First-Principles Study of Elastic Properties and Electronic Properties of Al-Ni-Ce Ternary Intermetallic Compounds
Abstract
:1. Introduction
2. Calculation Method
3. Results and Discussion
3.1. Crystal Structure
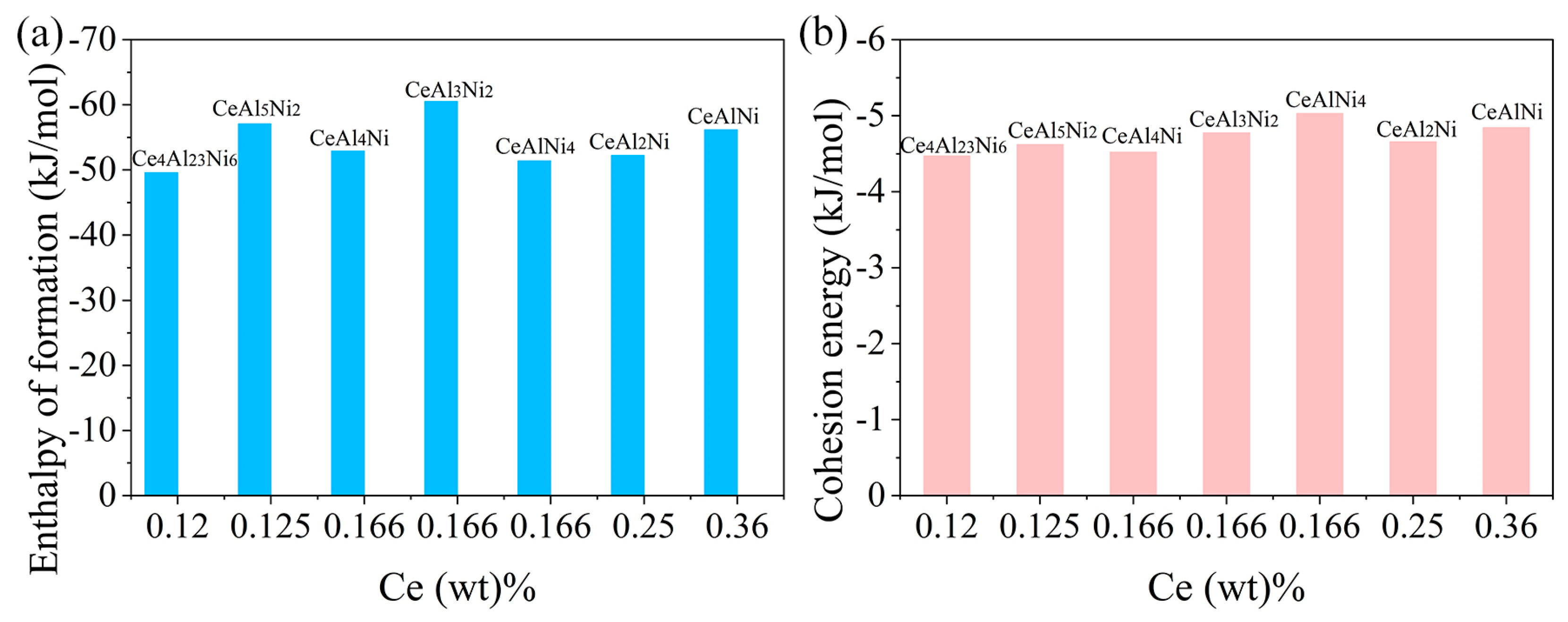
3.2. Enthalpy of Formation and Cohesion Energy
3.3. Elastic Constants and Elastic Modulus
3.3.1. Elastic Constants
3.3.2. Elastic Modulus
