Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting
Abstract
:1. Introduction
2. Experimental Methods and Materials
3. Results and Discussion
3.1. Thermodynamic Considerations of High-Temperature Reactions
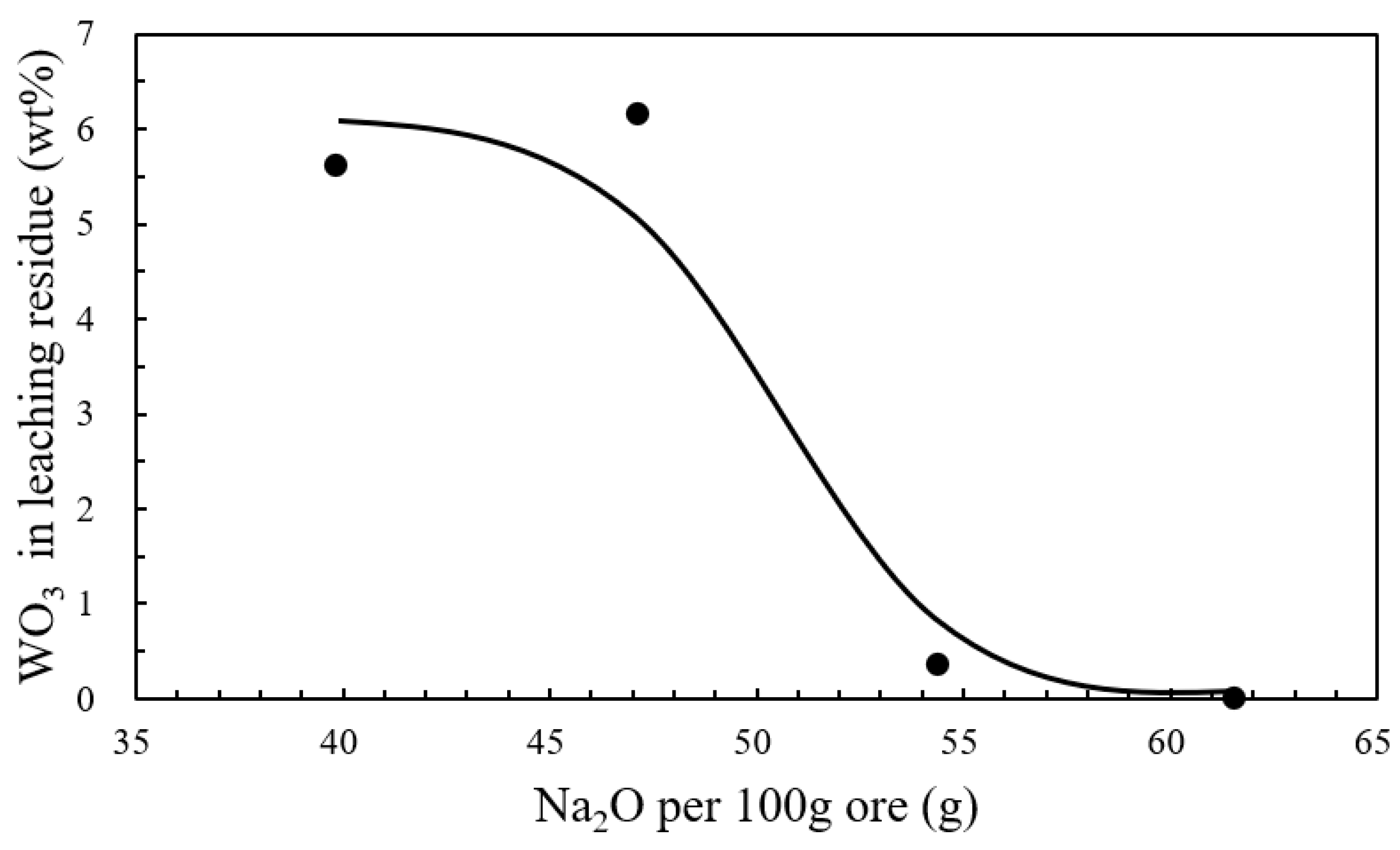
3.2. Experimental Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Li, X.Y.; Ye, Y.Q.; Zhang, F.L.; Wang, D. Recommended Management Strategies and Resources Status of Chinese Tungsten in the New Period. Mod. Min. 2018, 34, 25–28. [Google Scholar]
- Kvashnin, A.G.; Tantardini, C.; Zakaryan, H.A.; Kvashnina, Y.A.; Oganov, A.R. Computational Search for New W–Mo–B Compounds. Chem. Mater. 2020, 32, 7028–7035. [Google Scholar] [CrossRef]
- Pak, A.Y.; Shanenkov, I.I.; Mamontov, G.Y.; Kokorina, A.I. Vacuumless synthesis of tungsten carbide in a self-shielding atmospheric plasma of DC arc discharge. Int. J. Refract. Met. Hard Mater. 