Effect of Al2O3 Inclusions or Mold Flux Particles on Their Surrounding Microstructures of Sliver Defects on the Surface of Automobile Exposed Panel
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Production Process of Automobile Exposed Panel
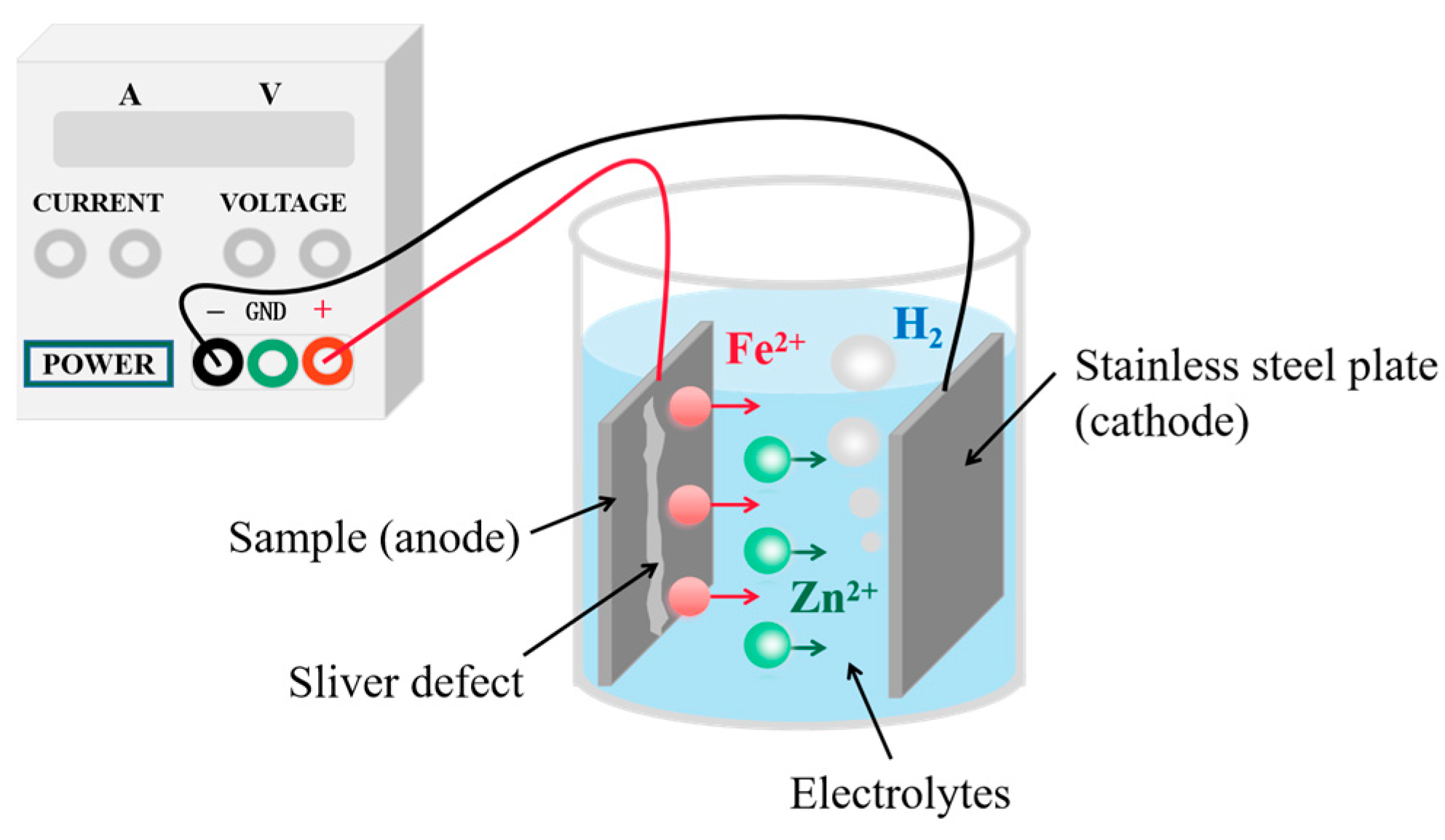
2.2. Sampling Methodology
- (1)
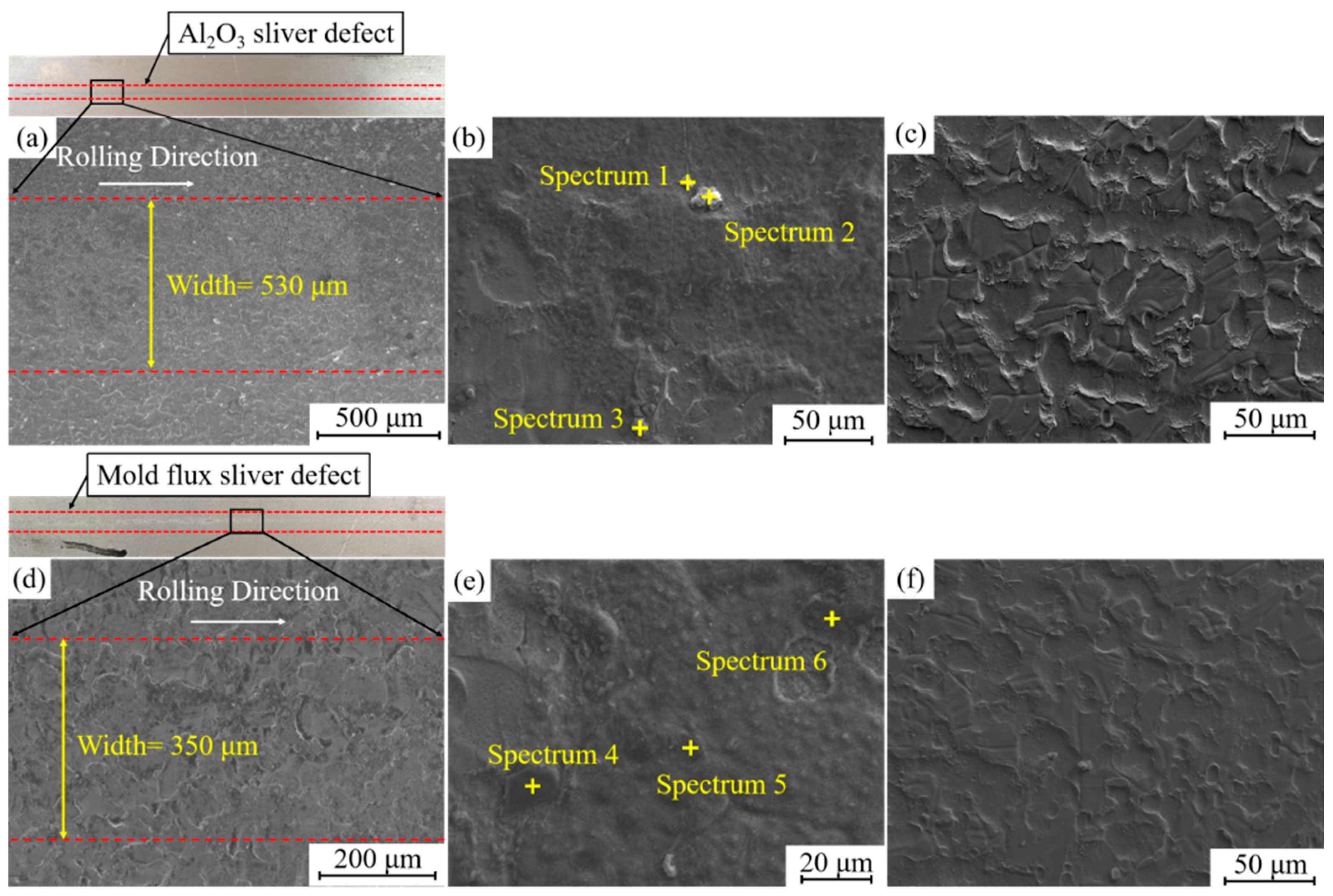
- Statistics of the morphology and size of inclusions on sliver defects
- (2)
- Statistics of grain information on defect zones and non-defect zones
3. Results and Discussion
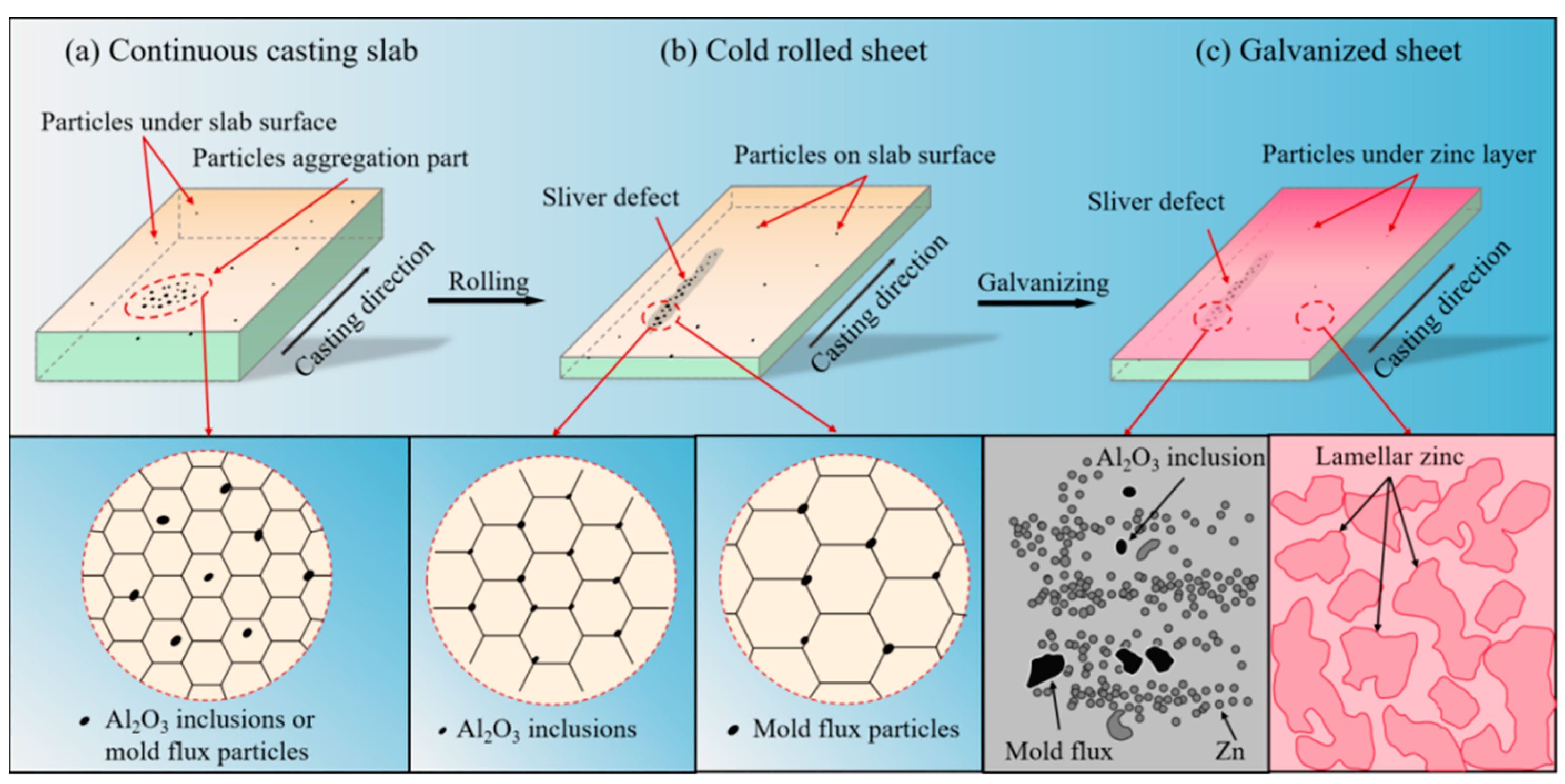
3.1. Morphology of Sliver Defects on Automobile Exposed Panel
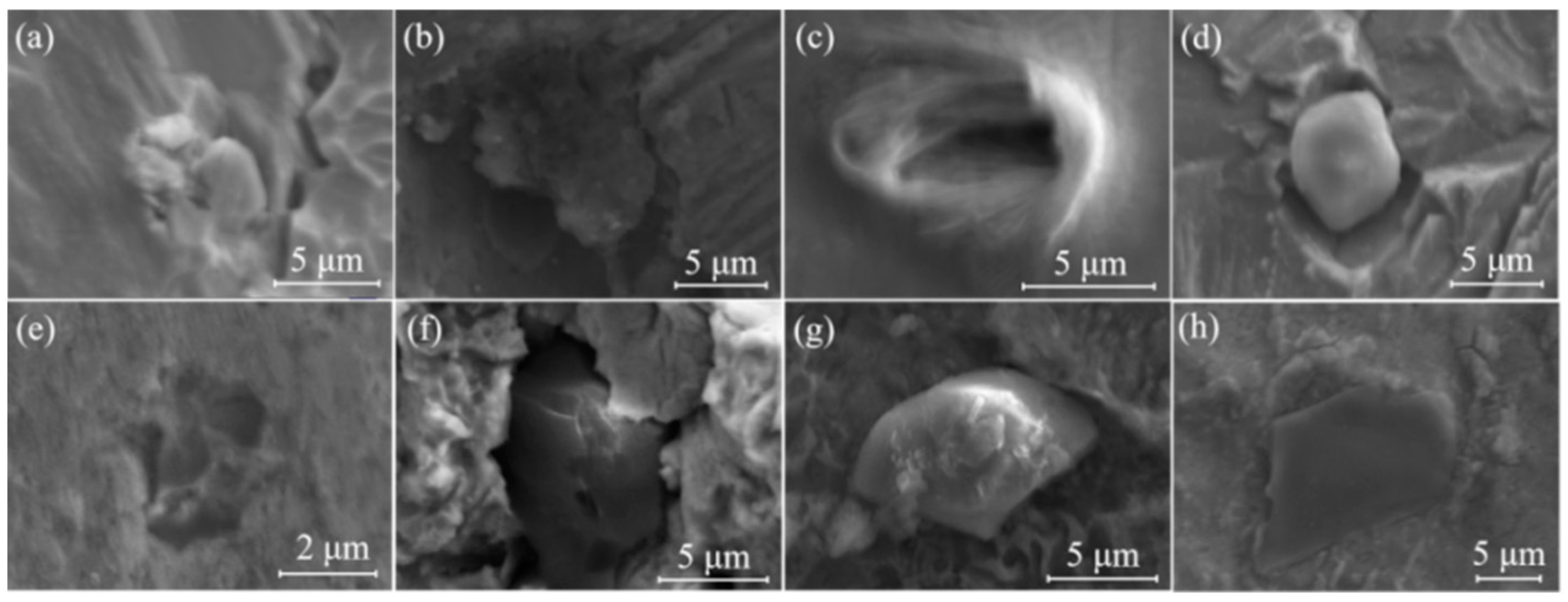
3.2. Three-Dimensional Morphology of Al2O3 Inclusions and Mold Flux Particles
3.3. EBSD Analysis of the Surface of Sliver Defect Zones and Surrounding Matrix
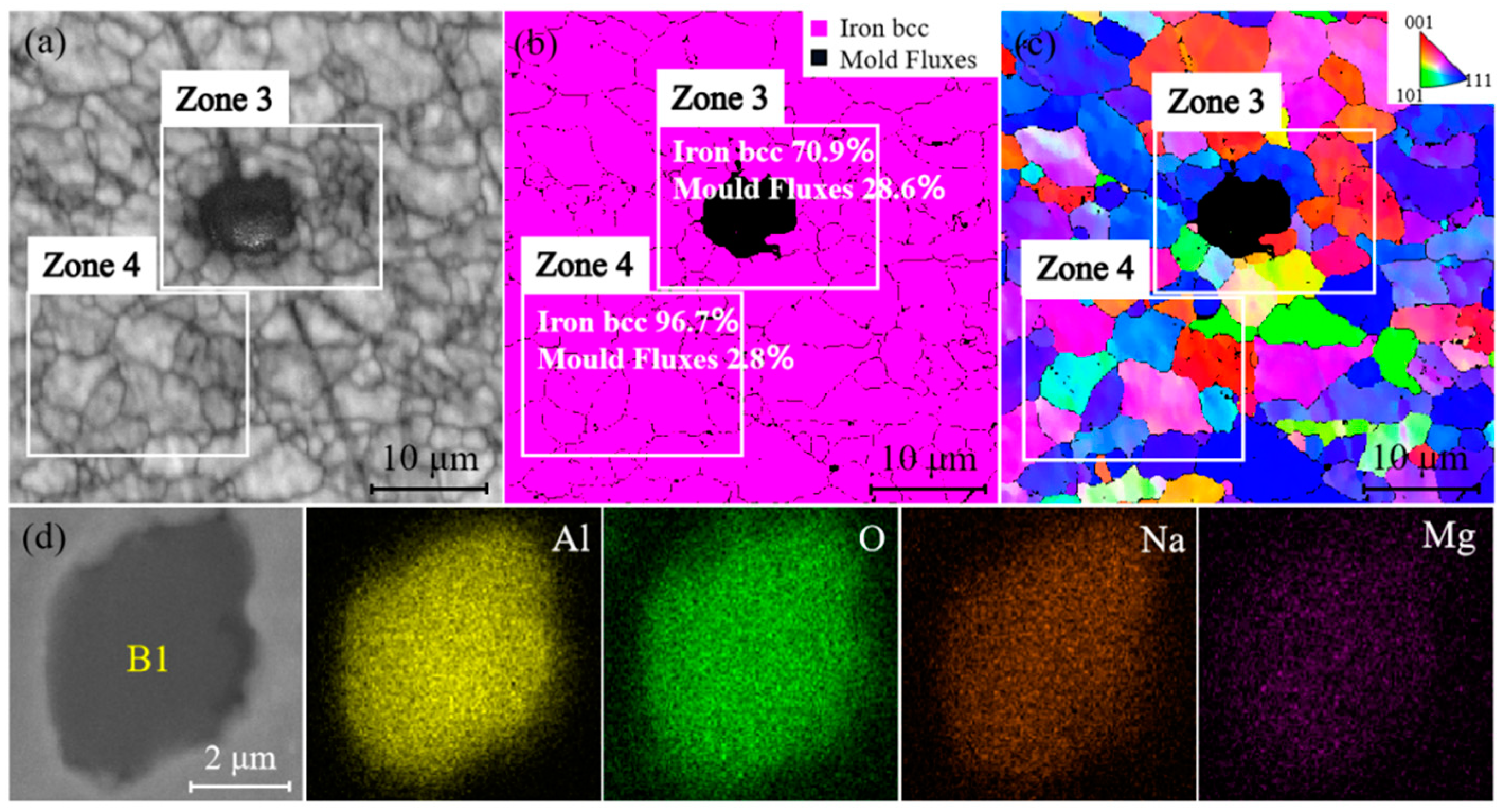
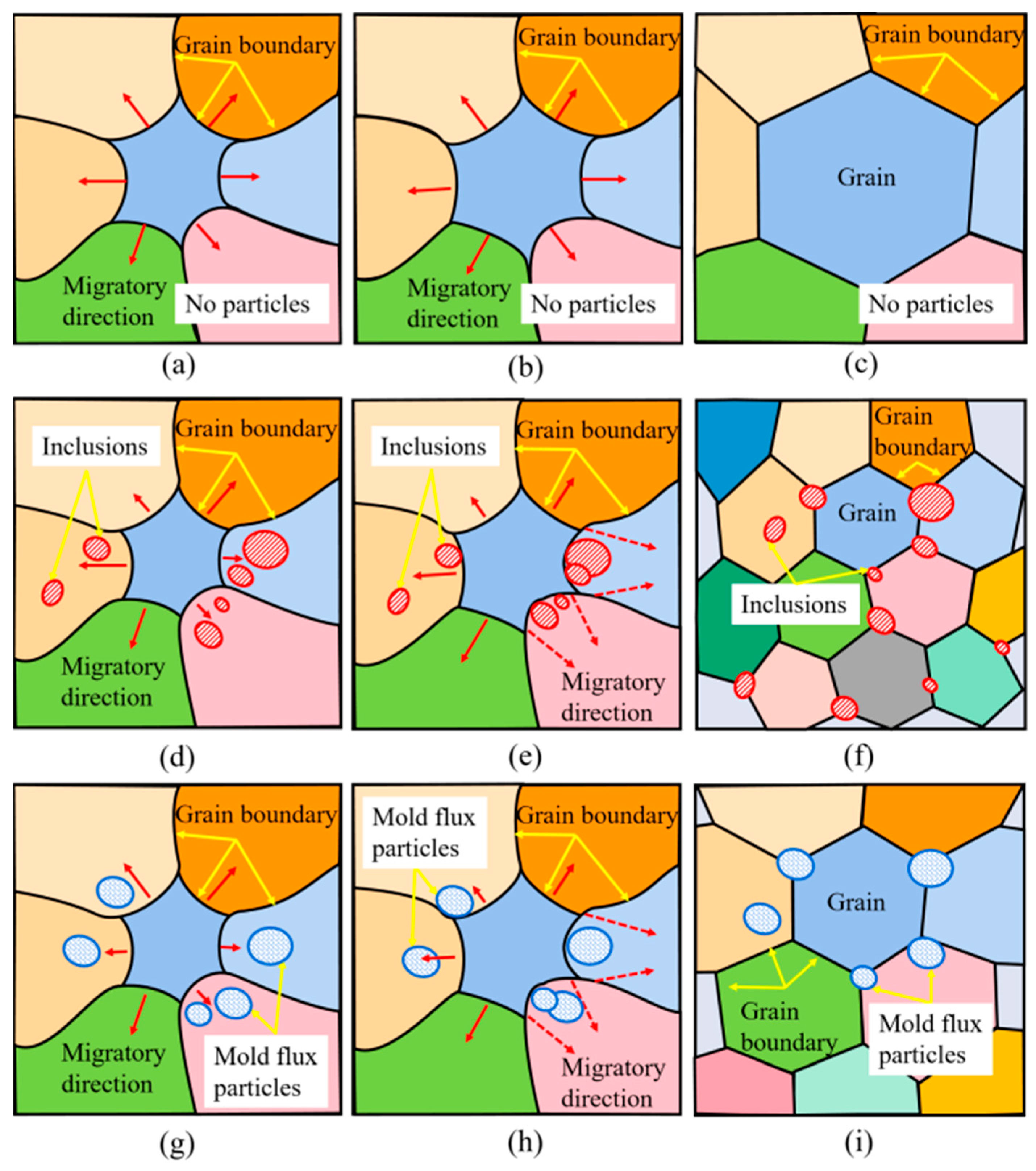
3.3.1. Analysis of Grain Orientation near Al2O3 Inclusions or Mold Flux Particles
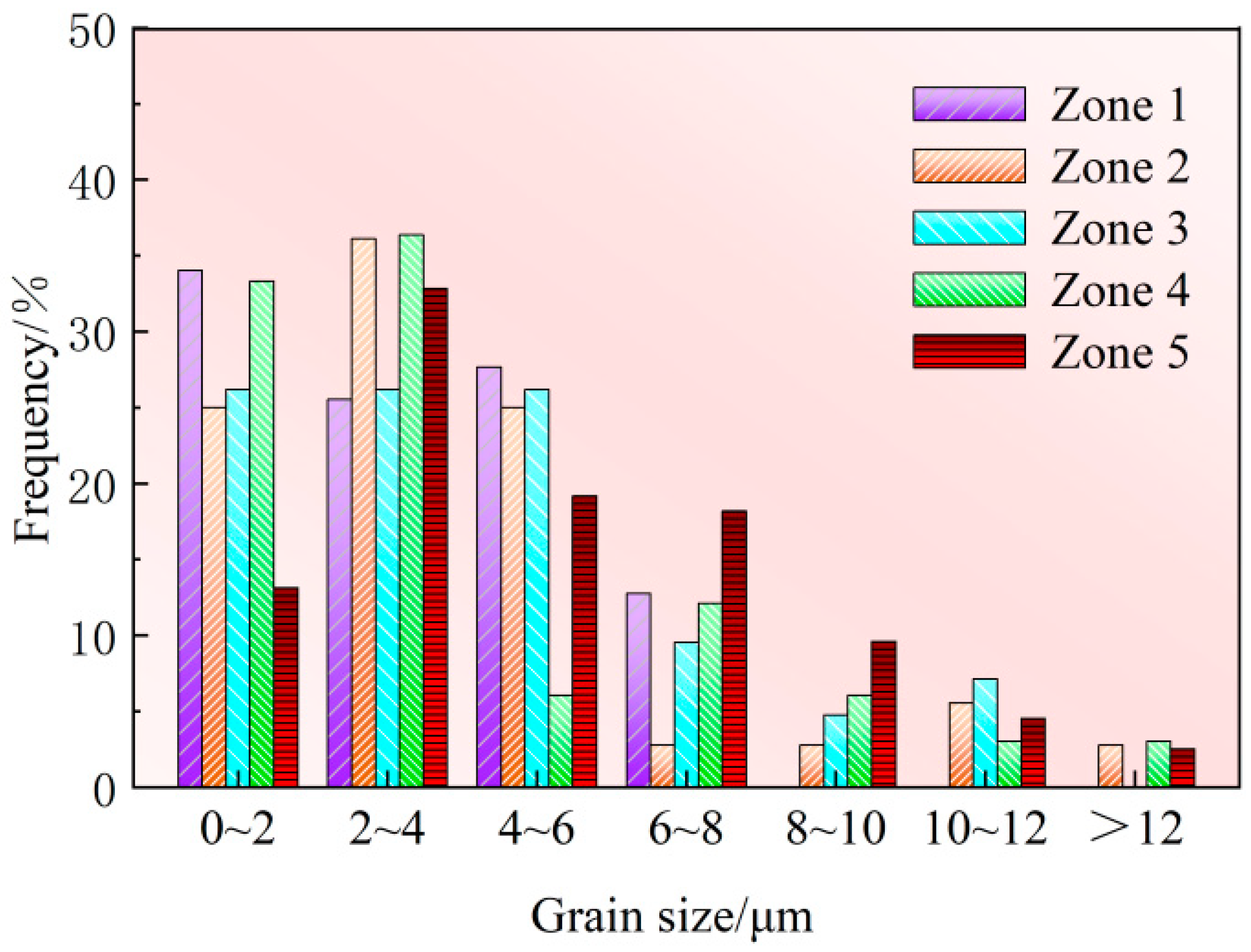
3.3.2. Grain Size Analysis of Defect Zones and Non-Defect Zone
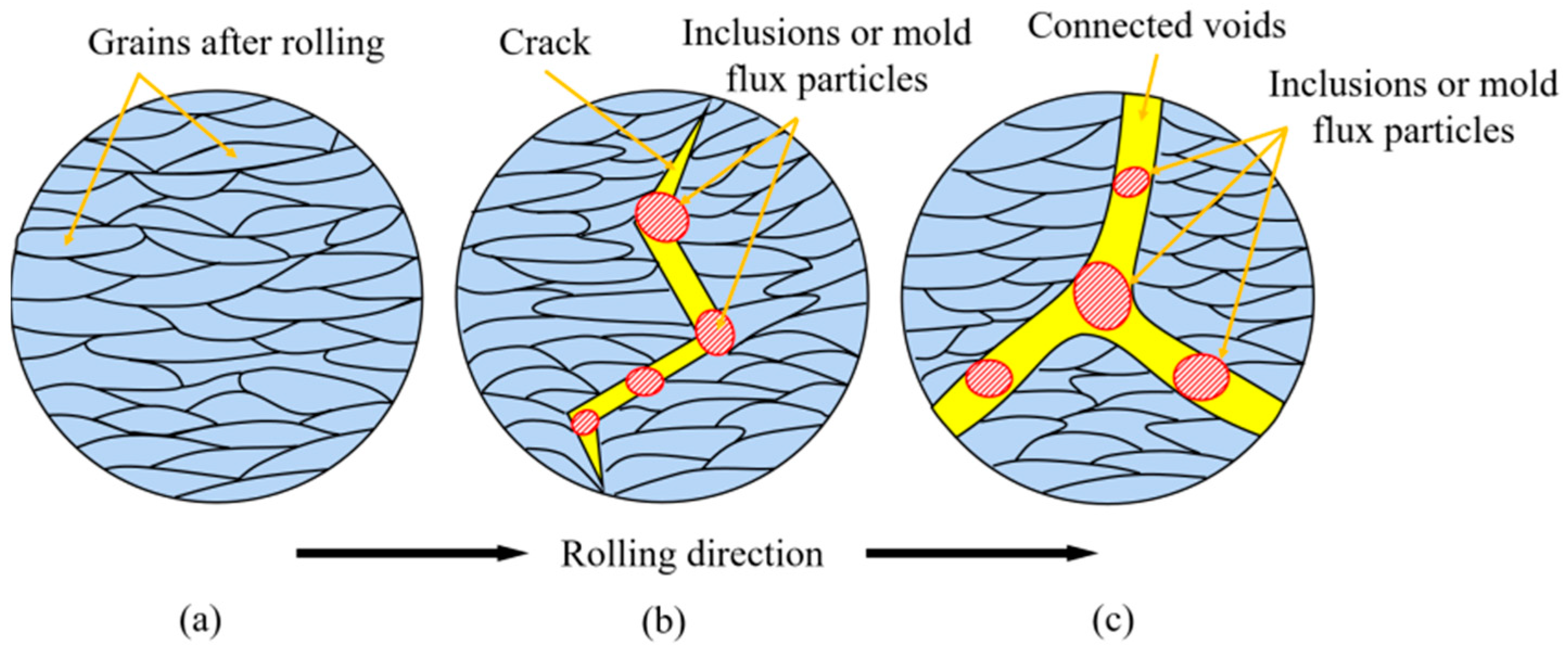
