Microstructure Evolution and Growth of Interfacial Intermetallic Compounds in NiCr/Ti Alloy Laminated Composite after Explosive Welding and Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
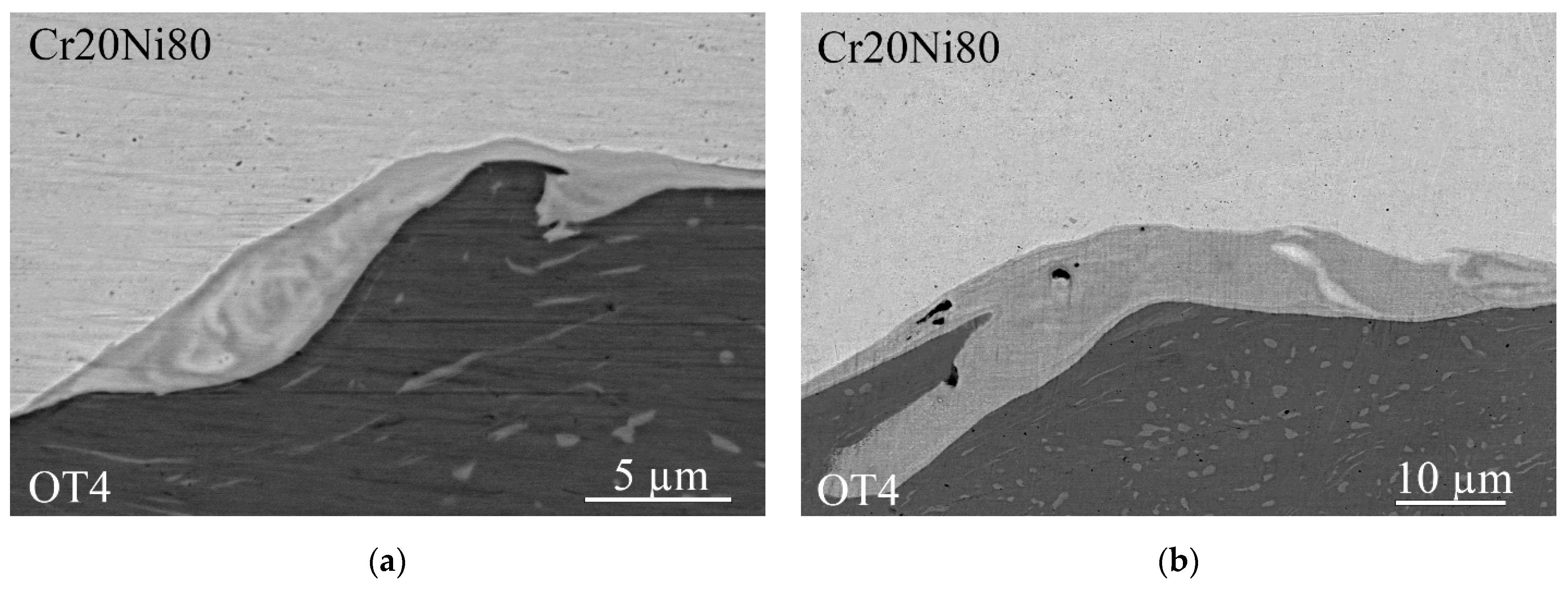
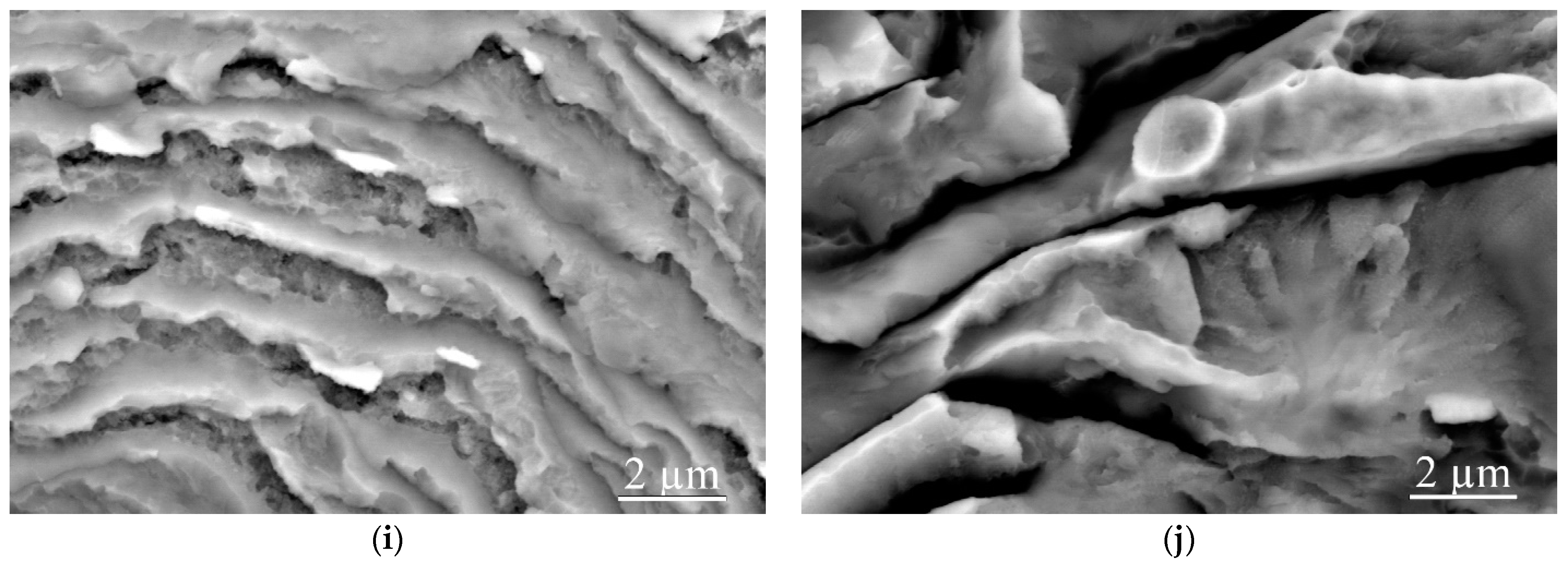
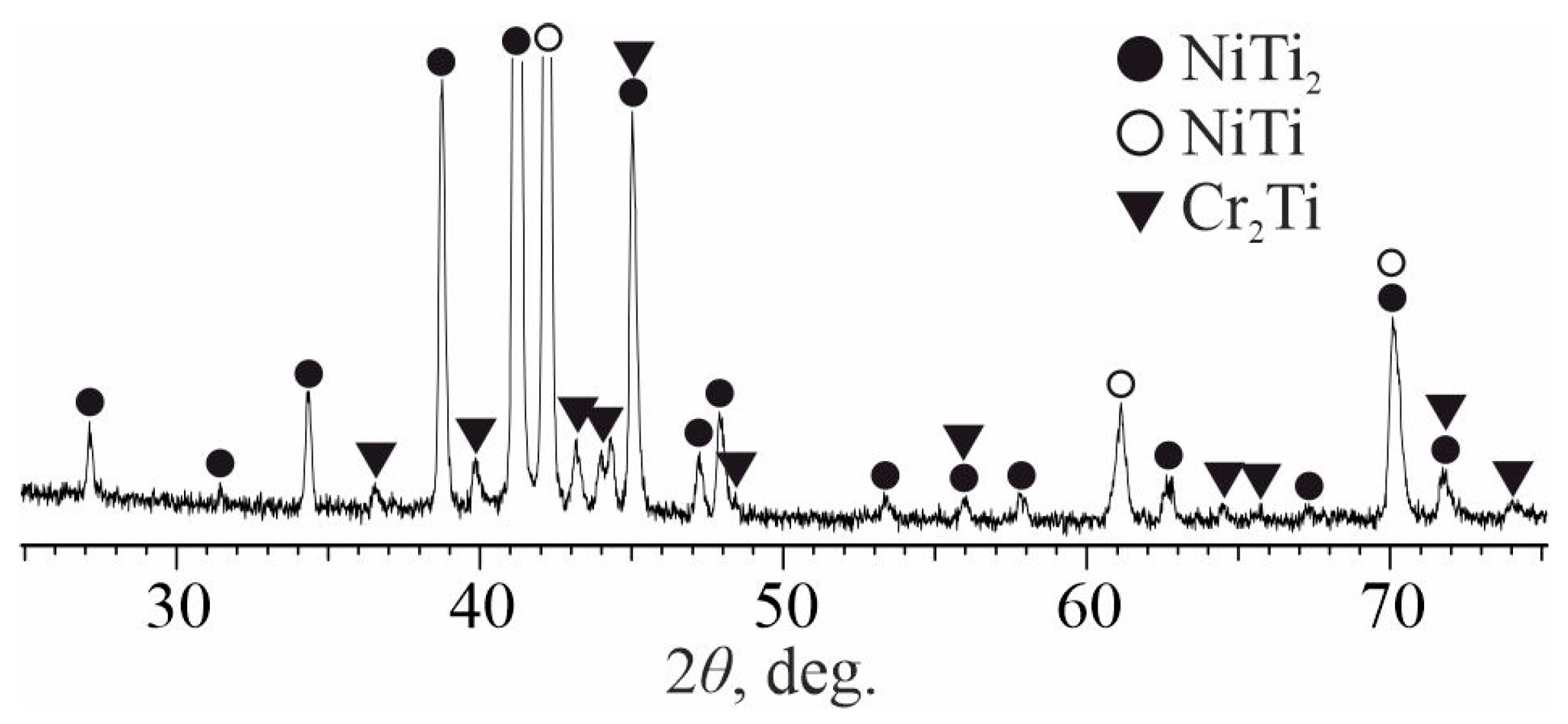
3.1. As-Welded State
3.2. Calculation of the Cooling Rate and the Lifetime of the Molten Metal in the Liquid State during the Welding Process
- -
- The values of γ, c, and Sm for the molten metal are determined by the mixture rule;
- -
- The values of the constants of the metals that make up the alloy are used at a normal temperature (Table 4).
