Insight into the Influence of Alloying Elements on the Elastic Properties and Strengthening of Copper: A High-Throughput First-Principles Calculations
Abstract
:1. Introduction
2. Computational Details and Theory
3. Results and Discussion
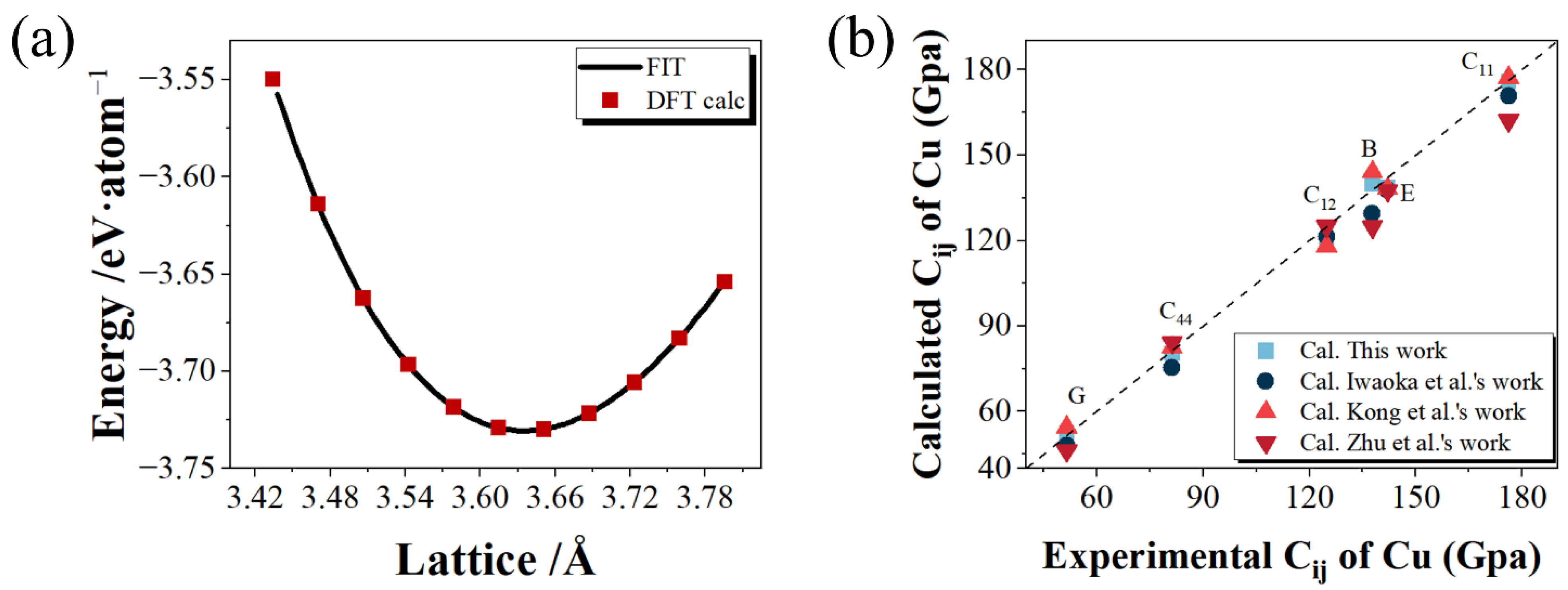
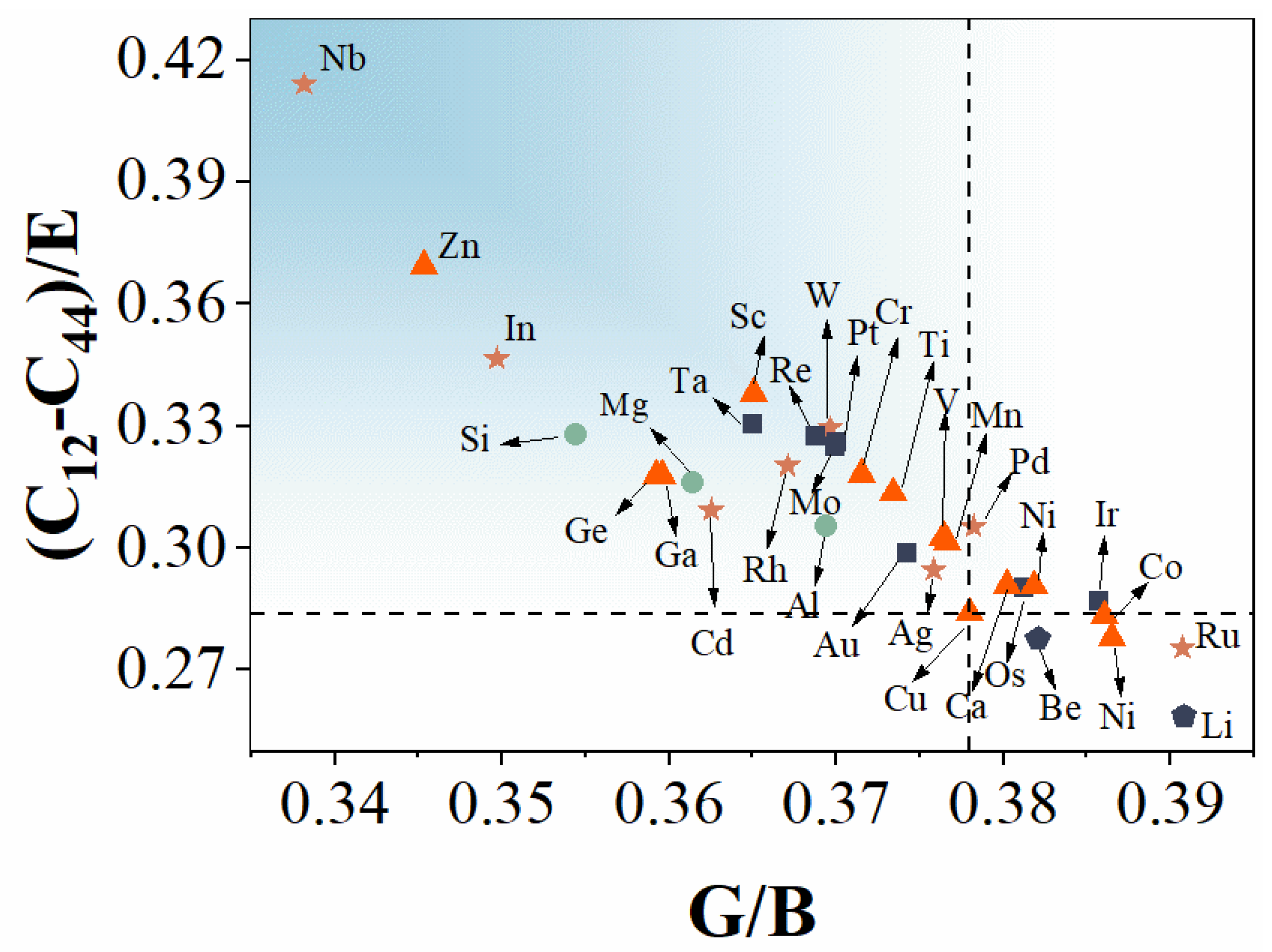
3.1. Elastic Properties
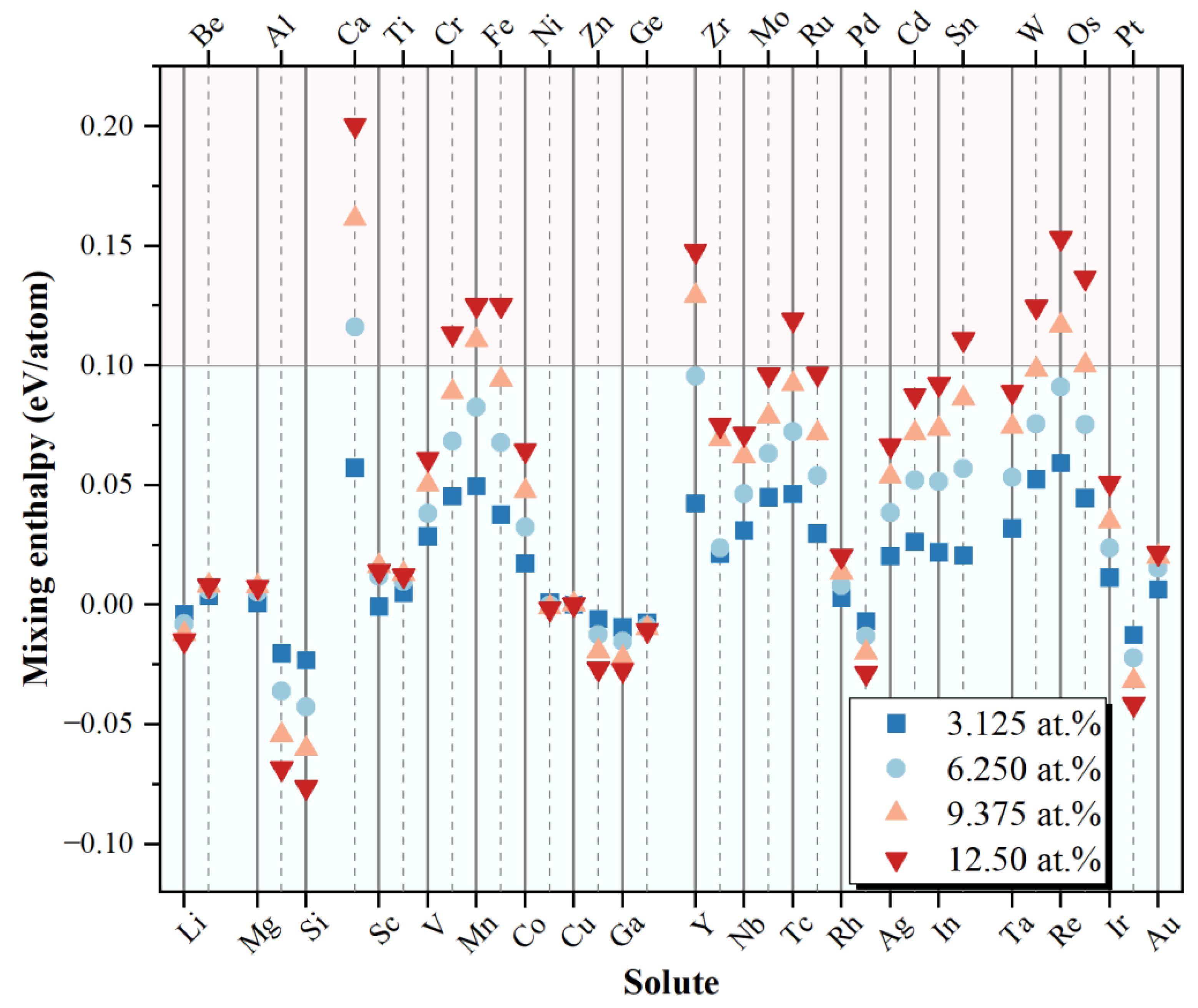
3.2. Stability and Energetics
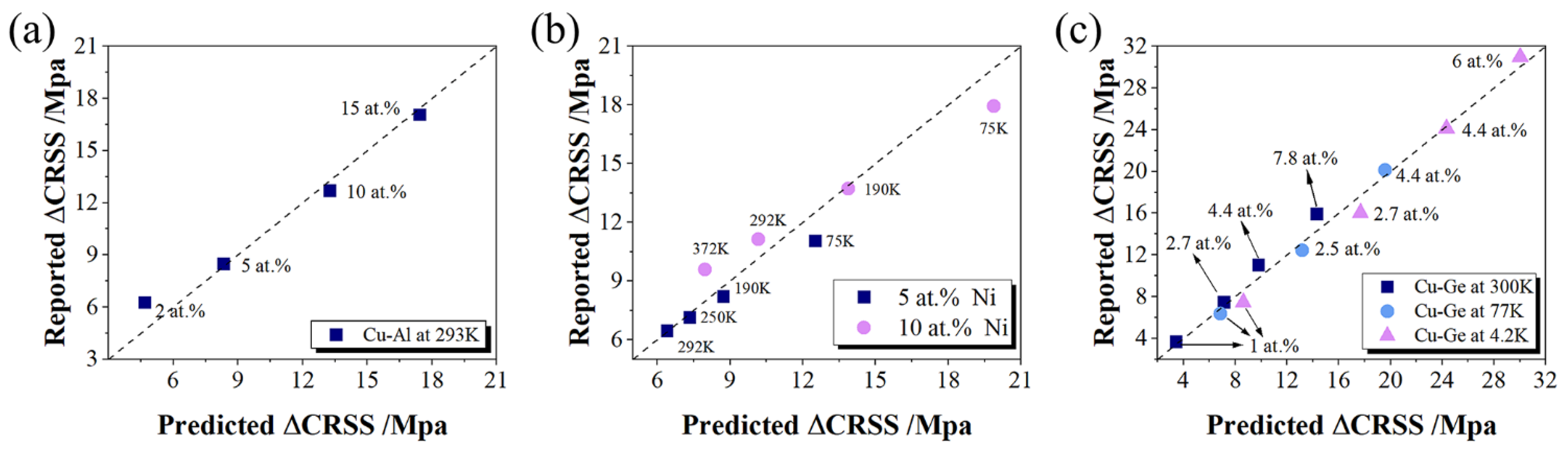
3.3. Solid Solution Strengthening
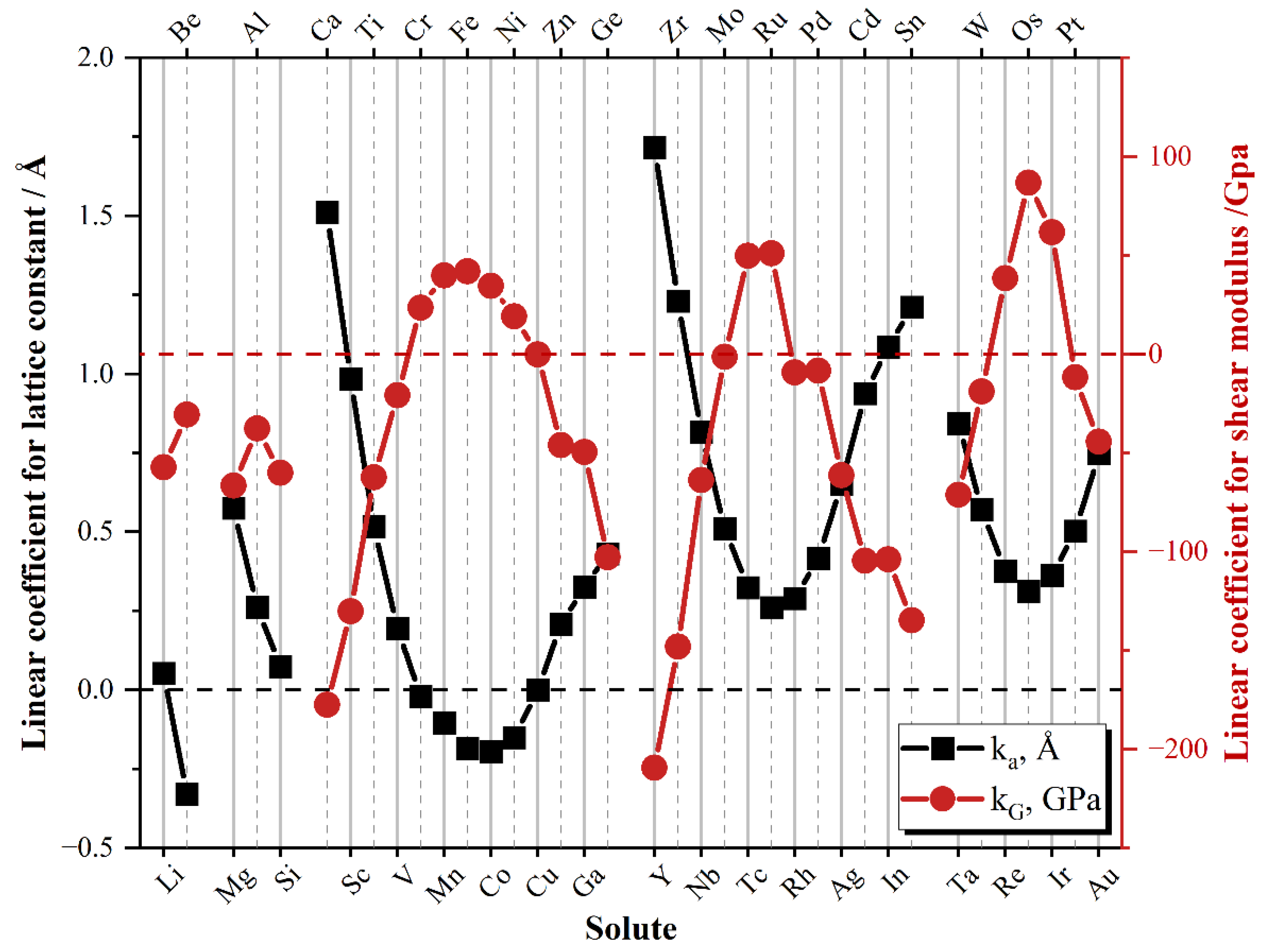
3.3.1. Linear Regression Coefficients of Lattice Parameter Change
3.3.2. Linear Regression Coefficients of Shear Modulus Change
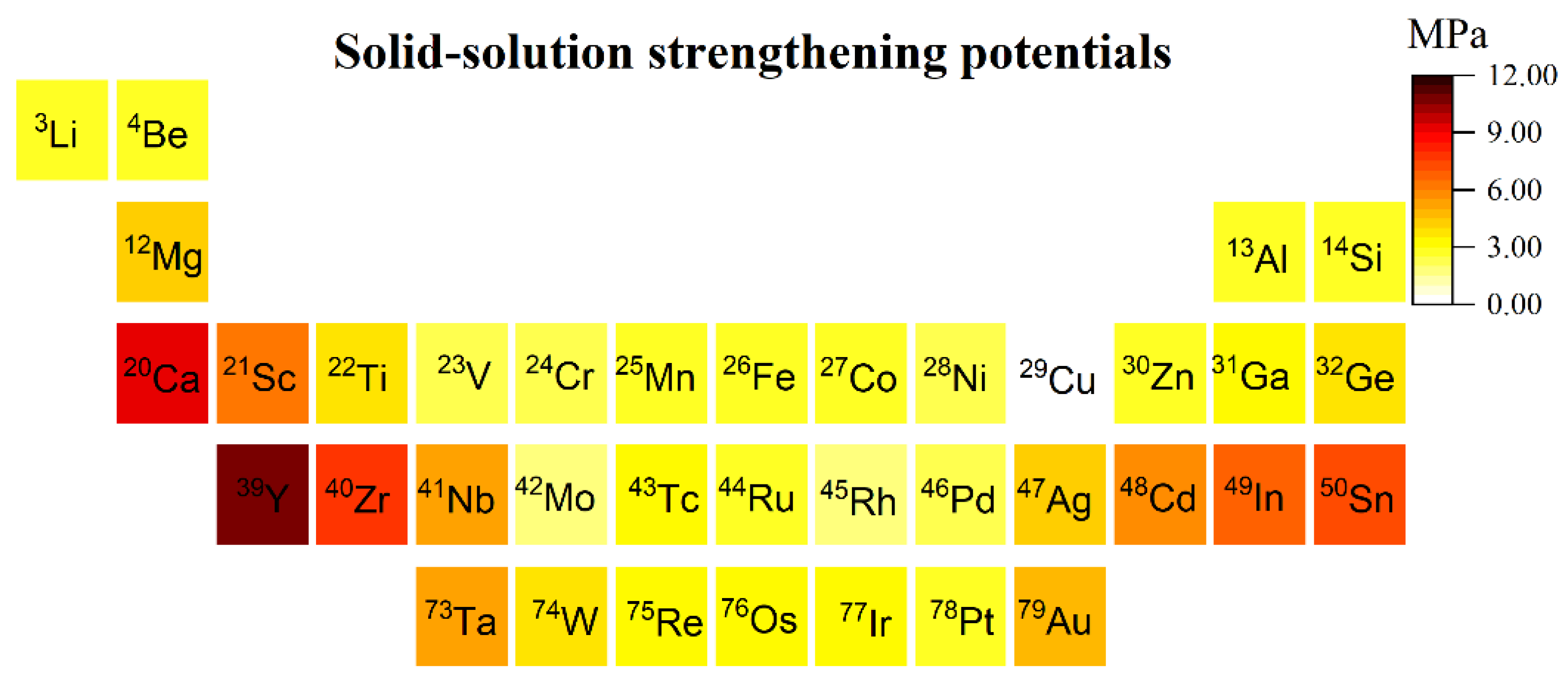
3.3.3. Solid Solution Strengthening Effects
4. Conclusions
- (1)
- The mixing enthalpy shows that most alloying elements can dissolve in copper at low concentrations, among which Li, Be, Al, Si, Ni, Zn, Ga, Ge, Rh, Pd, Pt, and Au are more thermodynamically favorable. The Born criterion states that all solid solution structures are mechanically stable.
- (2)
- The position of the alloying element in the periodic table of chemical elements determines the variation of the modulus of elasticity. For alloying elements of fourth to sixth periods, the modulus changes in a parabolic pattern within each period.
- (3)
- The lattice parameters and shear modulus of the solid solution showed a good linear relationship with the concentration, which matched the experimental values fairly well.
- (4)
- In the same period, the alloying elements at both ends have a higher strengthening ability than the elements in the middle.
- (5)