3.4. Formation Mechanism of Sliver Defect on Automobile Exposed Panel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasai, K. Agglomeration and Removal of Alumina Inclusions in Molten Steel with Controlled Concentrations of Interfacial Active Elements. ISIJ Int. 2020, 60, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Lopez, P.E.R.; Mills, K.C.; Lee, P.D. A Unified Mechanism for the Formation of Oscillation Marks. St. Metall. Mater. Trans. B. 2012, 43, 110–122. [Google Scholar]
- Zhang, Q.Y.; Wang, L.; Wang, X.H. Influence of casting speed variation during unsteady continuous casting. ISIJ Int. 2006, 46, 1421–1426. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Bao, Y.P. Source and negative effects of macro-inclusions in titanium stabilized ultra low carbon interstitial free (Ti-IF) steel. Met. Mater. Int. 2012, 18, 29–35. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, J.H.; Wu, G.R. Control for Surface Faint-Sliver Defects in Cold-Rolled IF Steel Sheet. Advanced Materials Research. Adv. Mater. Res. 2011, 396, 1145–1150. [Google Scholar] [CrossRef]
- Gao, W.F. Formation and Prevention of Sliver Defects on the Surface of Cold-Rolled Strip. Advanced Materials Research. Adv. Mater. Res. 2011, 402, 221–226. [Google Scholar] [CrossRef]
