Microstructures and Electrical Resistivity of Aluminum–Copper Joints
Abstract
:1. Introduction
2. Experimental Details
3. Results
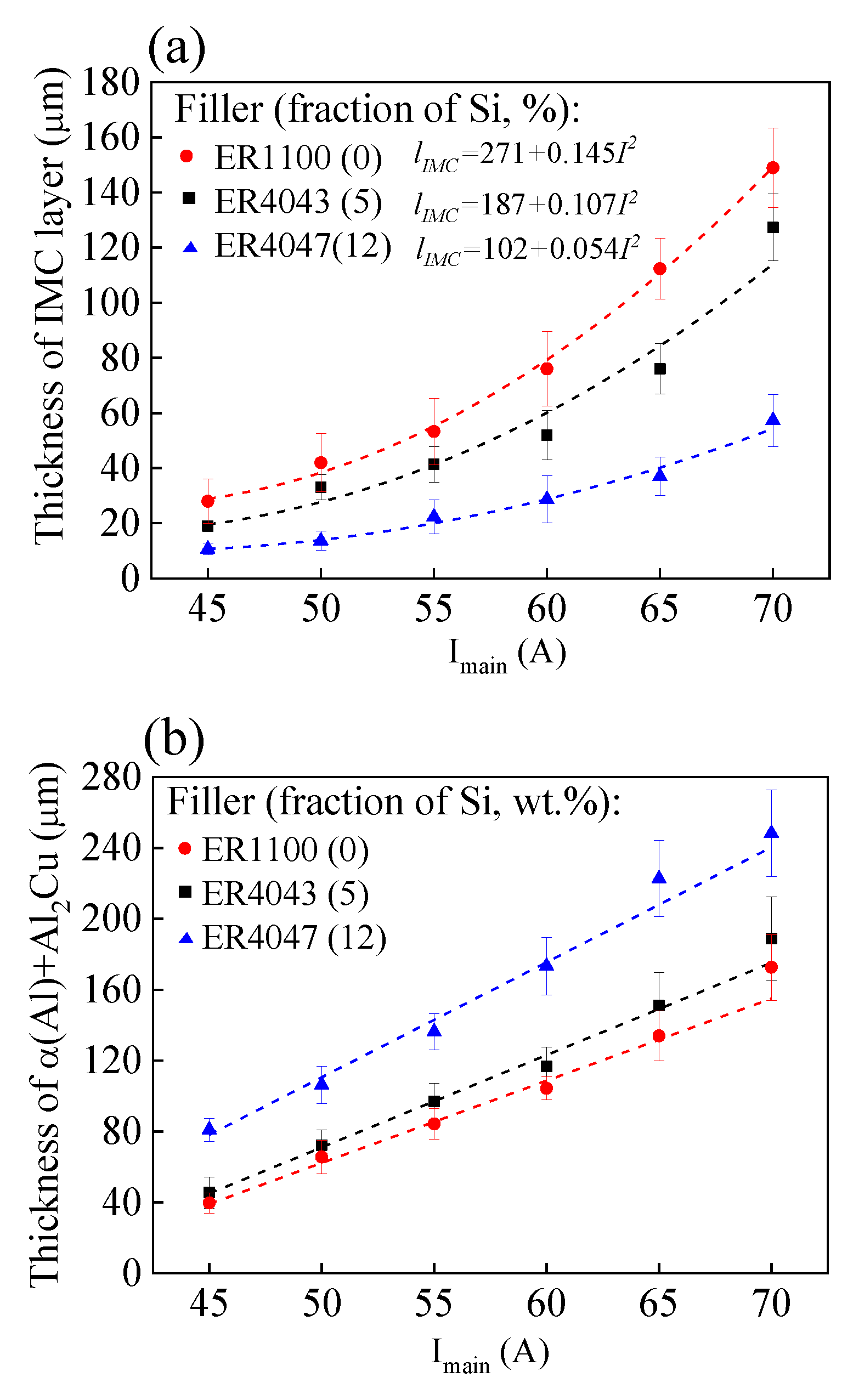
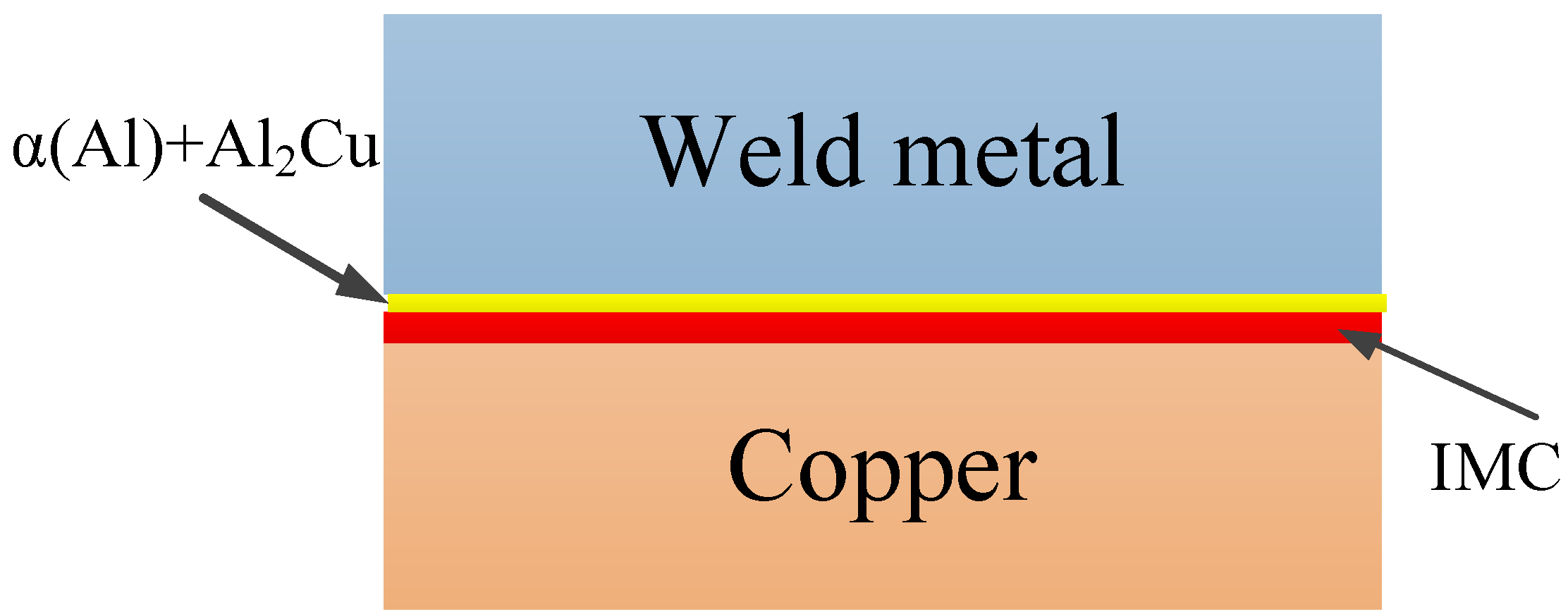
3.1. Al-Cu Interface
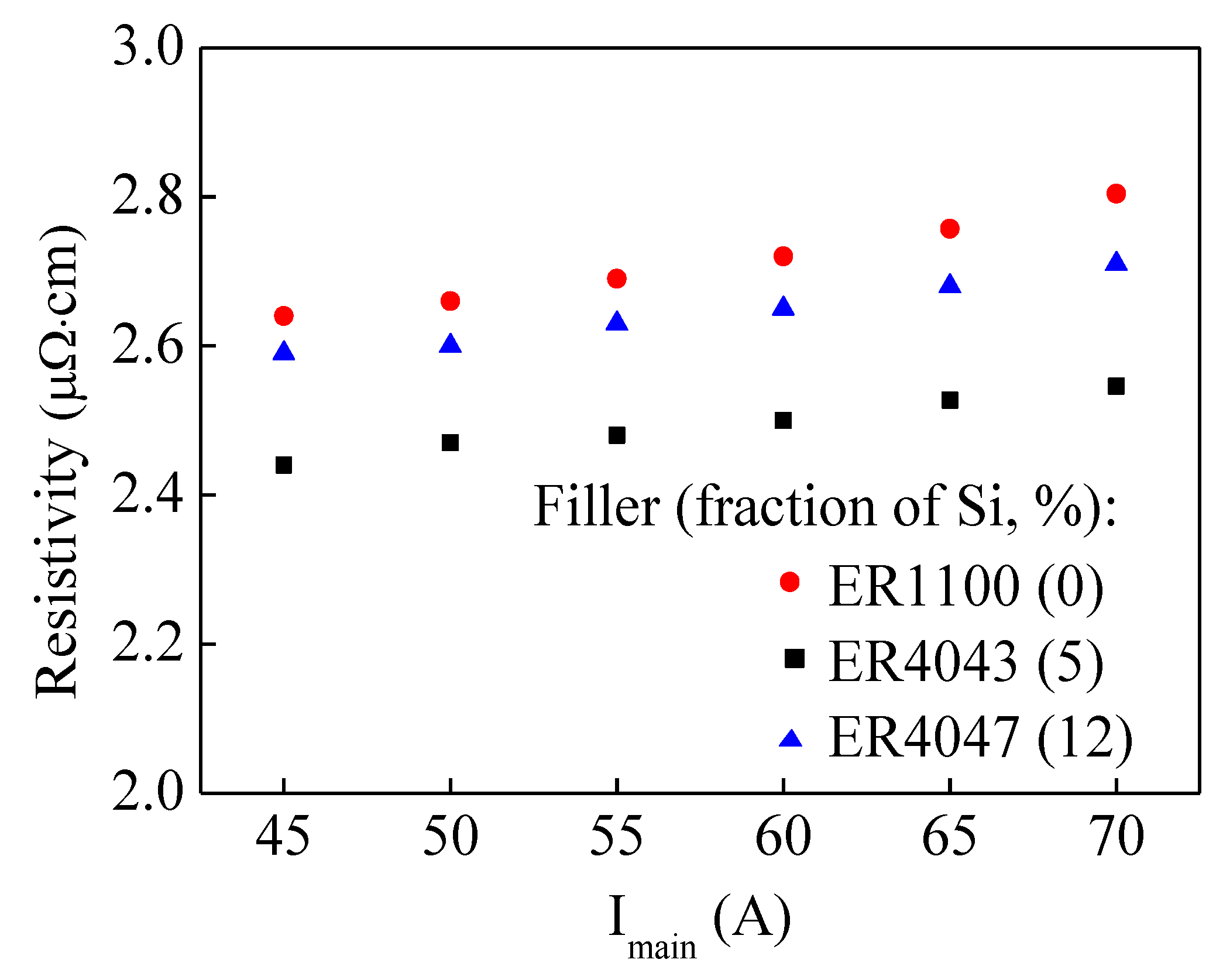
3.2. Electric Resistivity
4. Discussion
4.1. Al-Cu Interface
4.2. Electric Resistivity
5. Conclusions
- There are two layers formed around the Al-Cu interface during welding, which are sandwiched between the base metals of Al and Cu. One layer consists of the IMC of Al2Cu, and the other consists of α(Al) + Al2Cu.
- Si suppresses the growth of the IMC layer and assists the growth of the layer of α(Al) + Al2Cu.
- Increasing the welding current causes an increase in the apparent resistivity of the Al-Cu joints. Both the IMC layer and the layer of α(Al) + Al2Cu play important roles in the electric conduction of the Al-Cu joints.
- Increasing the fraction of Si leads to an increase in the apparent resistivity of the Al-Cu joints. The microstructure of the Al-Cu joint could be predicted based on the electric resistivity in the future.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Leon, M.; Shin, H. Review of the advancements in aluminum and copper ultrasonic welding in electric vehicles and superconductor applications. J. Mater. Process Technol. 2022, 307, 117691. [Google Scholar] [CrossRef]
- Paz Martínez-Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Song, L.; Zhang, Y.; Wei, S. Research and development status of laser micro-welding of aluminum-copper dissimilar metals: A review. Opt. Laser Eng. 2023, 161, 107312. [Google Scholar] [CrossRef]
- Bunaziv, I.; Akselsen, O.M.; Ren, X.; Nyhus, B.; Eriksson, M.; Gulbrandsen-Dahl, S. A review on laser-assisted joining of aluminium alloys to other metals. Metals 2021, 11, 1680. [Google Scholar] [CrossRef]
- Dimatteo, V.; Ascari, A.; Fortunato, A. Dissimilar laser welding of copper and aluminum alloys in multilayer configuration for battery applications. J. Laser Appl. 2021, 33, 4. [Google Scholar] [CrossRef]