- The predicted critical decomposition shear stresses are in good agreement with the experimental values. Considering the cost and strengthening ability of solute elements, Mg, Ca, Y, Zr, Cd, In, and Sn are potential candidates for solid solution strengthening, while Ru, Rh, and Pt have less potential as strengthening elements. This study provides theoretical insights into the design of high-performance copper alloys.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Fu, H.; Jiang, L.; Xue, D.; Xie, J. A Property-Oriented Design Strategy for High Performance Copper Alloys via Machine Learning. Npj Comput. Mater. 2019, 5, 87. [Google Scholar] [CrossRef]
- Geng, Y.; Ban, Y.; Wang, B.; Li, X.; Song, K.; Zhang, Y.; Jia, Y.; Tian, B.; Liu, Y.; Volinsky, A.A. A Review of Microstructure and Texture Evolution with Nanoscale Precipitates for Copper Alloys. J. Mater. Res. Technol. 2020, 9, 11918–11934. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; He, X.; Wang, C.; Jiang, L.; Chen, L.-Q.; Xie, J. Dramatically Enhanced Combination of Ultimate Tensile Strength and Electric Conductivity of Alloys via Machine Learning Screening. Acta Mater. 2020, 200, 803–810. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Z.; Lei, C.; Meng, L.; Fang, Y.; Liu, J.; Wang, H. High Strength and High Conductivity Cu Alloys: A Review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; Zhu, S.; Yong, W.; Xie, J. Machine Learning Assisted Composition Effective Design for Precipitation Strengthened Copper Alloys. Acta Mater. 2021, 215, 117118. [Google Scholar] [CrossRef]
- Xie, M.; Huang, W.; Chen, H.; Gong, L.; Xie, W.; Wang, H.; Yang, B. Microstructural Evolution and Strengthening Mechanisms in Cold-Rolled Cu–Ag Alloys. J. Alloys Compd. 2021, 851, 156893. [Google Scholar] [CrossRef]
- Xiong, H.; Ma, Y.; Zhang, H.; Chen, L. Design of Cu–Cr Alloys with High Strength and High Ductility Based on First-Principles Calculations. Metals 2022, 12, 1406. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Chen, X.; Zhu, Y.; Chen, M.; Wang, Y.; Shen, J.; Shi, J.; Wang, Z. In-Situ Fabrication, Microstructure and Mechanical Performance of Nano Iron-Rich Precipitate Reinforced Cu and Cu Alloys. Metals 2022, 12, 1453. [Google Scholar] [CrossRef]
