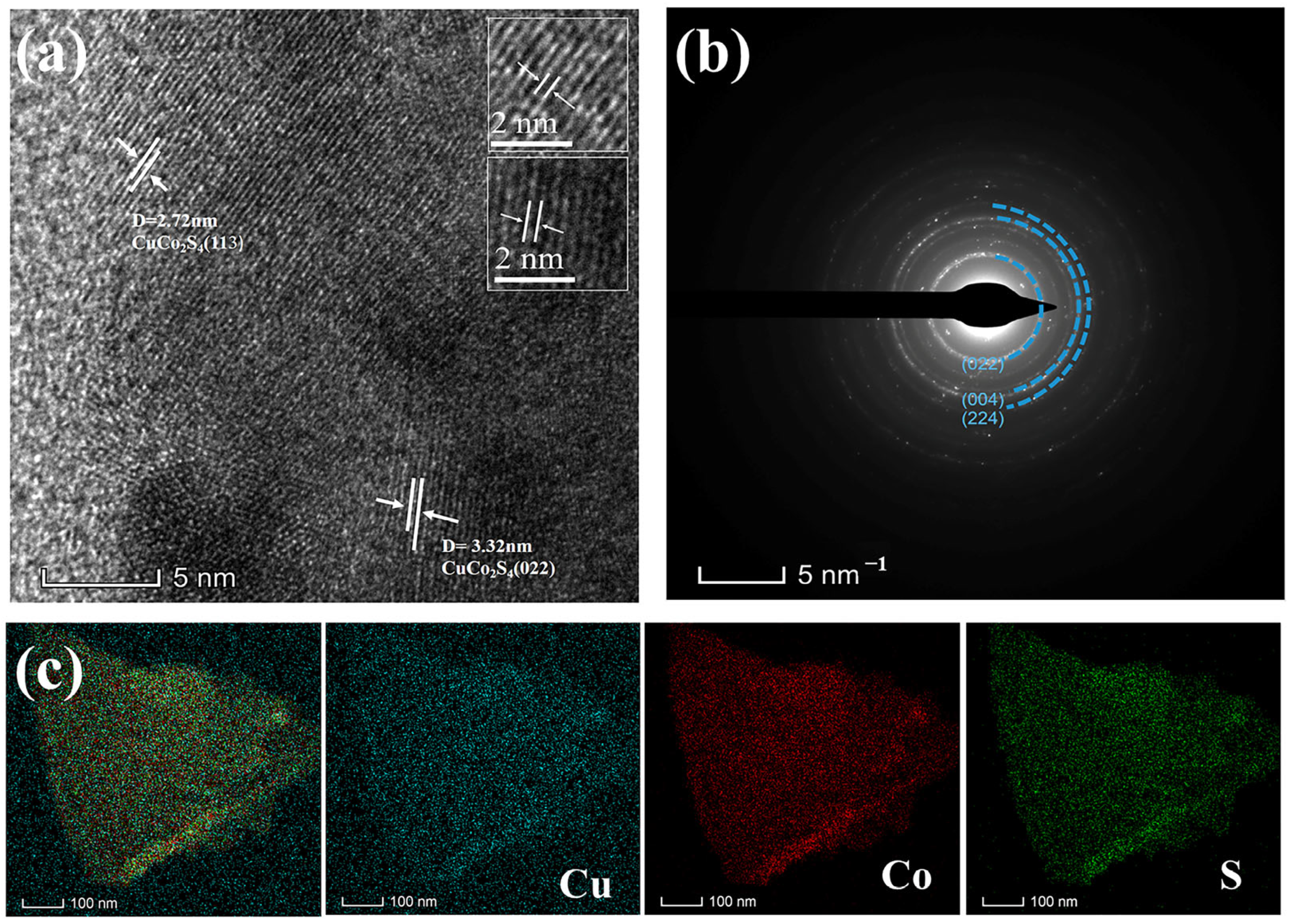
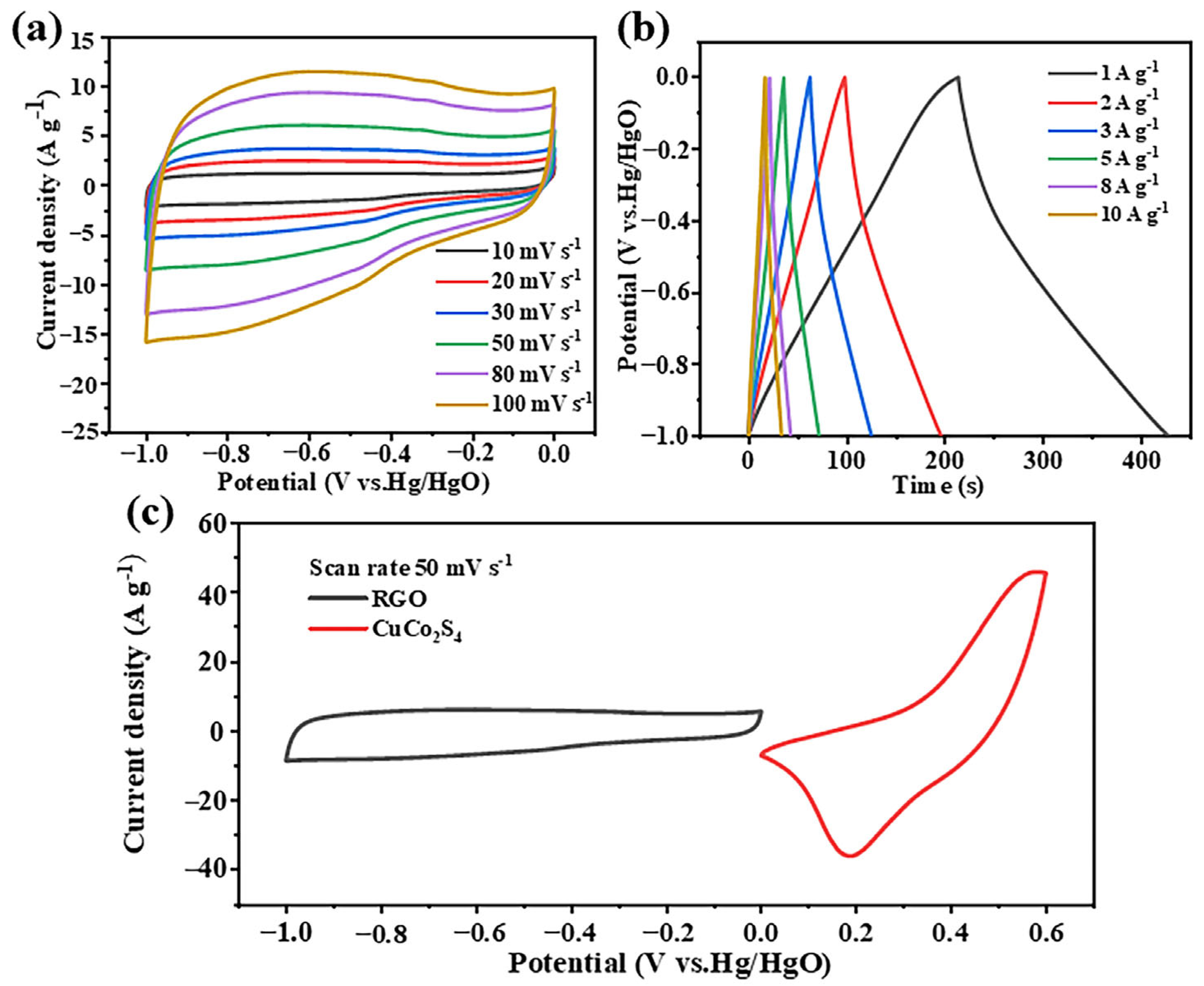
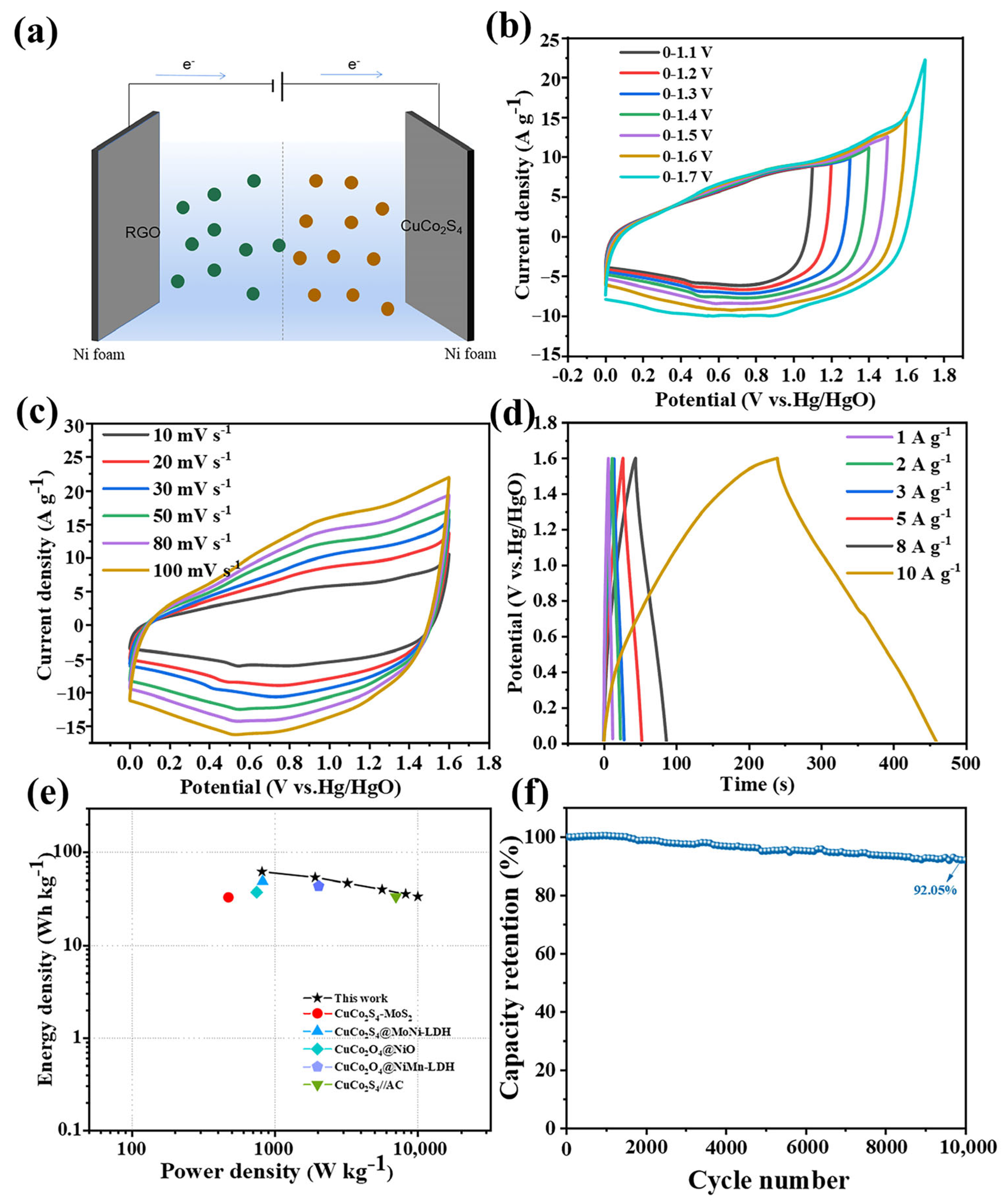
3. Results and Discussion

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yougbaré, S.; Chou, H.; Yang, C.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.; Kuo, T. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.; Kuo, T. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Chou, H.; Lin, I.; Yougbaré, S.; Chang, C.; Kuo, T. Phase-Dependent 1T/2H-MoS2 Nanosheets for Effective Photothermal Killing of Bacteria. ACS Sustain. Chem. Eng. 2022, 10, 8949–8957. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, J.; Wang, J. Morphology-controllable synthesis of CuCo2O4 arrays on Ni foam as advanced electrodes for supercapacitors. J. Alloys Compd. 2019, 789, 193–200. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ma, T.; Li, Z.; Tan, X.; Bateer, B. Design of thin-layer porous nickel cobalt sulfide for high-performance asymmetric supercapacitors. J. Alloys Compd. 2023, 945, 168902. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Yu, X.; Chen, X.; Jiang, J.; Han, S. Sponge-like 3D flower-like core-shell heterostructure CuCo2O4@CuCo2S4 as advanced electrodes for high-performance supercapacitor. J. Power Sources 2022, 551, 232186. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. High flexibility and large energy density asymmetric fibered-supercapacitor based on unique NiCo2O4@MnO2 core-shell nanobrush arrays electrode. Electrochim. Acta 2019, 295, 532–539. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Dhakal, G.; Lamiel, C.; Mohamed, S.G.; Sayed, M.S.; Lee, Y.R.; Lee, J.; Lee, M.; Shim, J. Growth of 2D nanoflakes from 1D long leaf arrays: Electrochemical influence of copper and nickel co-substituted cobalt oxide. J. Energy Storage 2020, 32, 101871. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, W.; Wang, Y.; Gao, W.; Li, J.; Liu, K.; Wang, X.; Jiang, J. Oxygen vacancies enhance supercapacitive performance of CuCo2O4 in high-energy-density asymmetric supercapacitors. J. Power Sources 2020, 458, 228005. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.; Lou, X.W.D. Nanostructured Electrode Materials for Advanced Sodium-Ion Batteries. Matter 2019, 1, 90–114. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Yu, W.; Dang, V.D.; Pandit, B.; Doong, R. Boosting supercapacitor performance with a cobalt hydroxide in-situ prepara-tion orange peel biochar flower-like composite. J. Energy Storage 2024, 81, 110302. [Google Scholar] [CrossRef]
- Saleki, F.; Mohammadi, A.; Moosavifard, S.E.; Hafizi, A.; Rahimpour, M.R. MOF assistance synthesis of nanoporous double-shelled CuCo2O4 hollow spheres for hybrid supercapacitors. J. Colloid Interface 2019, 556, 83–91. [Google Scholar] [CrossRef]
- Abo El-Reesh, G.Y.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 587. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Saeed, G.; Kim, N.H.; Lee, J.H. Zinc-nickel-cobalt oxide@NiMoO4 core-shell nanowire/nanosheet arrays for solid state asymmetric supercapacitors. Chem. Eng. J. 2020, 384, 123357. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Jia, K.; Liu, S.; Hu, C.; Qiu, J. Boosting supercapacitor performance of graphene by coupling with nitrogen-doped hollow carbon frameworks. Chem. Eur. J. 2020, 26, 2897–2903. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Zhang, Q.; Li, Y. High-performance flexible asymmetric supercapacitors based on 3D porous graphene/MnO2 nanorod and graphene/Ag hybrid thin-film electrodes. J. Mater. Chem. C 2013, 1, 1245–1251. [Google Scholar] [CrossRef]
- Wen, P.; Fan, M.; Yang, D.; Wang, Y.; Cheng, H.; Wang, J. An asymmetric supercapacitor with ultrahigh energy density based on nickle cobalt sulfide nanocluster anchoring multi-wall carbon nanotubes hybrid. J. Power Sources 2016, 320, 28–36. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Liu, X.; Wang, W.; Peng, T.; Guo, P.; Sun, H.; Yan, H.; Luo, Y. NiCo2S4/carbon nanotube nanocomposites with a chain-like architecture for enhanced supercapacitor performance. CrystEngComm 2016, 18, 7696–7706. [Google Scholar] [CrossRef]