- Alba-Galvín, J.J.; González-Rovira, L.; Bethencourt, M.; Botana, F.J.; Sánchez-Amaya, J.M. Influence of aerospace standard surface pretreatment on the intermetallic phases and CeCC of 2024-T3 Al-Cu alloy. Metals 2019, 9, 320. [Google Scholar] [CrossRef]
- Zoeram, A.S.; Anijdan, S.M.; Jafarian, H.R.; Bhattacharjee, T. Welding parameters analysis and microstructural evolution of dissimilar joints in Al/Bronze processed by friction stir welding and their effect on engineering tensile behavior. Mater. Sci. Eng. A 2017, 687, 288–297. [Google Scholar] [CrossRef]
- Tien, N.T.; Lo, Y.; Raza, M.M.; Chen, C.; Chiu, C. Optimization of processing parameters for pulsed laser welding of dissimilar metal interconnects. Opt. Laser Technol. 2023, 159, 109022. [Google Scholar] [CrossRef]
- Asemabadi, M.; Sedighi, M.; Honarpisheh, M. Investigation of cold rolling influence on the mechanical properties of explosive-welded Al/Cu bimetal. Mater. Sci. Eng. A 2012, 558, 144–149. [Google Scholar] [CrossRef]
- Paul, H.; Lityńska-Dobrzyńska, L.; Prażmowski, M. Microstructure and phase constitution near the interface of explosively welded aluminum/copper plates. Metall. Mater. Trans. A 2013, 44, 3836–3851. [Google Scholar] [CrossRef]
- Ouyang, J.; Yarrapareddy, E.; Kovacevic, R. Microstructural evolution in the friction stir welded 6061 aluminum alloy (T6-temper condition) to copper. J. Mater. Process. Technol. 2006, 172, 110–122. [Google Scholar]
- Tan, C.W.; Jiang, Z.G.; Li, L.Q.; Chen, Y.B.; Chen, X.Y. Microstructural evolution and mechanical properties of dissimilar Al–Cu joints produced by friction stir welding. Mater. Des. 2013, 51, 466–473. [Google Scholar] [CrossRef]
- Ni, Z.L.; Ye, F.X. Weldability and mechanical properties of ultrasonic joining of aluminum to copper alloy with an interlayer. Mater. Lett. 2016, 182, 19–22. [Google Scholar] [CrossRef]