- Thomas, S.; Yazdanparast, S.; Hildreth, O.; Asle Zaeem, M. Formation Energies, Electronic Properties and Elemental Diffusion of Cu–Cr–Nb (GRCop) Alloys. Phys. B Condens. Matter 2022, 637, 413909. [Google Scholar] [CrossRef]
- Wang, Y.; Su, C.; Konovalov, S. Microstructure and Mechanical Properties of Cu-6.5%Al Alloy Deposited by Wire Arc Additive Manufacturing. Metallogr. Microstruct. Anal. 2021, 10, 634–641. [Google Scholar] [CrossRef]
- Wei, H.; Hou, L.; Cui, Y.; Wei, Y. Effect of Ti Content on Corrosion Behavior of Cu-Ti Alloys in 3.5% NaCl Solution. Trans. Nonferrous Met. Soc. China 2018, 28, 669–675. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Jiang, Y.; Li, Z.; Xiao, Z.; Gong, S.; Wang, Y.; Guo, C.; Wei, H. Effects of Fe Content on Microstructure and Properties of Cu–Fe Alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3039–3049. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Volinsky, A.A.; Tian, B.; Song, K.; Wang, B.; Liu, Y. Hot Workability and Constitutive Model of the Cu-Zr-Nd Alloy. Vacuum 2017, 146, 35–43. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Qin, N.; Zhang, Y.; Li, S.; Xiao, Z.; Lei, Q.; Li, Z. Effects of Minor Rare Earths on the Microstructure and Properties of Cu-Cr-Zr Alloy. J. Alloys Compd. 2020, 847, 155762. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Tian, B.; Yakubov, V.; An, J.; Volinsky, A.A.; Liu, Y.; Song, K.; Li, L.; Fu, M. Effects of Ce and Y Addition on Microstructure Evolution and Precipitation of Cu-Mg Alloy Hot Deformation. J. Alloys Compd. 2019, 781, 118–130. [Google Scholar] [CrossRef]