- Dakshana, M.; Meyvel, S.; Malarvizhi, M.; Sathya, P.; Ramesh, R.; Prabhu, S.; Silambarasan, M. Facile synthesis of CuCo2S4 nanoparticles as a faradaic electrode for high performance supercapacitor applications. Vacuum 2020, 174, 109218. [Google Scholar] [CrossRef]
- Cheng, S.; Shi, T.; Chen, C.; Zhong, Y.; Huang, Y.; Tao, X.; Li, J.; Liao, G.; Tang, Z. Construction of porous CuCo2S4 nanorod arrays via anion exchange for high-performance asymmetric supercapacitor. Sci. Rep. 2017, 7, 6681. [Google Scholar] [CrossRef]
- Li, Q.; Lu, W.; Li, Z.; Ning, J.; Zhong, Y.; Hu, Y. Hierarchical MoS2/NiCo2S4@C urchin-like hollow microspheres for asymmetric supercapacitors. Chem. Eng. J. 2020, 380, 122544. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Sun, Z.; Shi, Y.; Cheng, H.; Li, F. A highly reversible Co3S4 microsphere cathode material for aluminum-ion batteries. Nano Energy 2019, 56, 100–108. [Google Scholar] [CrossRef]
- Bahaa, A.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Metal-organic framework derived hierarchical copper cobalt sulfide nanosheet arrays for high-performance solid-state asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 8620–8632. [Google Scholar] [CrossRef]
- Liu, S.; Jun, S.C. Hierarchical manganese cobalt sulfide core-shell nanostructures for high-performance asymmetric supercapacitors. J. Power Sources 2017, 342, 629–637. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Wu, H.; Yu, X.; Zhang, X.; Lou, X. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694. [Google Scholar] [CrossRef]
- Liu, S.; San Hui, K.; Hui, K.N.; Yun, J.M.; Kim, K.H. Vertically stacked bilayer CuCo2O4/MnCo2O4 heterostructures on functionalized graphite paper for high-performance electrochemical capacitors. J. Mater. Chem. A 2016, 4, 8061–8071. [Google Scholar] [CrossRef]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Dong, P.; Ajayan, P.M.; Shen, J.; Ye, M. Mesostructured CuCo2S4/CuCo2O4 nanoflowers as advanced electrodes for asymmetric supercapacitors. J. Power Sources 2018, 400, 96–103. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Wu, Z.; Sun, M.; Han, S.; Cai, C.; Shen, W.; Liu, X.; Fu, Y. Enhanced electrochemical performance of CuCo2S4/carbon nanotubes composite as electrode material for supercapacitors. J. Colloid Interface Sci. 2019, 549, 105–113. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Yin, Y.; Fan, L.; Zhang, N.; Sun, K. In Situ Synthesis of CuCo2S4 @N/S-Doped Graphene Composites with Pseudocapacitive Properties for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 11708–11714. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Y.; Wang, Z.; Zheng, X.; Li, H.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Sea urchin-like CuCo2S4 microspheres with a controllable interior structure as advanced electrode materials for high-performance supercapacitors. Inorg. Chem. Front. 2020, 7, 603–609. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Y.; Shen, J.; Ye, M. Facile synthesis of CuCo2S4 as a novel electrode material for ultrahigh supercapacitor performance. Chem. Commun. 2020, 52, 1509–1512. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, H.; Li, H.; Xu, S.; Lv, S.; Wang, M. Controllable preparation of CuCo2S4 nanotube arrays for high-performance hybrid supercapacitors. Electrochim. Acta 2022, 404, 139681. [Google Scholar] [CrossRef]
- Fan, L.; Pan, F.; Tu, Q.; Gu, Y.; Huang, J.; Huang, Y.; Wu, J. Synthesis of CuCo2S4 nanosheet arrays on Ni foam as binder-free electrode for asymmetric supercapacitor. Int. J. Hydrogen Energy 2018, 43, 23372–23381. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Zhou, T.; Pan, J.; Wei, T.; Sun, Y. Oriented CuCo2S4 nanograss arrays/Ni foam as an electrode for a high-performance all-solid-state supercapacitor. Nanotechnology 2020, 28, 465402. [Google Scholar] [CrossRef]
- Tsai, M.; Chen, T.; Juang, Y.; Hua, L.; Huang, C. High catalytic performance of CuCo/nickel foam electrode for ammonia electrooxidation. Electrochem. Commun. 2020, 121, 106875. [Google Scholar] [CrossRef]
- Guan, B.; Zhao, Y.; Zhang, N.; Zhang, J.; Sun, T.; Yi, T. Highly uniform platanus fruit-like CuCo2S4 microspheres as an electrode material for high performance lithium-ion batteries and supercapacitors. Dalton T 2021, 50, 13042–13051. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Cui, Z.; Hua, Y.; Wang, C.; Zhao, X.; Liu, X. Hierarchical copper cobalt sulfide nanobelt arrays for high performance asymmetric supercapacitors. Inorg. Chem. Front. 2021, 8, 325–336. [Google Scholar] [CrossRef]