- Cai, Z.P.; Ai, B.Q.; Cao, R.; Lin, Q.; Chen, J.H. Microstructure and properties of aluminum AA6061-T6 to copper (Cu)-T2 joints by cold metal transfer joining technology. J. Mater. Res. 2016, 31, 2876–2887. [Google Scholar] [CrossRef]
- Kraetzsch, M.; Standfuss, J.; Klotzbach, A.; Kaspar, J.; Brenner, B.; Beyer, E. Laser Beam Welding with High-Frequency Beam Oscillation: Welding of Dissimilar Materials with Brilliant Fiber Lasers; AIP Publishing: Long Island, NY, USA, 2011; pp. 169–178. [Google Scholar]
- Xue, P.; Ni, D.R.; Wang, D.; Xiao, B.L.; Ma, Z.Y. Effect of friction stir welding parameters on the microstructure and mechanical properties of the dissimilar Al-Cu joints. Mater. Sci. Eng. A 2011, 528, 4683–4689. [Google Scholar] [CrossRef]
- Muhammad, N.A.; Wu, C.S.; Tian, W. Effect of ultrasonic vibration on the intermetallic compound layer formation in Al/Cu friction stir weld joints. J. Alloys Compd. 2019, 785, 512–522. [Google Scholar] [CrossRef]
- Bergmann, J.P.; Petzoldt, F.; Schürer, R.; Schneider, S. Solid-state welding of aluminum to copper—Case studies. Weld. World 2013, 57, 541–550. [Google Scholar] [CrossRef]
- Chen, C.; Fan, C.; Cai, X.; Lin, S.; Yang, C. Analysis of droplet transfer, weld formation and microstructure in Al-Cu alloy bead welding joint with pulsed ultrasonic-GMAW method. J. Mater. Process. Technol. 2019, 271, 144–151. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, X.; Liu, J.; Li, P. Interfacial microstructure and reaction mechanism with various weld fillers on laser welding-brazing of Al/Cu lap joint. J. Manuf. Process 2021, 67, 226–240. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, H.; Jin, K.; He, J.; Ge, W.; Yan, W. Modeling of microstructure evolution coupled with molten pool oscillation during electron beam welding of an Al-Cu alloy. Int. J. Heat. Mass. Tran. 2022, 189, 122735. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.; Sun, Q.; Liu, J.; Wu, L. Microstructures and Properties of Aluminum–Copper Lap-Welded Joints by Cold Metal Transfer Technology. Adv. Eng. Mater. 2015, 17, 1480–1485. [Google Scholar] [CrossRef]