- Toda-Caraballo, I.; Rivera-Díaz-del-Castillo, P.E.J. Modelling Solid Solution Hardening in High Entropy Alloys. Acta Mater. 2015, 85, 14–23. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cai, D.D.; Sun, X.; Huang, H.; Lu, S.; Wang, Y.Z.; Hu, Q.M.; Vitos, L.; Ding, X.D. Solid Solution Strengthening of High-Entropy Alloys from First-Principles Study. J. Mater. Sci. Technol. 2022, 121, 105–116. [Google Scholar] [CrossRef]
- Fleisgher, R.L. Solution Hardening. Acta Metall. 1961, 9, 996–1000. [Google Scholar] [CrossRef]
- Labusch, R. A Statistical Theory of Solid Solution Hardening. Phys. Status Solidi B 1970, 41, 659–669. [Google Scholar] [CrossRef]
- Uesugi, T.; Higashi, K. First-Principles Studies on Lattice Constants and Local Lattice Distortions in Solid Solution Aluminum Alloys. Comput. Mater. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Yasi, J.A.; Hector, L.G.; Trinkle, D.R. First-Principles Data for Solid-Solution Strengthening of Magnesium: From Geometry and Chemistry to Properties. Acta Mater. 2010, 58, 5704–5713. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Shang, S.-L.; Wang, Y.; Darling, K.A.; Butler, B.G.; Kecskes, L.J.; Liu, Z.-K. Effects of Alloying Elements and Temperature on the Elastic Properties of W-Based Alloys by First-Principles Calculations. J. Alloys Compd. 2016, 671, 267–275. [Google Scholar] [CrossRef]
- Wang, H.; Gan, Y.C.; Geng, H.Y.; Chen, X.-R. MyElas: An Automatized Tool-Kit for High-Throughput Calculation, Post-Processing and Visualization of Elasticity and Related Properties of Solids. Comput. Phys. Commun. 2022, 281, 108495. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- van de Walle, A.; Tiwary, P.; de Jong, M.; Olmsted, D.L.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Efficient Stochastic Generation of Special Quasirandom Structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- van de Walle, A.; Asta, M.; Ceder, G. The Alloy Theoretic Automated Toolkit: A User Guide. Calphad 2002, 26, 539–553. [Google Scholar] [CrossRef]