- -
- The maximum molten metal temperature Tm was assumed to be equal to the liquidus temperature of the Ti-Ni-Cr system alloy with a similar chemical composition [36];
- -
- The solidus temperature Ts under conditions of high cooling rates is equal to 0.5 times the absolute melting temperature of the NiCr alloy;
- -
- The average molten metal cooling rate Vave in the crystallization interval is equal to the arithmetic mean of the cooling rates at the boundary points of the liquidus (VTm) and solidus (VTs) intervals;
- -
- Taking into account the heat transfer to two semi-infinite plates, we used the effective coefficient of thermal conductivity
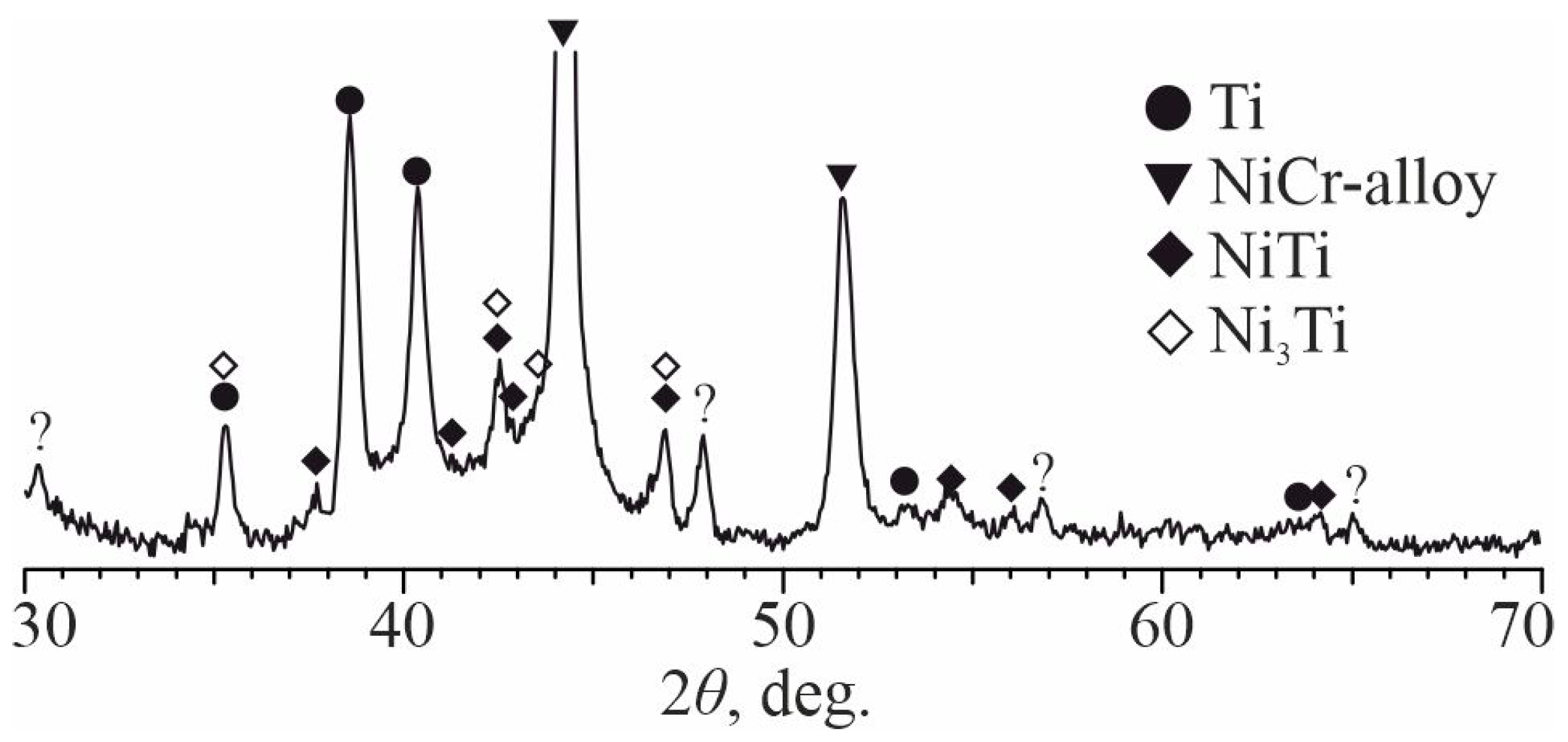
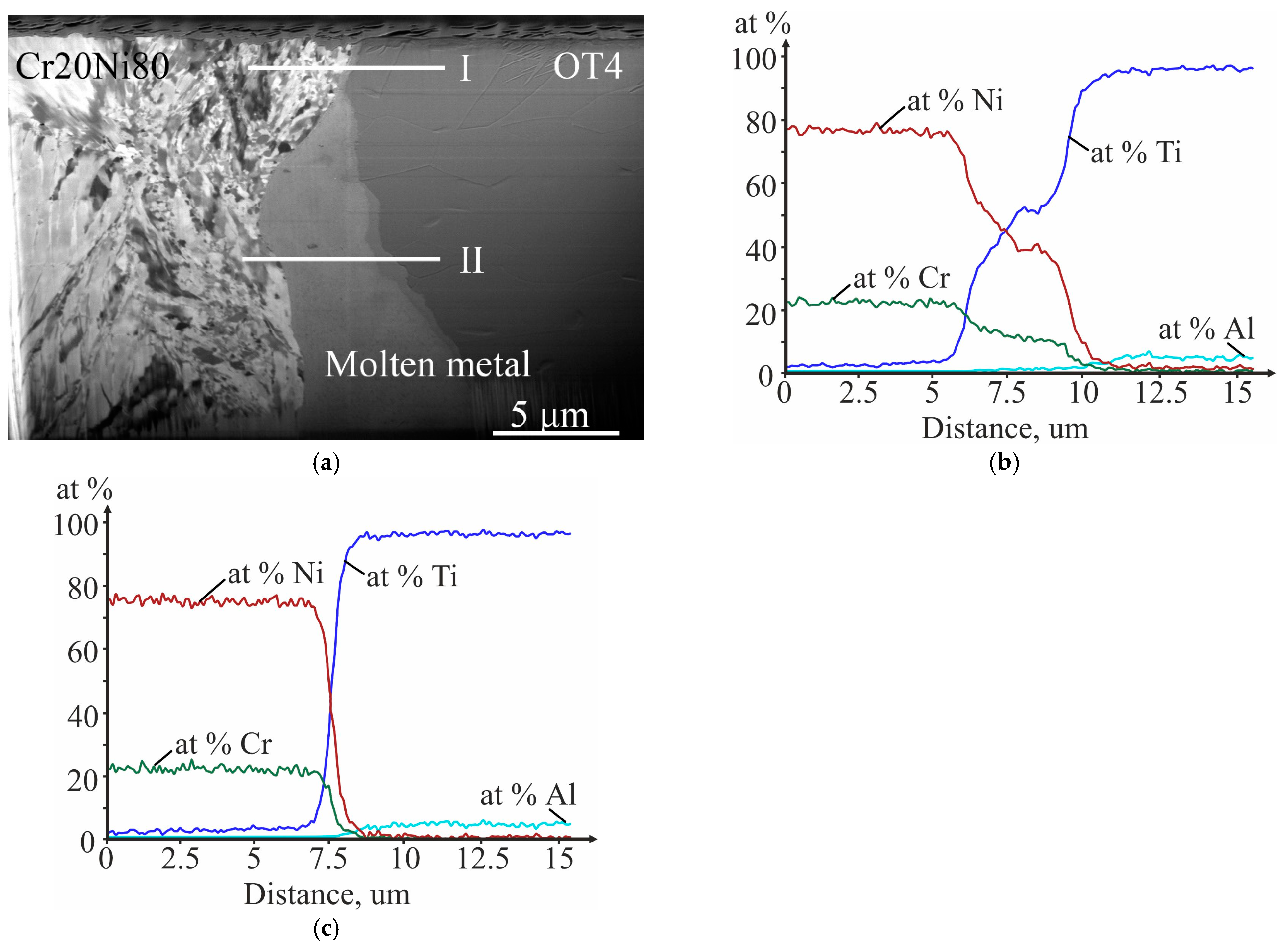
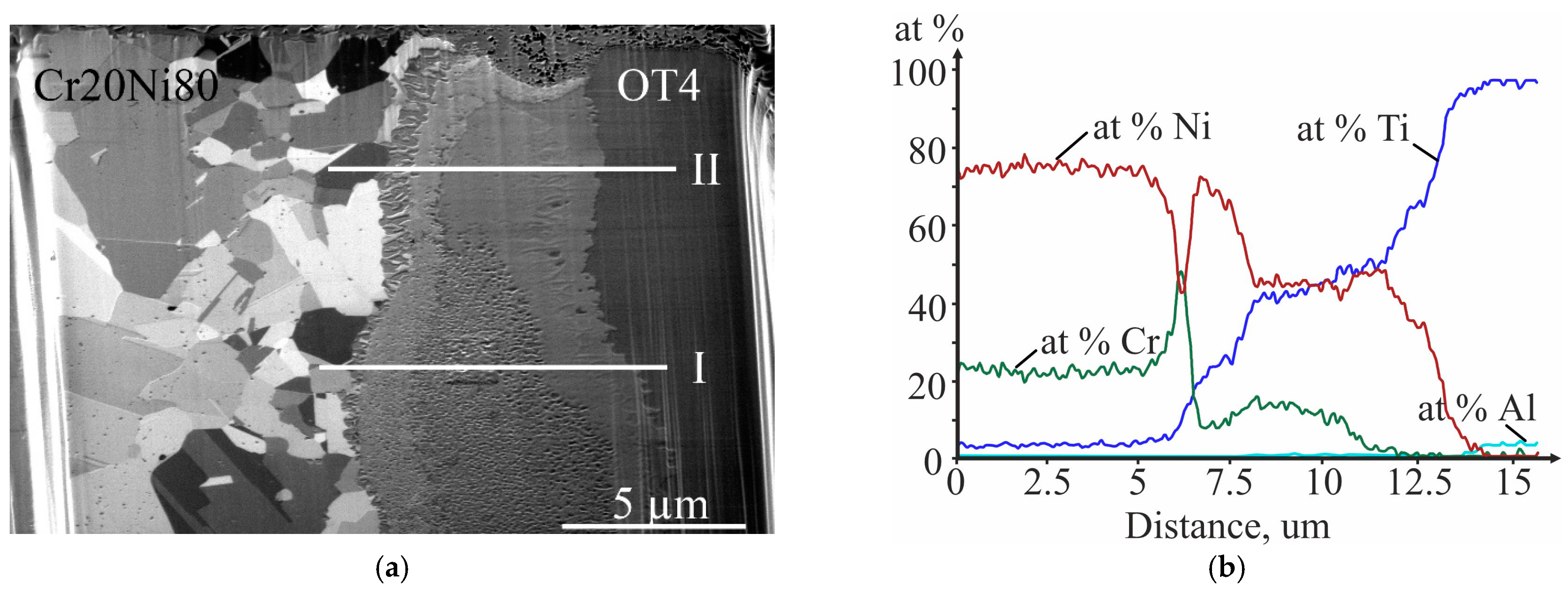
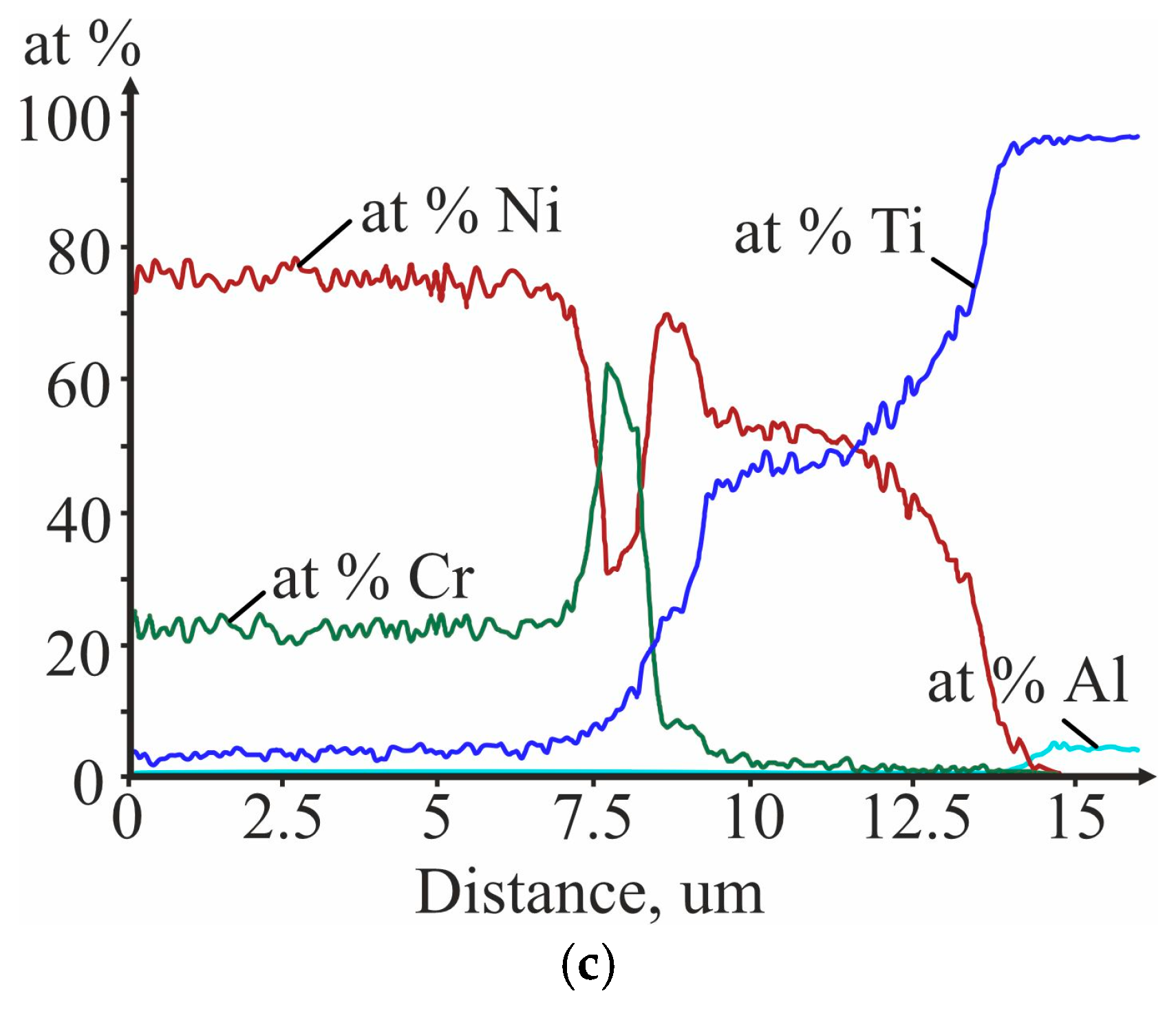
3.3. Investigation of the Initial Stages of Diffusion Interaction during Heating (In Situ)
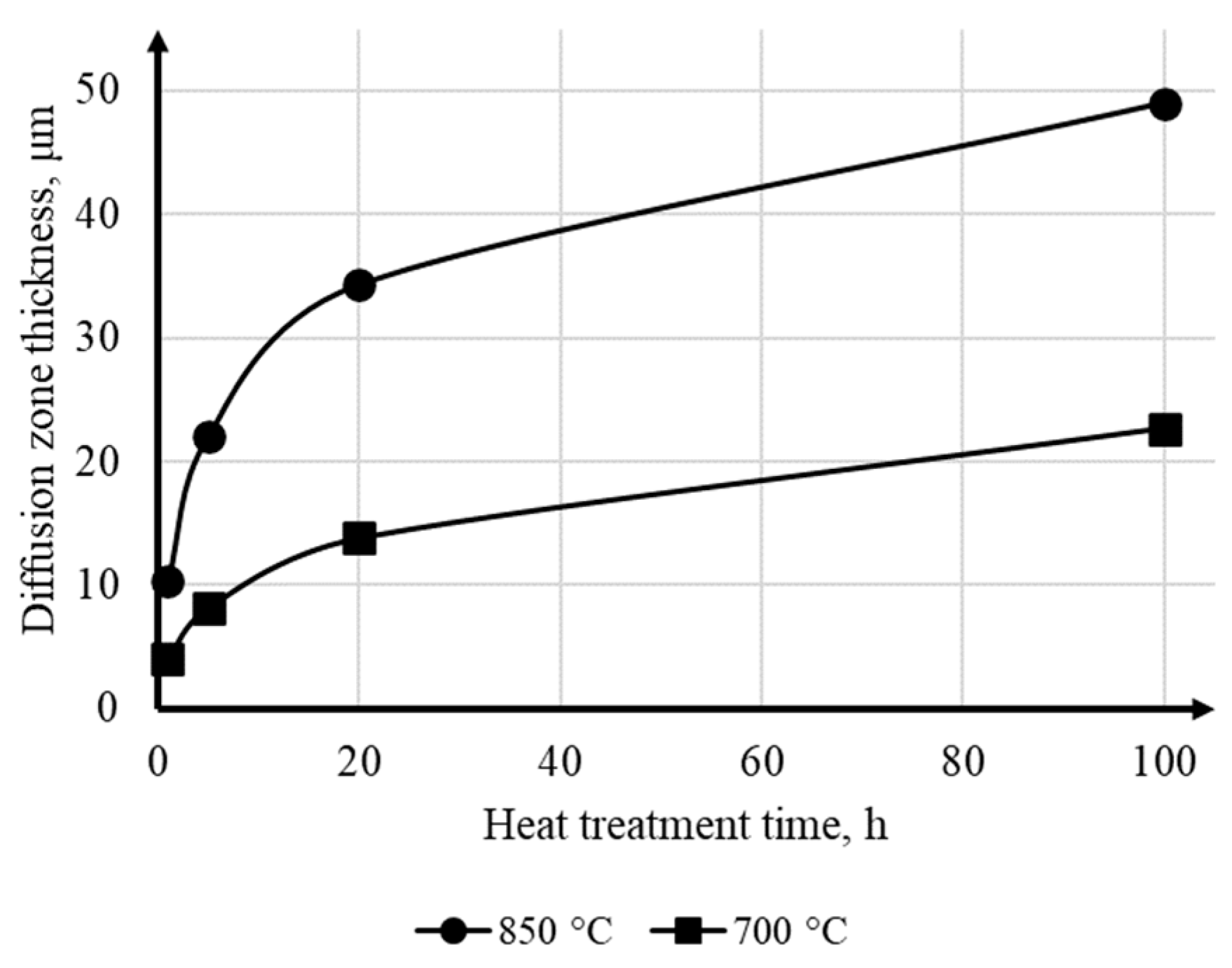