- Mohanraj, N.; Kumar, N.M.; Prathap, P.; Ganeshan, P.; Raja, K.; Mohanavel, V.; Karthick, A.; Muhibbullah, M. Mechanical properties and electrical resistivity of the friction stir spot-welded dissimilar Al–Cu joints. Int. J. Polym. Sci. 2022, 2022, 4130440. [Google Scholar] [CrossRef]
- Braunovic, M.; Alexandrov, N. Intermetallic compounds at aluminum-to-copper electrical interfaces: Effect of temperature and electric current. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1994, 17, 78–85. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Y.; Mehta, K.P. Metallurgical and mechanical properties of Al–Cu joint by friction stir spot welding and modified friction stir clinching. Met. Mater. Int. 2021, 27, 3085–3094. [Google Scholar] [CrossRef]
- Su, H.; Zhao, Q.; Chen, J.; Wu, C. Homogenizing the intermetallic compounds distribution in Al/Cu dissimilar friction stir welding joint with the assistance of ultrasonic vibration. Mater. Today Commun. 2022, 31, 103643. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Gu, Y.; Li, C. Effect of different filler wires on mechanical property and conductivity of aluminum-copper joints. Materials 2020, 13, 3648. [Google Scholar] [CrossRef]
- Shi, Y.; Shao, L.; Huang, J.; Gu, Y. Effects of Si and Mg elements on the microstructure of aluminum–steel joints produced by pulsed DE-GMA welding–brazing. Mater. Sci. Technol. 2013, 29, 1118–1124. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Shi, Y.; Zhu, M.; Yang, F. Microstructures and mechanical behavior of aluminum-copper lap joints. Mater. Sci. Eng. A 2017, 705, 105–113. [Google Scholar] [CrossRef]
- Ponweiser, N.; Lengauer, C.L.; Richter, K.W. Re-investigation of phase equilibria in the system Al–Cu and structural analysis of the high-temperature phase η1-Al1−δCu. Intermetallics 2011, 19, 1737–1746. [Google Scholar] [CrossRef]