- Yu, W.; Chong, X.; Gan, M.; Wei, Y.; Zhang, A.; Wang, Y.; Feng, J. Exploring the Solution Strengthening Effect of 33 Alloying Elements in Pt-Based Alloys by High-Throughput First-Principles Calculations. J. Appl. Phys. 2022, 131, 185103. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Shang, S.-L.; Wang, Y.; Kim, D.; Liu, Z.-K. First-Principles Thermodynamics from Phonon and Debye Model: Application to Ni and Ni3Al. Comput. Mater. Sci. 2010, 47, 1040–1048. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Y.; Liu, Z.-K. First-Principles Elastic Constants of α- and θ-Al2O3. Appl. Phys. Lett. 2007, 90, 101909. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Yang, J.; Ouyang, P.; Liu, T.; Yun, H.; Sun, W.; Wang, Y.; Liu, J.; Zhang, S. First-Principles Study of the Effect of Aluminum Content on the Elastic Properties of Cu-Al Alloys. Mater. Today Commun. 2022, 31, 103399. [Google Scholar] [CrossRef]
- Straumanis, M.E.; Yu, L.S. Lattice Parameters, Densities, Expansion Coefficients and Perfection of Structure of Cu and of Cu–In α Phase. Acta Crystallogr. Sect. A 1969, 25, 676–682. [Google Scholar] [CrossRef]
- Overton, W.C.; Gaffney, J. Temperature Variation of the Elastic Constants of Cubic Elements. I. Copper. Phys. Rev. 1955, 98, 969–977. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Yan, M.F.; Zhang, Y.X.; Zhang, C.S. First-Principles Investigation of Structural, Mechanical and Electronic Properties for Cu–Ti Intermetallics. Comput. Mater. Sci. 2016, 123, 70–78. [Google Scholar] [CrossRef]
- Iwaoka, H.; Hirosawa, S. First-Principles Calculation of Elastic Properties of Cu-Zn Intermetallic Compounds for Improving the Stiffness of Aluminum Alloys. Comput. Mater. Sci. 2020, 174, 109479. [Google Scholar] [CrossRef]
- Kong, G.-X.; Ma, X.-J.; Liu, Q.-J.; Li, Y.; Liu, Z.-T. Structural Stability, Elastic and Thermodynamic Properties of Au–Cu Alloys from First-Principles Calculations. Phys. B Condens. Matter 2018, 533, 58–62. [Google Scholar] [CrossRef]