- Záhumenský, P.; Merwin, M.J. Evolution of artificial defects from slab to rolled products, J. Mater. Process. Mater. Process. Tech. 2008, 196, 266–278. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Hara, K.; Matsumoto, R.; Azushima, A. Formation mechanism of surface scale defects in hot rolling process. CIRP Ann. 2014, 63, 261–264. [Google Scholar] [CrossRef]
- Yu, H.X.; Ji, C.X.; Chen, B.; Wang, C.; Zhang, Y.H. Characteristics and evolution of inclusion induced surface defects of cold rolled IF sheet. J. Iron Steel Res. 2015, 22, 17–23. [Google Scholar] [CrossRef]
- Pan, X.Q.; Yang, J.; Park, J.; Ono, H. Distribution characteristics of inclusions along with the surface sliver defect on the exposed panel of automobile: A quantitative electrolysis method. Int. J. Miner. Metall. Mater. 2020, 27, 1489–1498. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, J.; Wu, J.; Chen, J.J. Analysis of Orange Peel Defect in St14 Steel Sheet by Electron Backscattered Diffraction (EBSD). Mater. Sci. Technol. 2005, 21, 17–20. [Google Scholar]
- Dong, B.; Yang, C.Z.; Cai, J.; Huang, D.J. Formation mechanism of white linear defects on galvanized steel sheet. Journal of Iron and Steel Research. Iron Steel Res. 2015, 27, 56–61. [Google Scholar]
- Li, T.T.; Yang, J.; Li, G.B.; Kang, W.; Lin, Y.; Meng, J.S. Characteristics of sliver defects caused by mould flux entrainment on the surface of the hot-dip galvanised automobile exposed panel. Ironmak. Steelmak. 2022, 49, 456–471. [Google Scholar] [CrossRef]