- Doty, H.W.; Samuel, A.M.; Samuel, F.H. Factors Controlling the Type and Morphology of Cu-Containing Phases in the 319 Aluminum Alloy. In Proceedings of the 100th AFS Casting Congress, American Foundry Society, Philadelphia, PA, USA, 20–23 April 1996; pp. 20–23. [Google Scholar]
- Samuel, A.M.; Gauthier, J.; Samuel, F.H. Microstructural aspects of the dissolution and melting of Al 2 Cu phase in Al-Si alloys during sol, ution heat treatment. Metall. Mater. Trans. A 1996, 27, 1785–1798. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, J.; Lan, W.; Zeng, D. Effect of silicon phase on copper diffusion speed in the Al-Si-Cu alloys. Heat Treat. Met. 2006, 31, 13–16. [Google Scholar]







| Al Wires | Si | Cu | Mg | Fe | Zn | Mn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| ER1100 | 0.03 | 0.002 | - | 0.18 | 0.013 | 0.003 | - | Bal. |
| ER4043 | 5.00 | 0.30 | 0.05 | 0.80 | 0.10 | 0.05 | 0.20 | Bal. |
| ER4047 | 12.00 | 0.30 | 0.10 | 0.80 | 0.20 | 0.15 | - | Bal. |
| Al Wires | Points | Al | Cu | Si | Possible Phase |
|---|---|---|---|---|---|
| ER1100 | A | 69.3 | 30.7 | - | Al2Cu |
| B | 82.6 | 17.4 | - | α(Al) + Al2Cu | |
| ER4043 | C | 67.7 | 32 | 0.3 | Al2Cu |
| D | 84.7 | 13.8 | 1.5 | α(Al) + Al2Cu | |
| ER4047 | E | 66.2 | 33.1 | 0.7 | Al2Cu |
| F | 83.8 | 14.6 | 1.6 | α(Al) + Al2Cu | |
| G | 1.2 | 0.7 | 98.1 | Si |
| Materials | ER1100 | ER4043 | ER4047 | T2 Copper |
|---|---|---|---|---|
| Resistivity (μΩ·cm) | 2.86 | 3.18 | 3.52 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, C.; Bian, J.; Zhang, J.; Geng, B. Microstructures and Electrical Resistivity of Aluminum–Copper Joints. Metals 2023, 13, 1474. https://doi.org/10.3390/met13081474
Guo J, Li C, Bian J, Zhang J, Geng B. Microstructures and Electrical Resistivity of Aluminum–Copper Joints. Metals. 2023; 13(8):1474. https://doi.org/10.3390/met13081474
Chicago/Turabian StyleGuo, Jinchang, Chunkai Li, Jianxiao Bian, Jianrui Zhang, and Baolong Geng. 2023. "Microstructures and Electrical Resistivity of Aluminum–Copper Joints" Metals 13, no. 8: 1474. https://doi.org/10.3390/met13081474