- Mane, S.M.; Teli, A.M.; Yang, H.K.; Kwon, E.; Nimbalkar, N.A.; Patil, D.R.; Shin, J.C. Nanoneedles anchored ultrathin petals of CuCo layered double hydroxide with high areal capacitance and long cycle life for high-performance hybrid supercapacitors. J. Energy Storage 2023, 62, 106941. [Google Scholar] [CrossRef]
- Kumar, L.; Boruah, P.K.; Borthakur, S.; Saikia, L.; Das, M.R.; Deka, S. CuCo-Layered Double Hydroxide Nanosheet-Based Polyhedrons for Flexible Supercapacitor Cells. ACS Appl. Nano Mater. 2021, 4, 5250–5262. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Du, J.; Yan, Q.; Li, Y.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; Zhang, X.; Wang, G. Hierarchical copper cobalt sulfides nanowire arrays for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2019, 487, 198–205. [Google Scholar] [CrossRef]
- Tang, N.; You, H.; Li, M.; Chen, G.Z.; Zhang, L. Cross-linked Ni(OH)2/CuCo2S4/Ni networks as binder-free electrodes for high performance supercapatteries. Nanoscale 2018, 10, 20526–20532. [Google Scholar] [CrossRef]
- You, H.; Zhang, L.; Jiang, Y.; Shao, T.; Li, M.; Gong, J. Bubble-supported engineering of hierarchical CuCo2S4 hollow spheres for enhanced electrochemical performance. J. Mater. Chem. A 2018, 6, 5265–5270. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Yang, H.; Zhang, W.; Qin, T.; Wang, Z.; Molin, S.; Jasinski, P.; Zheng, W. Cu-Doped Layered Double Hydroxide Constructs the Performance-Enhanced Supercapacitor Via Band Gap Reduction and Defect Triggering. ACS Appl. Energy Mater. 2022, 5, 2192–2201. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Lu, G. Controlled Synthesis of Flower-like Hierarchical NiCo-Layered Double Hydroxide Integrated with Metal-Organic Framework-Derived Co@C for Supercapacitors. ACS Appl. Mater. Interface 2023, 15, 36143–36153. [Google Scholar] [CrossRef]