- Li, H.B.; Yuan, P.; Chen, B.; Liu, F.G. The influence of slag chemistry on the formation of sliver defects. Ironmak. Steelmak. 2019, 46, 463–468. [Google Scholar] [CrossRef]
- Moir, S.; Preston, J.J. Surface defects-evolution and behaviour from cast slab to coated strip. Mater. Process. Tech. 2002, 125, 720–724. [Google Scholar] [CrossRef]
- Ravinath, H.; Ahammed, I.; Harigovind, P.; VR, A.S.; Shankar, K.V.; Nandakishor, S. Nandakishor, impact of aging temperature on the metallurgical and dry sliding wear behaviour of LM25/Al2O3 metal matrix composite for automotive application. Int. J. Lightweight Mater. Manuf. 2023, in press. [Google Scholar]
- Jiang, B.; Wu, M.; Sun, H.; Wang, Z.L.; Zhao, Z.G.; Liu, Y.Z. Prediction Model of Austenite Growth and the Role of MnS Inclusions in Non-Quenched and Tempered Steel. Met. Mater. Int. 2018, 24, 15–22. [Google Scholar] [CrossRef]
- Gao, L.F.; Kang, Y.L.; Lv, G.; Zhu, G.M.; Liu, R.D.; Lin, L.J. Study on Microstructure Evolution and Micro-Texture inTi-IF Steel During Continuous Annealing. Iron Steel Res. 2011, 23, 33–36. [Google Scholar]