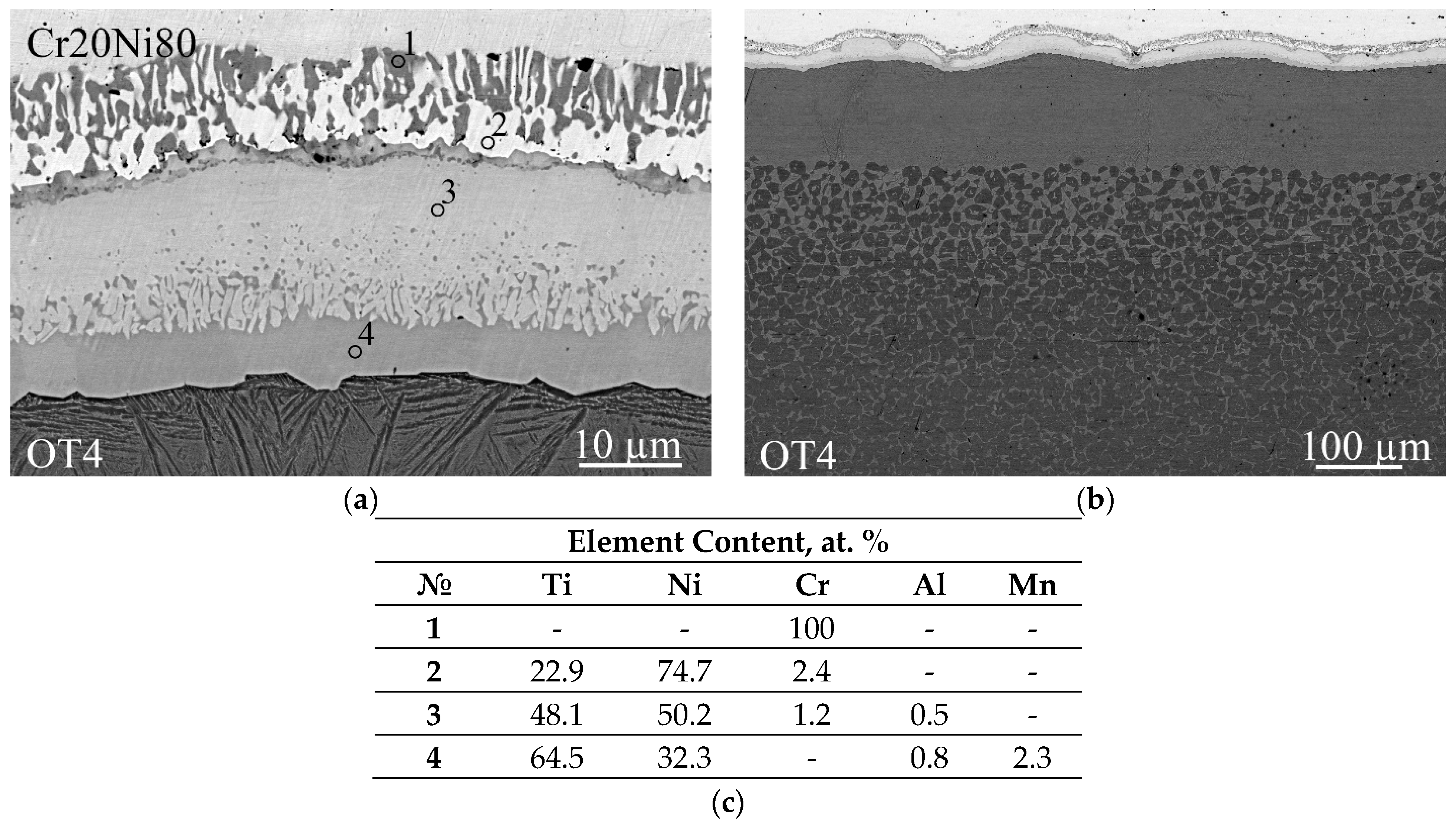
3.4. Investigation of Diffusion Interaction during Heat Treatment
4. Conclusions
- The local areas and thin continuous layers of molten metal formed during explosive welding of the Cr20Ni80 alloy with OT4 titanium alloy are heterogeneous mixtures based on Ni(Cr,Ti) and Ti(Ni,Al) solid solutions and NiTi and Ni3Ti intermetallic compounds. When welding at optimal conditions, an increase in collision point velocity leads to an increase in the average content of titanium in the molten metal.
- The lifetime of molten metal in the liquid state is comparable with the time of arrival of the unloading wave on the contact surface, which is the reason for the presence of fracture areas in the form of detachments on the fracture surface. Continuous nanometer size molten metal layers with an amorphous structure, due to the ultra-high cooling rate, induce viscous destruction of the interface.