- Du, X.; Su, H.; Zhang, X. Metal-Organic Framework-Derived Cu-Doped Co9S8 Nanorod Array with Less Low-Valence Co Sites as Highly Efficient Bifunctional Electrodes for Overall Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 16917–16926. [Google Scholar] [CrossRef]
- Peng, H.; Wei, C.; Wang, K.; Meng, T.; Ma, G.; Lei, Z.; Gong, X. Ni0.85Se@MoSe2 Nanosheet Arrays as the Electrode for High-Performance Supercapacitors. ACS Appl. Mater. Interface 2017, 9, 17067–17075. [Google Scholar] [CrossRef]
- Hasan, S.; Reaz, A.H.; Das, S.; Roy, C.K.; Basith, M.A. CuCo2S4-MoS2 nanocomposite: A novel electrode for high-performance supercapacitors. J. Mater. Chem. C 2022, 10, 7980–7996. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Q.; Lu, L.; Zou, Y.; Xu, F.; Sun, L.; Cai, D.; Xiang, C. Hollow core–shell CuCo2O4@MoNi-layered double hydroxides as an electrode material for supercapacitors. J. Energy Storage 2023, 61, 106691. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, X.; Wu, N.; Zhao, X.; Gong, J. Dandelion-like CuCo2O4@ NiMn LDH Core/Shell Nanoflowers for Excellent Battery-Type Supercapacitor. Nanomaterials 2023, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ma, S.; Shen, Y.; Ren, Q.; Yang, J.; Chen, X.; Hu, J. CuCo2O4 nanowire arrays wrapped in metal oxide nanosheets as hierarchical multicomponent electrodes for supercapacitors. Chem. Eng. J. 2019, 369, 363–369. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yu, N.; Xu, L.; Wang, W.; Wei, F.; Qi, J.; Sui, Y. Controllable Synthesis of Flower-like Hierarchical CuCo2S4 Nanostructure Arrays for High-Performance Hybrid Supercapacitors. Metals 2024, 14, 145. https://doi.org/10.3390/met14020145
Li M, Yu N, Xu L, Wang W, Wei F, Qi J, Sui Y. Controllable Synthesis of Flower-like Hierarchical CuCo2S4 Nanostructure Arrays for High-Performance Hybrid Supercapacitors. Metals. 2024; 14(2):145. https://doi.org/10.3390/met14020145
Chicago/Turabian StyleLi, Man, Ningning Yu, Lei Xu, Wenyu Wang, Fuxiang Wei, Jiqiu Qi, and Yanwei Sui. 2024. "Controllable Synthesis of Flower-like Hierarchical CuCo2S4 Nanostructure Arrays for High-Performance Hybrid Supercapacitors" Metals 14, no. 2: 145. https://doi.org/10.3390/met14020145