- Muraki, M.; Toge, T.; Sakata, K.; Obara, T.; Furubayashi, E. Formation mechanism of {111} recrystallization texture in ferritic steels. Tetsu Hagane 1999, 85, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Ji, C.; Zhu, G.; Liu, Q.; Huang, F.; Tian, Z.; Wang, X. Quantitative Evaluations of Surface Cleanliness in IF Steel Slabs at Unsteady Casting. Metall. Mater. Trans. B 2019, 50, 1974–1987. [Google Scholar] [CrossRef]
- Leao, P.B.P.; Klug, J.L.; de Abreu, H.F.G.; Carneiro, C.A.R.; Ferreira, H.C.; Bielefeldt, W.V. Sliver defects in an ultra-low carbon Al-killed steel caused by low steel level in the tundish. Ironmak. Steelmak. 2021, 48, 978–987. [Google Scholar] [CrossRef]
- Lee, G.G.; Shin, H.J.; Kim, S.H.; Kim, S.K.; Choi, W.Y.; Thomas, B.G. Prediction and control of subsurface hooks in continuous cast ultra-low-carbon steel slabs. Ironmak. Steelmak. 2009, 36, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.M.; Haldar, A.; Chakrabarti, D. Microstructure and mechanical property of cold rolled low carbon steel after prolonged annealing treatment. Mater. Sci. Technol. 2012, 28, 823–828. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.L.; Zhang, H.L.; Wang, X.T.; Wang, Y.L. Characterization of Impact Deformation Behavior of a Thermally Aged Duplex Stainless Steel by EBSD. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.T.; Yao, S.J.; Sun, Y.; Gao, F.; Song, H.Y.; Liu, G.H.; Li, L.; Geng, D.Q.; Liu, Z.Y.; Wang, G.D. Evolution of microstructure, texture and inhibitor along the processing route for grain-oriented electrical steels using strip casting. Mater. Charact. 