- Heat treatment at temperatures of 700 and 850 °C leads to the formation of a reaction zone with a layered structure at the NiCr/Ti interface. The reaction zone consists of interlayers of solid solutions based on Ti2Ni, TiNi, and TiNi3 intermetallic compounds, as well as inclusions of a Cr(Ti) solid solution. The diffusion flow gradient is predominantly directed into the titanium alloy. Heat treatment at 700 °C (below the eutectoid transformation point) does not lead to significant changes in the structure of the titanium alloy. Increasing the heat treatment temperature to 850 °C (above the eutectoid transformation point) intensifies diffusion processes at the NiCr/Ti interface and leads to a change in the ratio of intermetallic interlayer thicknesses without affecting their composition. In this case, a near-boundary region with a eutectoid structure (Ti + Ti2Ni) is formed in titanium alloy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Chen, J.; Ma, F.; Ameyama, K.; Xiao, W.; Ma, C. Tensile and flexural properties of multilayered metal/intermetallics composites. Mater. Charact. 2015, 102, 165–172. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, E.; Li, W.; Niu, Z.; Chang, Y.; Guo, C.; Jiang, F. Improved fracture toughness of NiTi shape memory alloy fiber-reinforced Ti-Al metal-intermetallic-laminate composite. J. Alloys Compd. 2018, 739, 669–677. [Google Scholar] [CrossRef]
- Wang, H.; Harrington, T.; Zhu, C.; Vecchio, K.S. Design, fabrication and characterization of FeAl-based metallic-intermetallic laminate (MIL) composites. Acta Mater. 2019, 175, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Yuan, M.; Wang, H.; Pei, X.; Zhou, X.; Li, M. Microstructure evolution and mechanical properties of Ti-Ni-Al ternary Metallic-Intermetallic Laminate (MIL) composites. J. Alloys Compd. 2023, 935, 167853. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Shi, F. Intermetallic formation and mechanical properties of Ni-Ti diffusion couples. Mater. Des. 2017, 130, 175–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Cai, H. Fabrication, characterization and tensile property of a novel Ti2Ni/TiNi micro-laminated composite. Mater. Des. 2016, 92, 486–493. [Google Scholar] [CrossRef]
- Mo, T.; Chen, J.; Chen, Z.; He, W.; Liu, Q. Microstructure evolution during roll bonding and growth of interfacial intermetallic compounds in Al/Ti/Al laminated metal composites. JOM 2019, 71, 4769–4777. [Google Scholar] [CrossRef]
- Chang, D.; Wang, P.; Zhao, Y. Effects of asymmetry and annealing on interfacial microstructure and mechanical properties of Cu/Al laminated composite fabricated by asymmetrical roll bonding. J. Alloys Compd. 2020, 815, 152453. [Google Scholar] [CrossRef]
- Sun, W.; Yang, F.; Kong, F.; Wang, X.; Chen, Y. Interface characteristics of Ti6Al4V-TiAl metal-intermetallic laminate (MIL) composites prepared by a novel hot-pack rolling. Mater. Charact. 2018, 144, 173–181. [Google Scholar] [CrossRef]
- Sun, W.; You, F.; Kong, F.; Wang, X.; Chen, Y. Effect of residual stresses on the mechanical properties of Ti-TiAl laminate composites fabricated by hot-pack rolling. Mater. Charact. 2020, 166, 110394. [Google Scholar] [CrossRef]
- Szachogluchowicz, I.; Sniezek, L.; Hutsaylyuk, V. Low cycle fatigue properties of AA2519–Ti6Al4V laminate bonded by explosion welding. Eng. Fail. Anal. 2016, 69, 77–87. [Google Scholar] [CrossRef]
- Mali, V.I.; Bataev, A.A.; Maliutina, I.N.; Kurguzov, V.D.; Bataev, I.A.; Esikov, M.A.; Lozhkin, V.S. Microstructure and mechanical properties of Ti/Ta/Cu/Ni alloy laminate composite materials produced by explosive welding. Int. J. Adv. Manuf. Technol. 2017, 93, 4285–4294. [Google Scholar] [CrossRef]
- Kuz’min, E.V.; Lysak, V.I.; Kuz’min, S.V.; Korolev, M.P. Influence of structure formation and properties of bimetal produced by ultrasound-assisted explosive welding. J. Manuf. Process. 2021, 71, 734–742. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Yang, Y.; Wang, Y.; Sheng, L.; Li, Y.; Huo, M.; Zhang, K.; Xing, L.; Zhang, G. Study on the Microstructure and Mechanical Properties of a Ti/Mg Alloy Clad Plate Produced by Explosive Welding. Metals 2022, 12, 399. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Y.; Huang, X.; Li, C.; Zhang, Z. Microstructure characteristics of a high-entropy-alloy intermetallic laminate composite. Mater. Lett. 2020, 273, 127937. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Xia, C.; Wang, J. Microstructural evolution and properties of TLP diffusion bonding super-Ni/NiCr laminated composite to Ti-6Al-4V alloy with Cu interlayer. Mater. Des. 2017, 135, 184–196. [Google Scholar] [CrossRef]
- Assari, A.H.; Eghbali, B. Solid state diffusion bonding characteristics at the interfaces of Ti and Al layers. J. Alloys Compd. 2019, 773, 50–58. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Yanov, V.; Saevarsdottir, G. Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals 2022, 12, 319. [Google Scholar] [CrossRef]
- Noro, J.; Ramos, A.S.; Vieira, M.T. Intermetallic phase formation in nanometric Ni/Al multilayer thin films. Intermetallics 2008, 16, 1061–1065. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, H.; Zhang, Y.; Guo, Y.; Zhu, L.; Huang, G.; Chen, P. Intermetallic Reaction of the Bonding Interface of TA2/Q235 Explosive Welding Composite. Metals 2023, 13, 571. [Google Scholar] [CrossRef]
- Malakhov, A.; Epishin, A.; Denisov, I.; Saikov, I.; Nolze, G. Morphology and Structure of Brass–Invar Weld Interface after Explosive Welding. Materials 2022, 15, 8587. [Google Scholar] [CrossRef]