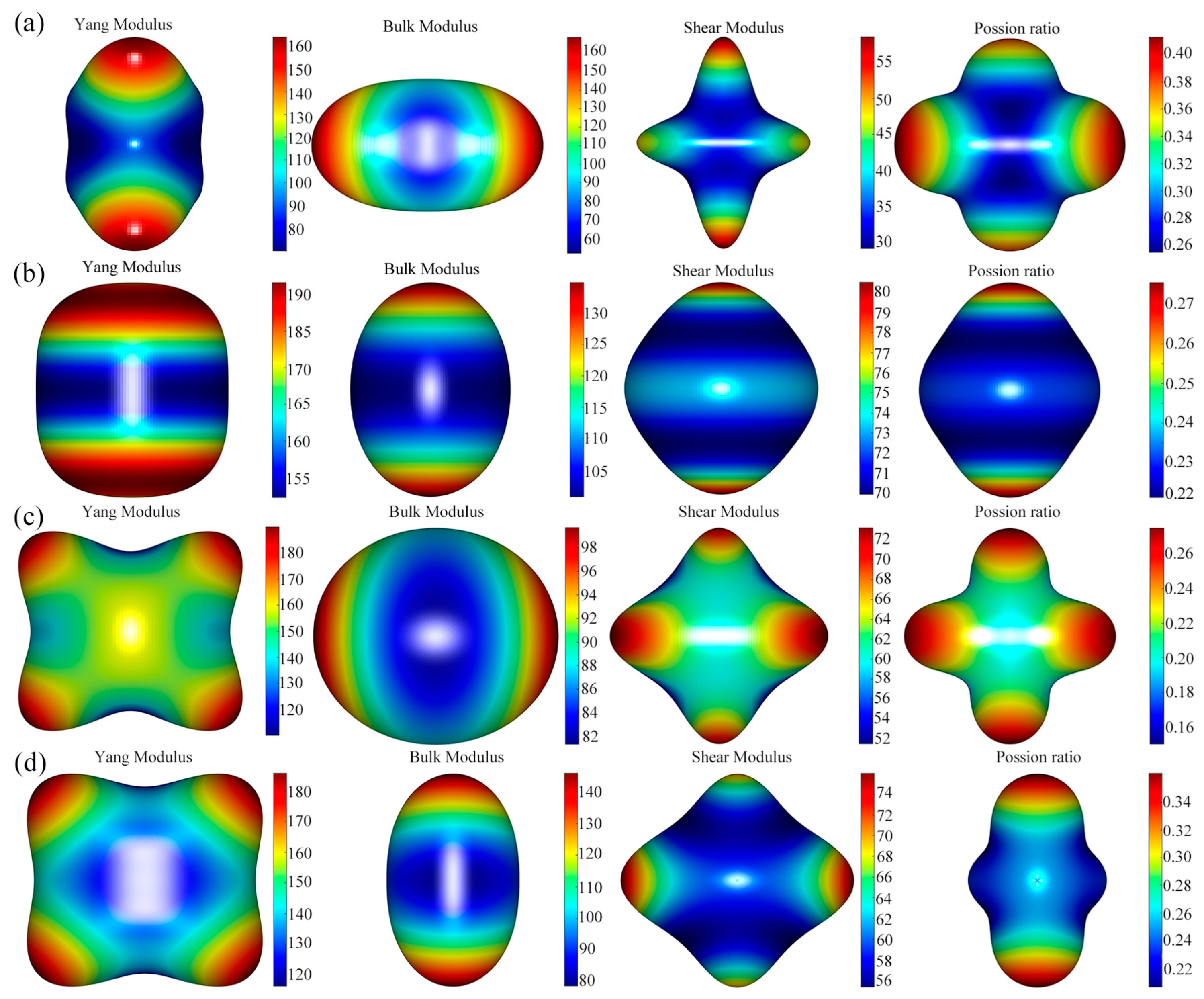
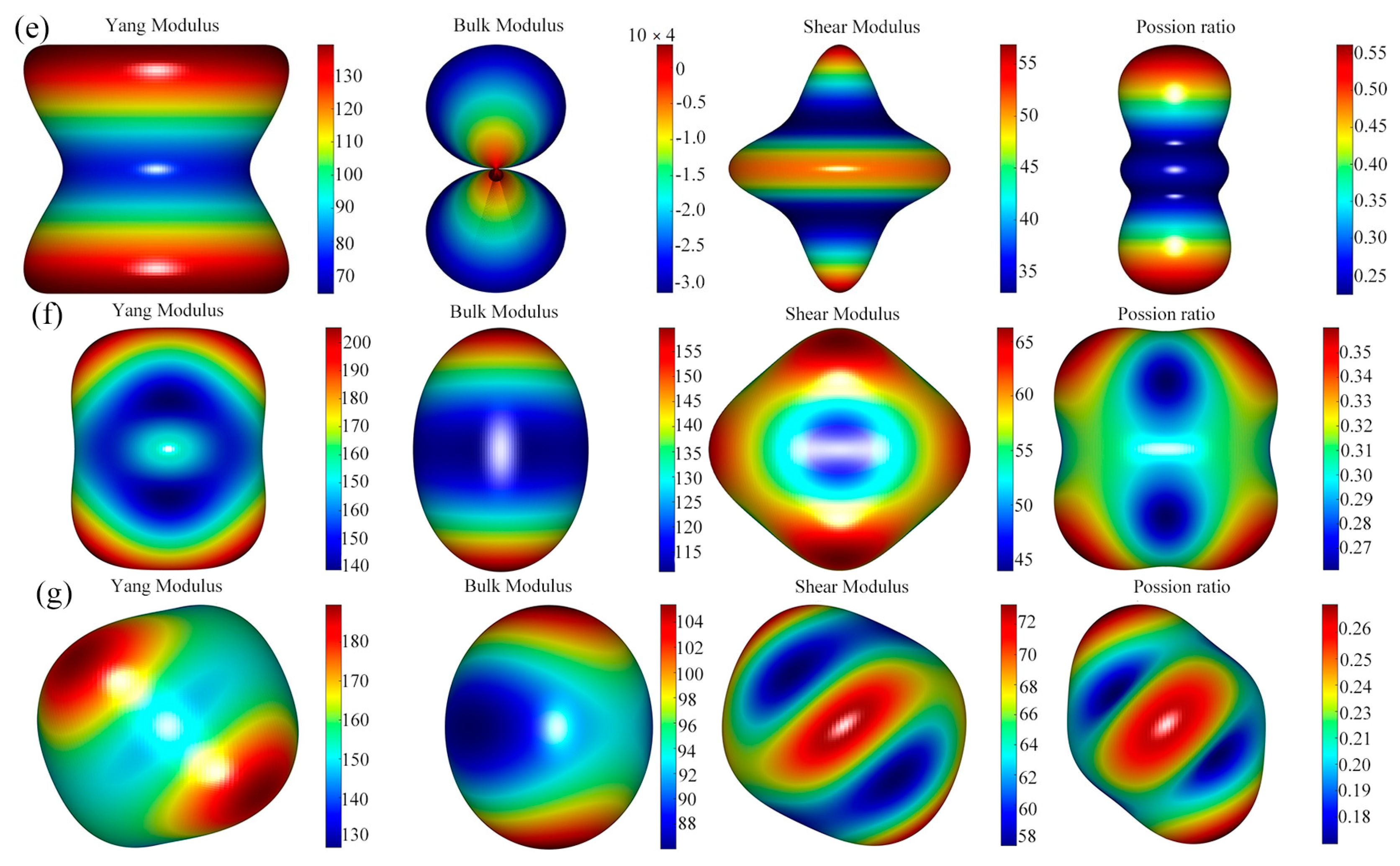
3.4. Anisotropic Elastic Properties
3.5. Partial Density of States
4. Conclusions
- The calculated results of lattice parameters were verified to be close to the experimental values.
- The calculated enthalpy of formation and cohesion energy shows that the Al-Ni-Ce IMCs have good thermodynamic stability.
- The elastic modulus results show that CeAl3Ni2 has the highest Young’s modulus, CeAlNi4 has the highest bulk modulus, and the Poisson’s ratios of CeAlNi and CeAl2Ni are the highest.
- The analysis of the Al-Ni-Ce IMCs modulus of elasticity showed that CeAl3Ni2, CeAl4Ni, CeAl5Ni2, and Ce4Al23Ni6 exhibited brittleness, while CeAlNi, CeAlNi4, and CeAl2Ni exhibited toughness.