2015, 106, 273–282. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five Decadesof the Zener Equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.Q.; Jiang, B.; Mei, Z.; Zhang, C.L.; Zhou, L.Y.; Liu, Y.Z. Effect of Carbide Distribution on the Grain Refinement in the Steel for Large-Size Bearing Ring. steel research international. Steel Res. Int. 2016, 87, 745–751. [Google Scholar] [CrossRef]
- Song, H.Y.; Wang, Y.P.; Esling, C.; Wang, G.D.; Liu, H.T. The role of grain colony on secondary recrystallization in grain-oriented electrical steel: New insights from an original tracking experiment. Acta Mater. 2021, 206, 116611. [Google Scholar] [CrossRef]
- Kozisek, Z.J. Crystallization in small droplets: Competition between homogeneous and heterogeneous nucleation. Journal Of Crystal Growth. Cryst. Growth 2019, 522, 53–60. [Google Scholar] [CrossRef]




| C | Si | Mn | Al | P | S | N | T.O |
|---|---|---|---|---|---|---|---|
| 0.0011–0.0023 | 0.003–0.133 | 0.15–0.63 | 0.017–0.081 | 0.011–0.045 | 0.0039–0.015 | 0.0012–0.0023 | 0.0012–0.0019 |
| SiO2 | CaO | Al2O3 | MgO | Na2O/K2O | F- | Csolid | Basicity |
|---|---|---|---|---|---|---|---|