- Fürth, R. On the Stability of Crystal Lattices: VI. The Properties of Matter under High Pressure and the Lattice Theory of Crystals. Math. Proc. Camb. Philos. Soc. 1941, 37, 177–185. [Google Scholar] [CrossRef]
- Ghosh, D.; Biswas, R. Theoretical Calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii. Int. J. Mol. Sci. 2002, 3, 87–113. [Google Scholar] [CrossRef]
- Wang, M.-X.; Zhu, H.; Yang, G.-J.; Liu, K.; Li, J.-F.; Kong, L.-T. Solid-Solution Strengthening Effects in Binary Ni-Based Alloys Evaluated by High-Throughput Calculations. Mater. Des. 2021, 198, 109359. [Google Scholar] [CrossRef]
- Liu, T.; Chong, X.-Y.; Yu, W.; Zhou, Y.-X.; Huang, H.-G.; Zhou, R.-F.; Feng, J. Changes of Alloying Elements on Elasticity and Solid Solution Strengthening of α-Ti Alloys: A Comprehensive High-Throughput First-Principles Calculations. Rare Met. 2022, 41, 2719–2731. [Google Scholar] [CrossRef]
- Huang, C.X.; Hu, W.; Yang, G.; Zhang, Z.F.; Wu, S.D.; Wang, Q.Y.; Gottstein, G. The Effect of Stacking Fault Energy on Equilibrium Grain Size and Tensile Properties of Nanostructured Copper and Copper–Aluminum Alloys Processed by Equal Channel Angular Pressing. Mater. Sci. Eng. A 2012, 556, 638–647. [Google Scholar] [CrossRef]
- Pearson, W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys: International Series of Monographs on Metal Physics and Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2013; Volume 4, ISBN 1-4832-2661-1. [Google Scholar]
- Vegard, L. The Constitution of Mixed Crystal and the Space Occupied by Atom. Z. Phys. 1921, 5, 17. [Google Scholar] [CrossRef]
- Cyrot, M.; Cyrot-Lackmann, F. Energy of Formation of Binary Transitional Alloys. J. Phys. F Met. Phys. 1976, 6, 2257–2265. [Google Scholar] [CrossRef]