2020, 93, 105343. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Rybkovskiy, D.V.; Filonenko, V.P.; Bugakov, V.I.; Zibrov, I.P.; Brazhkin, V.V.; Oganov, A.R.; Osiptsov, A.A.; Zakirov, A.Y. WB5−x: Synthesis, Properties, and Crystal Structure—New Insights into the Long-Debated Compound. Adv. Sci. 2020, 7, 2000775. [Google Scholar] [CrossRef] [PubMed]
- Kvashnin, A.G.; Allahyari, Z.; Oganov, A.R. Computational discovery of hard and superhard materials. J. Appl. Phys. 2019, 126, 040901. [Google Scholar] [CrossRef]
- Geoscience Australia. Available online: https://www.ga.gov.au/about/projects/resources/critical-minerals (accessed on 22 December 2022).
- Gao, Y.D. The characteristics and research advances of mineral processing technologies of Chinese tungsten resources. China Tungsten Ind. 2016, 31, 35–39. [Google Scholar]
- Zhao, Z.W.; Sun, F.L.; Yang, J.H.; Fang, Q.; Jiang, W.W.; Liu, X.H.; Chen, X.Y.; Li, J.T. Status and prospect for tungsten resources, technologies and industrial development in China. Trans. Nonferrous Met. Soc. China 2019, 29, 1902–1916. [Google Scholar]
- Rao, N.K. Beneficiation of tungsten ores in India: A review. Bull. Mater. Sci. 1996, 19, 201–205. [Google Scholar]
- Yao, Z.G. Research on NaF autoclave process for scheelite leaching. China Tungsten Ind. 1999, 14, 167–170. [Google Scholar]
- Zhang, W.J.; Wen, P.C.; Xia, L.; Chen, J.; Che, J.Y.; Wang, C.Y.; Ma, B.Z. Understanding the role of hydrogen peroxide in sulfuric acid system for leaching low-grade scheelite from the perspective of phase transformation and kinetics. Sep. Purif. Technol. 2021, 277, 119407. [Google Scholar] [CrossRef]
- Johansson, Ö.; Pamidi, T.; Shankar, V. Extraction of tungsten from scheelite using hydrodynamic and acoustic cavitation. Ultrason. Sonochem. 