| 41.95 | 35.71 | 4.77 | 3.66 | 5.15 | 4.51 | 0.49 | 0.85 |
| Defect | Element | Al | O | Mg | Ca | Si | Na | K | F | C | Fe | Zn | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | 1 | 20.2 | 21.6 | / | / | / | / | / | / | 0.49 | 35.3 | 22.4 | 100 |
| 2 | 12.0 | 13.6 | / | / | / | / | / | / | 9.73 | 34.5 | 30.2 | 100 | |
| 3 | 26.4 | 23.8 | / | / | / | / | / | / | 1.47 | 26.2 | 22.1 | 100 | |
| Mold flux | 4 | 8.00 | 27.9 | 6.57 | / | 11.11 | / | 4.63 | / | / | 5.94 | 35.9 | 100 |
| 5 | 4.78 | 24.6 | / | 3.90 | 2.82 | 4.82 | / | / | 19.4 | 13.2 | 26.5 | 100 | |
| 6 | 11.17 | 23.2 | / | 23.4 | 8.21 | / | / | 6.09 | 6.16 | 11.20 | 10.5 | 100 |
| Sample | Zone | Zone Types | X (RD) | Y (TD) | Z (ND) | Max PD | Microtexture | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | PD | OR | PD | OR | PD | |||||
| 1 | Zone 1 | Al2O3 DZ + Al2O3 | <114> | 2.05 | <001> | 2.03 | <001> | 2.65 | 2.68 | {001}<114> |
| 1 | Zone 2 | Al2O3 DZ − Al2O3 | <313> | 1.75 | <101> | 2.05 | <111> | 4.11 | 4.10 | {111}<313> |
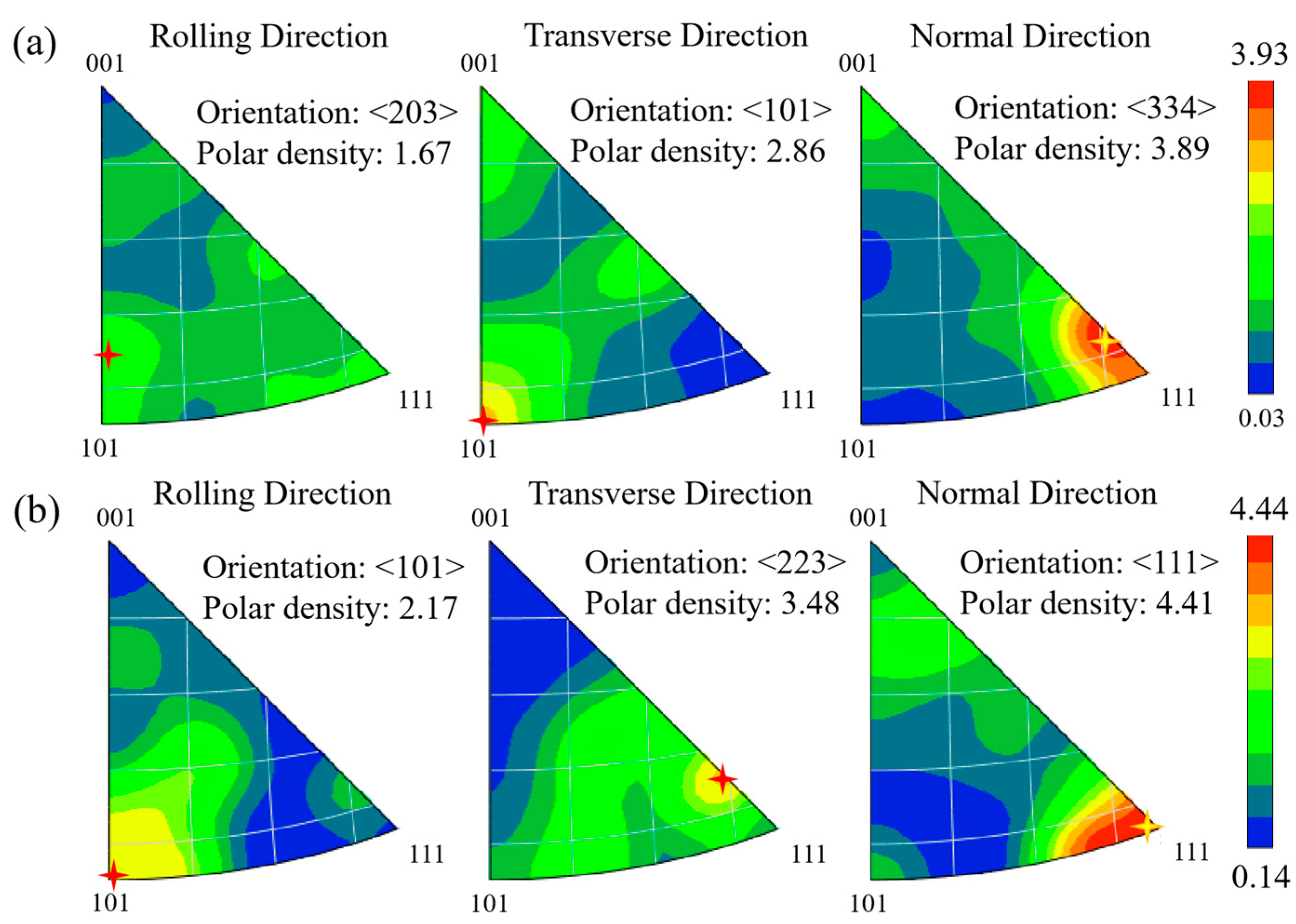
| 5 | Zone 3 | MFDZ + MFP | <203> | 1.67 | <101> | 2.86 | <334> | 3.89 | 3.93 | {001}<203> |
| 5 | Zone 4 | MFDZ − MFP | <101> | 2.17 | <223> | 3.48 | <111> | 4.41 | 4.44 | {111}<101> |
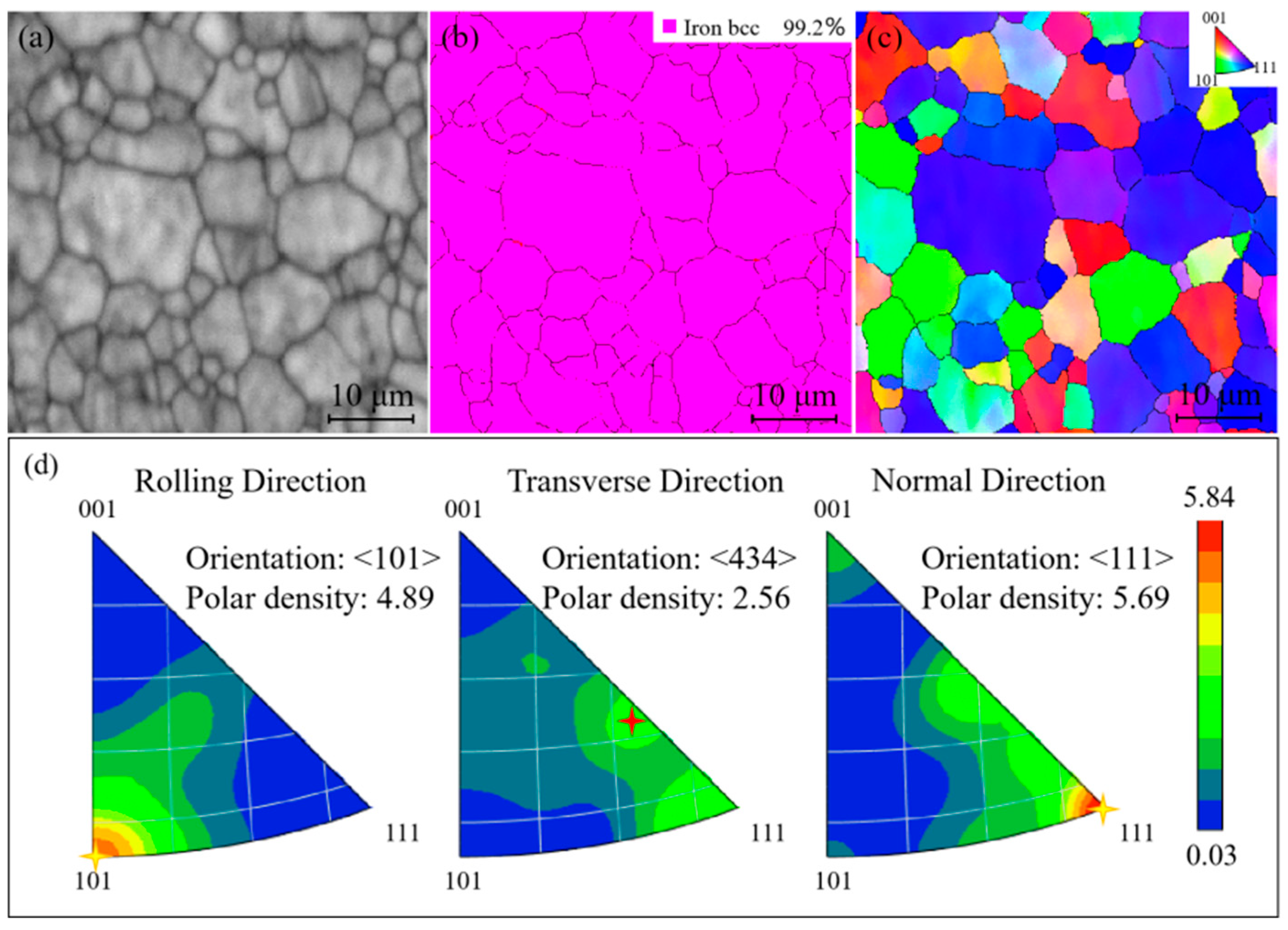