- Cyrot-Lackmann, F. Sur le calcul de la cohésion et de la tension superficielle des métaux de transition par une méthode de liaisons fortes. J. Phys. Chem. Solids 1968, 29, 1235–1243. [Google Scholar] [CrossRef]
- Sutton, A.P. Electronic Structure of Materials; Clarendon Press: Oxford, UK, 1993; ISBN 0-19-158853-9. [Google Scholar]
- Wen, C.; Wang, C.; Zhang, Y.; Antonov, S.; Xue, D.; Lookman, T.; Su, Y. Modeling Solid Solution Strengthening in High Entropy Alloys Using Machine Learning. Acta Mater. 2021, 212, 116917. [Google Scholar] [CrossRef]
- Leyson, G.P.M.; Hector, L.G.; Curtin, W.A. Solute Strengthening from First Principles and Application to Aluminum Alloys. Acta Mater. 2012, 60, 3873–3884. [Google Scholar] [CrossRef]
- Ma, D.; Friák, M.; von Pezold, J.; Raabe, D.; Neugebauer, J. Computationally Efficient and Quantitatively Accurate Multiscale Simulation of Solid-Solution Strengthening by Ab Initio Calculation. Acta Mater. 2015, 85, 53–66. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, Y.; Bei, H. Thermal Activation Mechanisms and Labusch-Type Strengthening Analysis for a Family of High-Entropy and Equiatomic Solid-Solution Alloys. Acta Mater. 2016, 120, 108–119. [Google Scholar] [CrossRef]
- Pöhl, C.; Schatte, J.; Leitner, H. Solid Solution Hardening of Molybdenum–Hafnium Alloys: Experiments and Modeling. Mater. Sci. Eng. A 2013, 559, 643–650. [Google Scholar] [CrossRef]
- Agustianingrum, M.P.; Yoshida, S.; Tsuji, N.; Park, N. Effect of Aluminum Addition on Solid Solution Strengthening in CoCrNi Medium-Entropy Alloy. J. Alloys Compd. 2019, 781, 866–872. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Plastic Deformation of Ni-Cr Single Crystals. Metall. Trans. 1971, 2, 2757–2763. [Google Scholar] [CrossRef]
- Čižek, L.; Kratochvíl, P.; Smola, B. Solid Solution Hardening of Copper Crystals. J. Mater. Sci. 1974, 9, 1517–1520. [Google Scholar] [CrossRef]