2020, 71, 105408. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.M.; Li, H.G.; Li, Y.J.; Zhao, Z.W.; Huo, G.S.; Sun, Z.M.; Liu, M.S. Decomposing scheelite and scheelite-wolframite mixed concentrate by caustic soda digestion. J. Cent. South Univ. Technol. 2003, 10, 297–300. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Li, J.T.; Wang, S.B.; Li, H.G.; Liu, M.S.; Sun, P.M.; Li, Y.J. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Zhao, B.J.; Su, K.; Ma, X.D. Phase Equilibria in the Na2O-WO3 System. Ceram. Int. 2022, 48, 15098–15104. [Google Scholar] [CrossRef]
- Zhao, B.J.; Su, K.; Ma, X.D. Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals 2021, 11, 2014. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermodynamic software and databases 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.D.; Liao, C.F.; Zhao, B.J. High Temperature Processing of Tungsten Slag. In 11th International Symposium on High-Temperature Metallurgical Processing; Peng, Z., Hwang, J.Y., Downey, J.P., Gregurek, D., Zhao, B.J., Yucel, O., Keskinkilic, E., Jiang, T., White, J.F., Mahmoud, M.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 289–294. ISBN 978-3030365394. [Google Scholar]


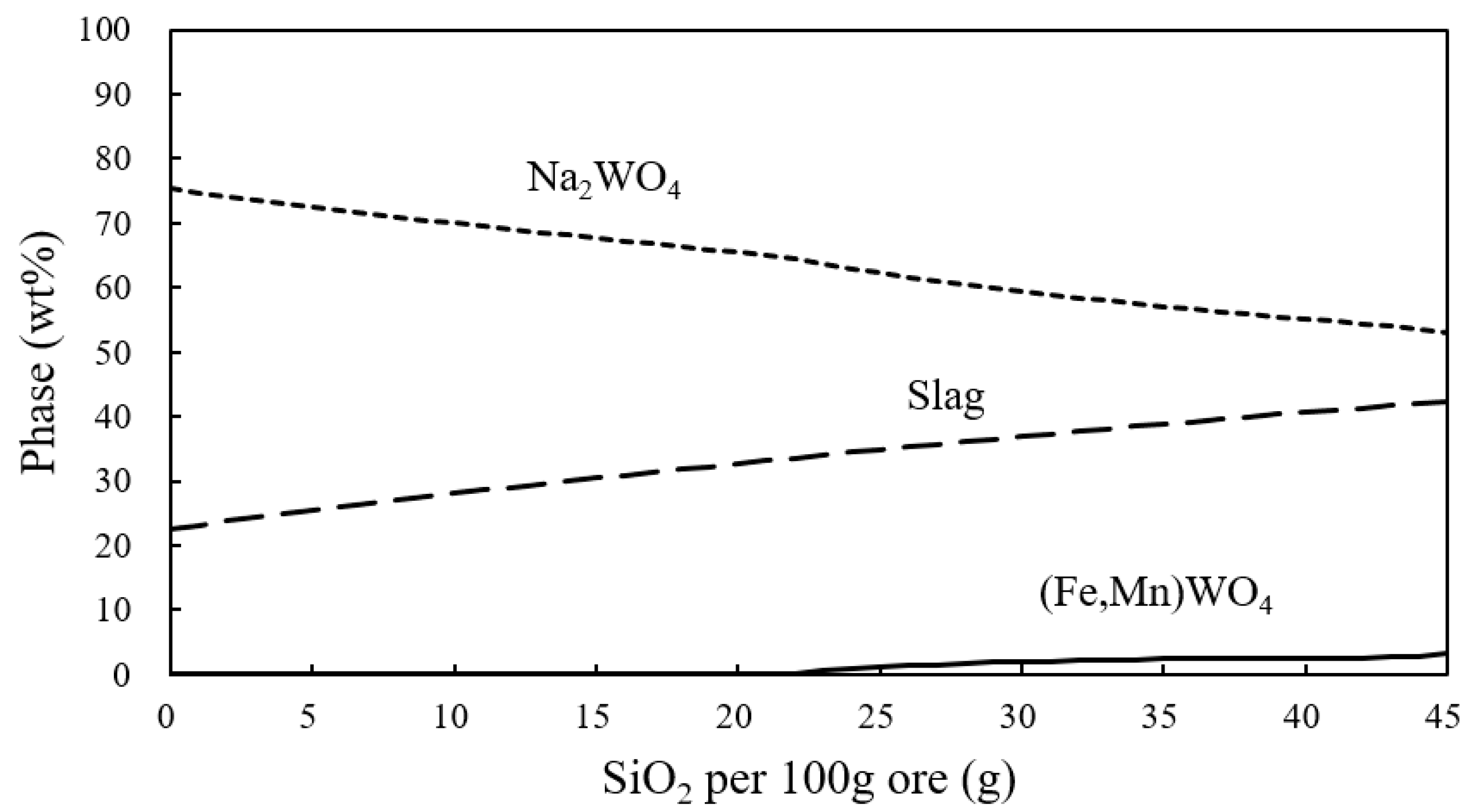
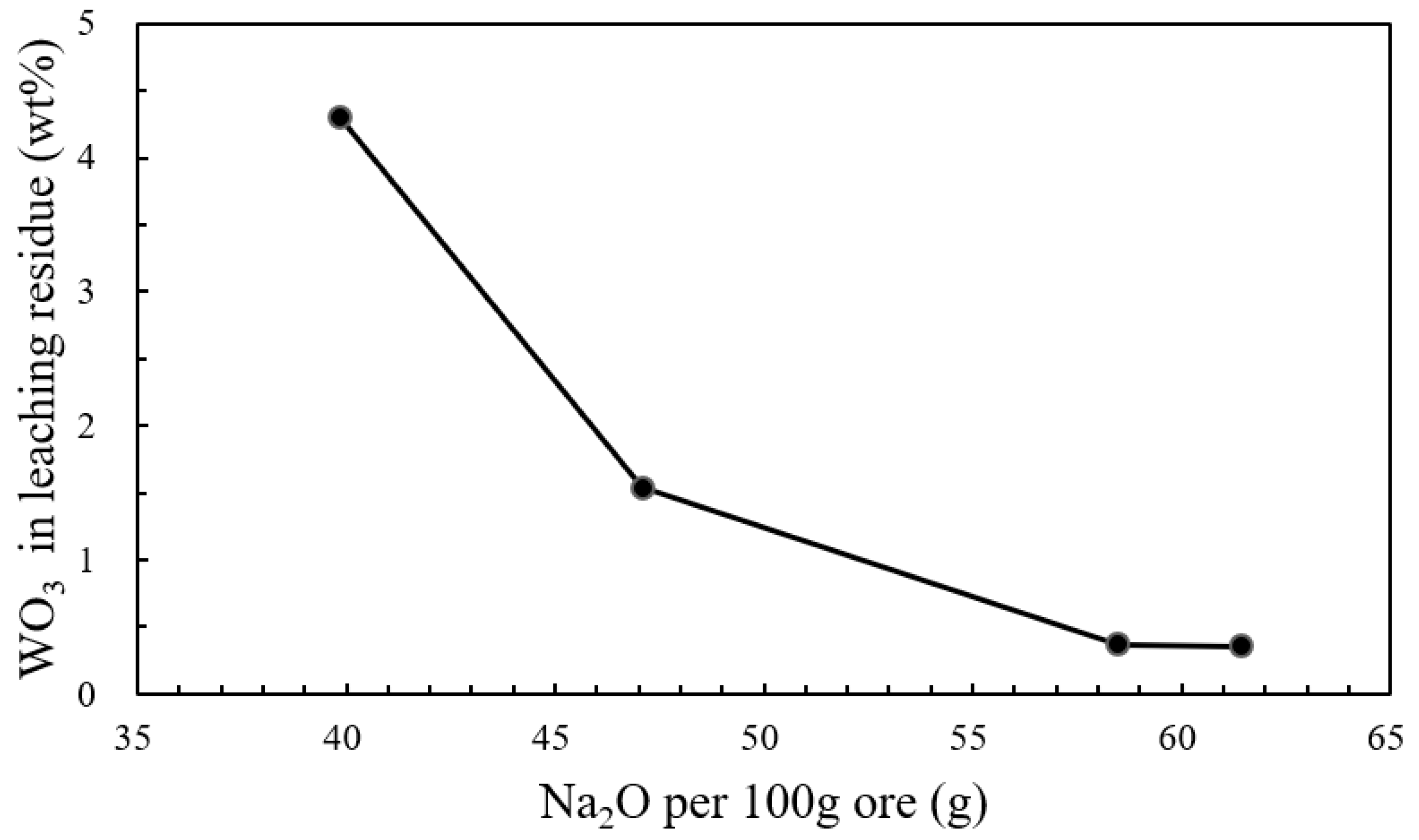
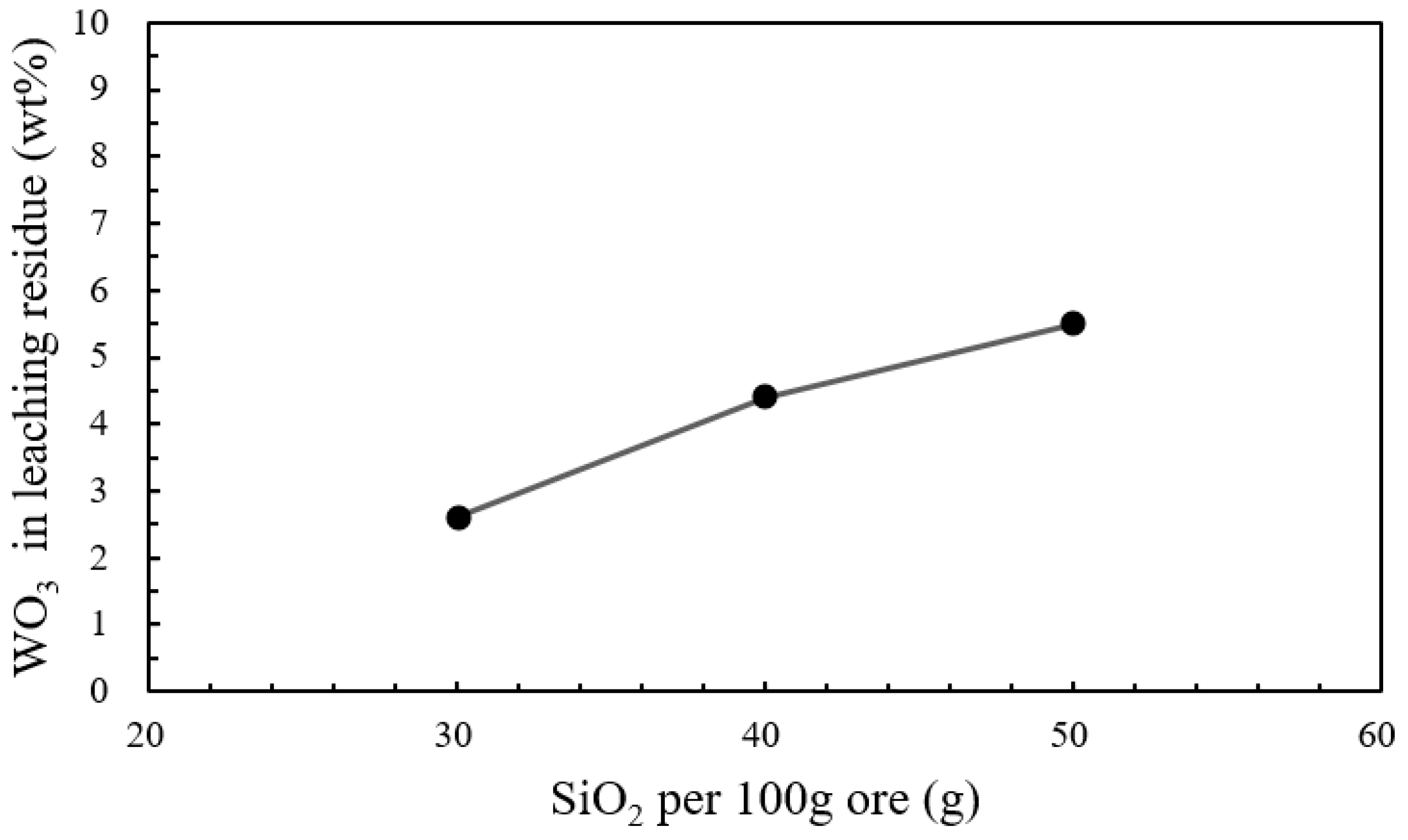

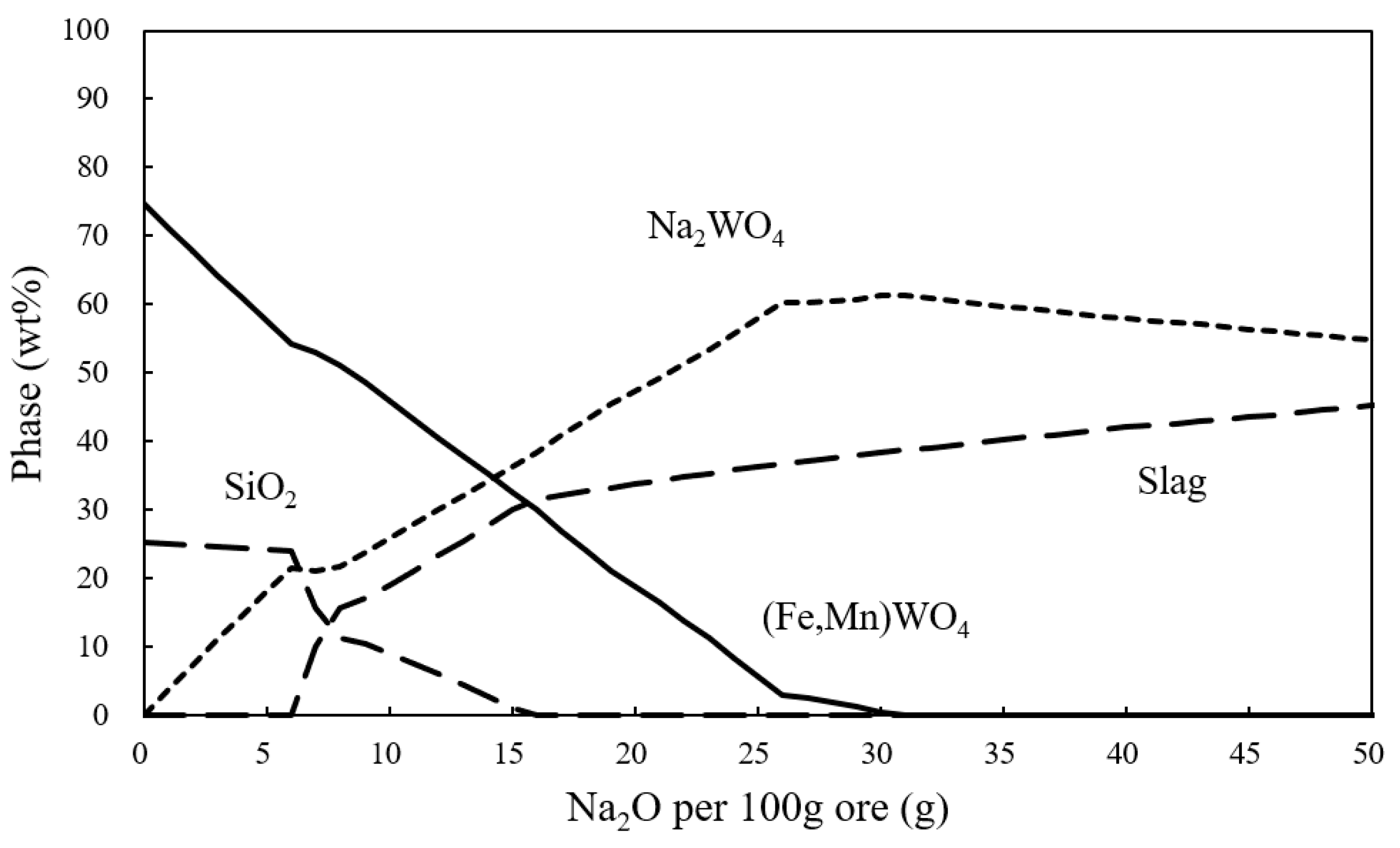
| Wolframite | WO3 | CaO | FeO | MnO | SiO2 | S |
|---|---|---|---|---|---|---|
| wt% | 78.04 | 1.06 | 10.69 | 7.88 | 1.73 | 0.61 |
| Exp No | Ore (g) | Temp (°C) | Time (min) | Na2CO3 (g) | SiO2 (g) | Atmosphere |
|---|---|---|---|---|---|---|
| 1 | 10 | 1200 | 60 | 10.5 | 3 | Air |
| 2 | 10 | 1200 | 60 | 10 | 3 | Air |
| 3 | 10 | 1200 | 60 | 8.05 | 3 | Air |
| 4 | 10 | 1200 | 60 | 6.81 | 3 | Air |
| 5 | 10 | 1050 | 60 | 6.81 | 4 | Air |
| 6 | 10 | 1100 | 60 | 6.81 | 4 | Air |
| 7 | 10 | 1200 | 60 | 6.81 | 4 | Air |
| 8 | 10 | 1200 | 60 | 9.29 | 3 | Air |
| 9 | 10 | 1200 | 60 | 9.29 | 4 | Air |
| 10 | 10 | 1200 | 60 | 9.29 | 5 | Air |
| 11 | 10 | 1200 | 60 | 10.52 | 3 | Ar |
| 12 | 10 | 1200 | 60 | 9.29 | 3 | Ar |
| 13 | 10 | 1200 | 60 | 8.05 | 3 | Ar |
| 14 | 10 | 1200 | 60 | 6.81 | 3 | Ar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhao, B. Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting. Metals 2023, 13, 312. https://doi.org/10.3390/met13020312
Xu L, Zhao B. Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting. Metals. 2023; 13(2):312. https://doi.org/10.3390/met13020312
Chicago/Turabian StyleXu, Liqiang, and Baojun Zhao. 2023. "Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting" Metals 13, no. 2: 312. https://doi.org/10.3390/met13020312