| 9 | Zone 5 | NDZ | <101> | 4.89 | <434> | 2.56 | <111> | 5.69 | 5.84 | {111}<101> |
| Sample | Zone | Zone Types | Min | Max | Difference between Max and Min | Average |
|---|---|---|---|---|---|---|
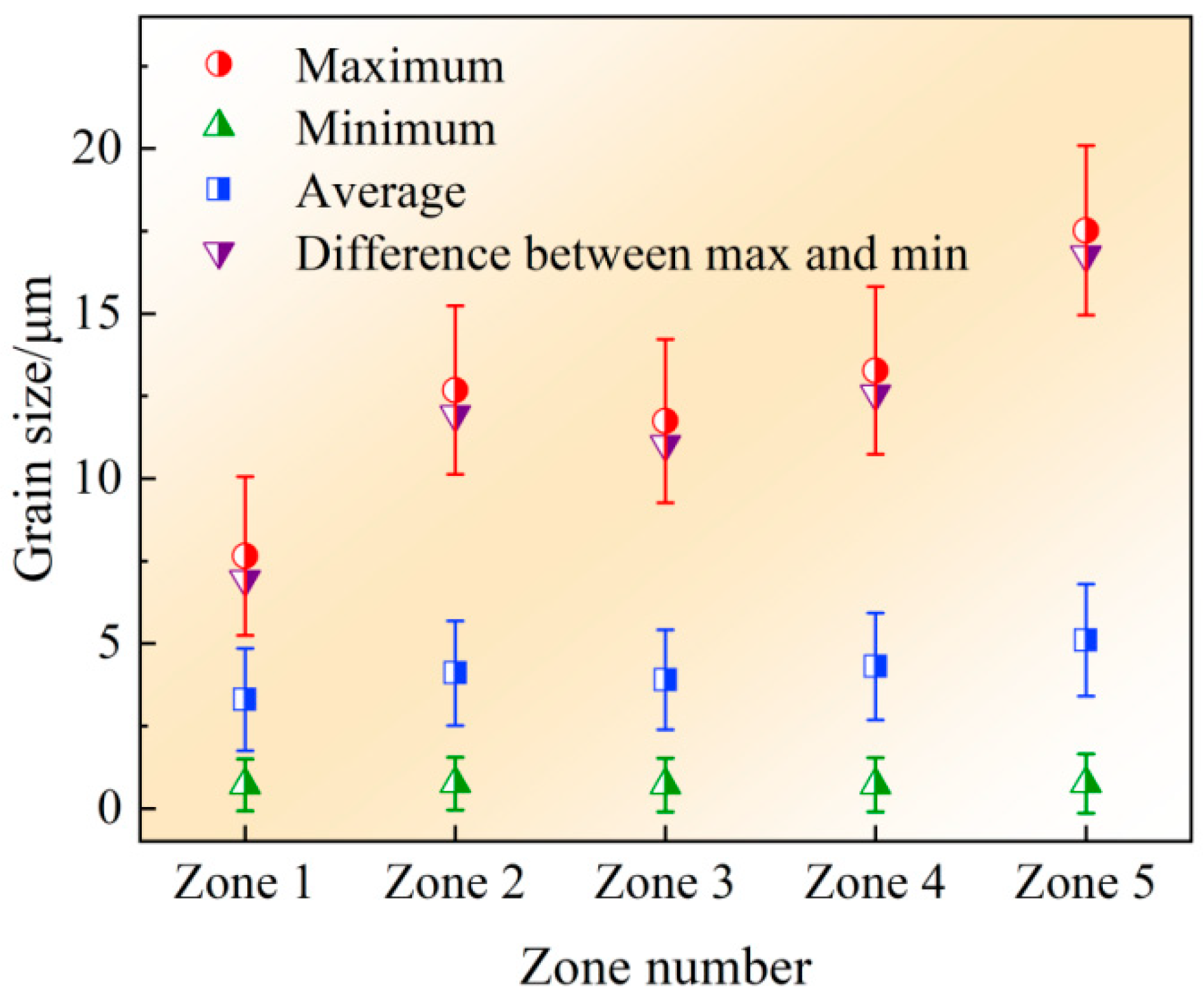
| 1 | Zone 1 | Al2O3 DZ with Al2O3 | 0.71 | 7.65 | 6.94 | 3.3 |
| 1 | Zone 2 | Al2O3 DZ without Al2O3 | 0.75 | 12.68 | 11.93 | 4.1 |
| 5 | Zone 3 | MFDZ with MFP | 0.71 | 11.74 | 11.03 | 3.9 |
| 5 | Zone 4 | MFDZ without MFP | 0.71 | 13.27 | 12.56 | 4.3 |
| 9 | Zone 5 | NDZ | 0.75 | 17.52 | 16.77 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, T.; Yang, J. Effect of Al2O3 Inclusions or Mold Flux Particles on Their Surrounding Microstructures of Sliver Defects on the Surface of Automobile Exposed Panel. Metals 2023, 13, 661. https://doi.org/10.3390/met13040661
Zhang Q, Li T, Yang J. Effect of Al2O3 Inclusions or Mold Flux Particles on Their Surrounding Microstructures of Sliver Defects on the Surface of Automobile Exposed Panel. Metals. 2023; 13(4):661. https://doi.org/10.3390/met13040661
Chicago/Turabian StyleZhang, Qing, Tingting Li, and Jian Yang. 2023. "Effect of Al2O3 Inclusions or Mold Flux Particles on Their Surrounding Microstructures of Sliver Defects on the Surface of Automobile Exposed Panel" Metals 13, no. 4: 661. https://doi.org/10.3390/met13040661