- Butt, M.Z. Investigation of the Activation-Parameters of Low-Temperature Slip in Cubic Metals. Czechoslov. J. Phys. 1999, 49, 1177–1184. [Google Scholar] [CrossRef]
- Butt, M.Z.; Ghauri, I.M. Effect of short-range order on the temperature dependence of plastic flow in α-brasses. Phys. Status Solidi A 1988, 107, 187–195. [Google Scholar] [CrossRef]
- Kamada, K.; Yoshizawa, I. The Anomalies in Temperature Dependence of the Yield Stress of Cu Base Solid Solutions. J. Phys. Soc. Jpn. 1971, 31, 1056–1068. [Google Scholar] [CrossRef]
- Miura, S.; Ono, N.; Nishimura, Y. Hall-Petch Relation and Twin Boundaries in Pure Copper and Cu-Al Alloys. J. Soc. Mater. Sci. Jpn. 2009, 58, 865–872. [Google Scholar] [CrossRef]
- Butt, M.Z.; Aziz, F.; Ali, D. Correlation between the Temperature Dependence of Yield Stress and the Nature of Solute Distribution in Cu–Ni Solid Solutions. J. Alloys Compd. 2010, 498, 102–106. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, Y.; Wang, A.; Liang, T.; Mao, Z.; Su, B.; Li, H.; Xie, J. Insight into the Influence of Alloying Elements on the Elastic Properties and Strengthening of Copper: A High-Throughput First-Principles Calculations. Metals 2023, 13, 875. https://doi.org/10.3390/met13050875
Zhang J, Zhang Y, Wang A, Liang T, Mao Z, Su B, Li H, Xie J. Insight into the Influence of Alloying Elements on the Elastic Properties and Strengthening of Copper: A High-Throughput First-Principles Calculations. Metals. 2023; 13(5):875. https://doi.org/10.3390/met13050875
Chicago/Turabian StyleZhang, Jinhao, Youcheng Zhang, Aiqin Wang, Tingting Liang, Zhiping Mao, Bo Su, Haisheng Li, and Jingpei Xie. 2023. "Insight into the Influence of Alloying Elements on the Elastic Properties and Strengthening of Copper: A High-Throughput First-Principles Calculations" Metals 13, no. 5: 875. https://doi.org/10.3390/met13050875