- Szachogłuchowicz, I.; Śnieżek, L.; Ślęzak, T. Mechanical Properties Analysis of Explosive Welded Sheet of AA2519-Ti6Al4V with Interlayer of AA1050 Subjected to Heat-Treatment. Materials 2022, 15, 4023. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Wierzbicka-Miernik, A.; Szczerba, M.; Bobrowski, P.; Szulc, Z.; Wojewoda-Budka, J. On the Disintegration of A1050/Ni201 Explosively Welded Clads Induced by Long-Term Annealing. Materials 2021, 14, 2931. [Google Scholar] [CrossRef]
- Li, H.; Cao, L.; Liang, X.; Zhang, W.; Wu, C.; Zeng, Z.; Zhou, C. Influence of Rolling Temperatures on Interface Microstructure and Mechanical Properties of Multi-Pass Rolling TA1/Q235B Explosive Welded Sheets. Metals 2020, 10, 1654. [Google Scholar] [CrossRef]
- Trykov, Y.P.; Shmorgun, V.G. Evaluation of the impact toughness of explosion-welded joints. Weld. Int. 2002, 16, 415–416. [Google Scholar] [CrossRef]
- Trykov, Y.P.; Shmorgun, V.G.; Abramenko, S.A. Influence of rolling on the properties of titanium—Steel composite obtained by explosive welding. Steel Transl. 2005, 35, 61–62. [Google Scholar]
- Greenberg, B.A.; Ivanov, M.A.; Pushkin, M.S.; Inozemtsev, A.V.; Patselov, A.M.; Tankeyev, A.P.; Kuzmin, S.V.; Lysak, V.I. Formation of Intermetallic Compounds During Explosive Welding. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 5461–5473. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Bogdanov, A.I.; Arisova, V.N.; Trykov, Y.P. Growth kinetics of the diffusion zone at the interface of the explosion-welded nickel–aluminium composite. Weld. Int. 2016, 30, 625–629. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Trykov, Y.P.; Slautin, O.V.; Metelkin, V.V.; Bogdanov, A.I. The kinetics of diffusion processes in the nickel-aluminum composition. Russ. J. Non-Ferr. Met. 2009, 50, 286–289. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Slautin, O.V.; Kulevich, V.P.; Serov, A.G. Formation of the Interaction Zone on the Interlayer Boundary of the Explosion-Welded Composite VT1-0–NP2. Inorg. Mater. Appl. Res. 2020, 11, 98–102. [Google Scholar] [CrossRef]
- Gurevich, L.M.; Shmorgun, V.G.; Bogdanov, A.I.; Taube, A.O.; Storozheva, E.I. Kinetics of the diffusion interaction on the “melt–solid body” boundary in explosive welded Al-Ni composite. Mater. Today Proc. 2019, 19, 1904–1907. [Google Scholar] [CrossRef]
- Sha, W.; Malinov, S. (Eds.) Titanium Alloys: Modelling of Microstructure, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2009; p. 569. [Google Scholar]
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef]
- Shmorgun, V.G.; Bogdanov, A.I.; Kulevich, V.P.; Taube, A.O. Experimental research of the explosive welding of metal plates with different initial hardness. Mater. Manuf. Process. 2023. [Google Scholar] [CrossRef]
- Kasuya, T.; Yurioka, N. Prediction of Welding Thermal History by a Comprehensive Solution. Weld. J. Res. Suppl. 1993, 72, 107–115. [Google Scholar]
- Hu, B.; Du, Y.; Schuster, J.C.; Sun, W.; Liu, S.; Tang, C. Thermodynamic modeling of the Cr–Ni–Ti system using a four-sublattice model for ordered/disordered bcc phases. Thermochim. Acta 2014, 578, 35–42. [Google Scholar] [CrossRef]






| Ti | Al | V | Mo | Zr | Mn | Si | Fe |
|---|---|---|---|---|---|---|---|
| 91.638–95.7 | 3.5–5 | - | - | ≤0.3 | 0.8–2 | ≤0.15 | ≤0.3 |
| Al | C | Cr | Fe | Mn | Ni | P | S | Si | Ti | Zr |
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.20 | ≤0.06 | 20.0–23.0 | ≤1.0 | ≤0.60 | base | ≤0.020 | ≤0.015 | 1.00–1.50 | ≤0.20 | 0.20–0.50 |
| Mode | Height of the Explosive Charge, mm | Stand-Off Distance, mm | Vc, m/s | Vi, m/s |
|---|---|---|---|---|
| I | 40 | 3.5 | 2000 | 620 |
| II | 3 | 3350 | 1000 |
| Metals | Density, kg/m3 | Specific Heat of Melting, kJ/kg | Specific Heat, J/(kg × K) |
|---|---|---|---|
| Nickel | 8900 | 303 | 0.46 |
| Titanium | 4540 | 358 | 0.54 |
| Chromium | 7190 | 264 | 0.448 |
| Average Area of Local Molten Metal | Weighted Average Nickel Content | Liquidus Temperature | Solidus Temperature | Cooling Rate | Molten Metal Lifetime |
|---|---|---|---|---|---|
| F × 10−9, m2 | mNi, at. % | Tm, K | TS, K | V × 1010, K/s | τm × 10−8, s |
| 0.6 | 42 | 1600 | 836 | 3.2 | 2.3 |
| Average Area of Continuous Molten Metal | Weighted Average Nickel Content | Liquidus Temperature | Solidus Temperature | Cooling Rate | Molten Metal Lifetime |
|---|---|---|---|---|---|
| F × 10−9, m2 | mNi, at. % | Tm, K | TS, K | V × 1014, K/s | τm × 10−11, s |
| 3.0 | 28 | 1370 | 836 | 1.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, A.I.; Kulevich, V.P.; Shmorgun, V.G. Microstructure Evolution and Growth of Interfacial Intermetallic Compounds in NiCr/Ti Alloy Laminated Composite after Explosive Welding and Heat Treatment. Metals 2023, 13, 1417. https://doi.org/10.3390/met13081417
Bogdanov AI, Kulevich VP, Shmorgun VG. Microstructure Evolution and Growth of Interfacial Intermetallic Compounds in NiCr/Ti Alloy Laminated Composite after Explosive Welding and Heat Treatment. Metals. 2023; 13(8):1417. https://doi.org/10.3390/met13081417
Chicago/Turabian StyleBogdanov, Artem Igorevich, Vitaliy Pavlovich Kulevich, and Victor Georgievich Shmorgun. 2023. "Microstructure Evolution and Growth of Interfacial Intermetallic Compounds in NiCr/Ti Alloy Laminated Composite after Explosive Welding and Heat Treatment" Metals 13, no. 8: 1417. https://doi.org/10.3390/met13081417