- The results of elastic anisotropy analysis showed that the Young’s modulus of CeAl4Ni and CeAlNi4 was the same, the bulk modulus of Ce4Al23Ni6 and CeAl4Ni was the same, and the Poisson’s ratio of CeAlNi4 and CeAl2Ni was the same.
- The analysis of the fractional density of states reveals the prevalence of covalent and metallic bonds in the Al-NI-Ce IMCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, P.; Su, J.; He, Q.; Chen, R.; Chai, S.; Yu, D.; Dai, Q.; Lu, J. Solid-state bonding process induced highly synergistic mechanical properties of 6061 Al alloy joint by shear deformation. J. Mater. Sci. Technol. 2023, 164, 168–178. [Google Scholar] [CrossRef]
- Tang, W.; Yang, X.; Wang, R.; Luo, T. Tailoring microstructure of additive friction stir-deposited Al–Mg alloy through post-processing deformation treatment for enhancing mechanical performance. Mater. Sci. Eng. A 2023, 885, 145632. [Google Scholar] [CrossRef]
- Meng, Y.; Li, J.; Gao, M.; Chen, H. Preparation of Ni–Al intermetallic compounds by plasma arc melting deposition through double-wire feeding. J. Mater. Res. Technol. 2023, 24, 6174–6186. [Google Scholar] [CrossRef]
- Perrin, A.E.; Michi, R.A.; Leonard, D.N.; Sisco, K.D.; Plotkowski, A.J.; Shyam, A.; Poplawsky, J.D.; Allard, L.F.; Yang, Y. Effect of Mn on eutectic phase equilibria in Al-rich Al-Ce-Ni alloys. J. Alloys Compd. 2023, 965, 171455. [Google Scholar] [CrossRef]
- Michi, R.A.; Sisco, K.; Bahl, S.; Yang, Y.; Poplawsky, J.D.; Allard, L.F.; Dehoff, R.R.; Plotkowski, A.; Shyam, A.A. Creep-resistant additively manufactured Al-Ce-Ni-Mn alloy. Acta Mater. 2022, 227, 117699. [Google Scholar] [CrossRef]
- Wu, T.; Plotkowski, A.; Shyam, A.; Dunand, D.C. Microstructure, and creep properties of cast near-eutectic Al–Ce–Ni alloys. Mater. Sci. Eng. A 2022, 833, 142551. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Chen, Z.; Yang, B. Thermodynamic Optimization of the Ni-Al-Ce Ternary System. J. Phase Equilib. Diffus. 2016, 37, 222–228. [Google Scholar] [CrossRef]
- Tang, C.; Du, Y.; Xu, H.H.; Xiong, W.; Zhang, L.J.; Zheng, F.; Zhou, H.Y. Experimental investigation of the Al–Ce–Ni system at 800 °C. Intermetallics 2008, 16, 432–439. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Yan, M.; Li, C.; Wang, L.; Zhou, Y. First-principles Study of NiAl Alloyed with Rare Earth Element Ce. J. Mater. Sci. Technol. 2011, 27, 719–724. [Google Scholar] [CrossRef]
- Delsante, S.; Parodi, N.; Borzone, G. Effect of the Rare-Earth Addition (R = Ce, Sm) on the Phase Equilibria and Microstructure of Ni-Al Alloys. J. Phase Equilib. Diffus. 2014, 35, 421–428. [Google Scholar] [CrossRef]
- Qiao, J.; Wu, F.; Chen, H.; Yang, Z.; Yan, R.; Bai, H.; Pan, F.; Lin, X. Investigation on electronic structures, elastic and thermodynamic properties of MNi3 (M=Be, Mg, Ca) intermetallic compound. Phys. Rev. B Condens. Matter Mater. Phys. 2023, 668, 415217. [Google Scholar] [CrossRef]
- Cheng, Z.; Peng, Z.; Zhong, B.; Liu, H.; Lu, Z.; Zhu, S.; Liu, J. Substitution behavior of Cu doped into Fe3Si and its effect on the electronic structure and mechanical properties based on first-principles calculation. Intermetallics 2023, 160, 107918. [Google Scholar] [CrossRef]
- Hao, L.Y.; Shen, S.K.; Zhang, S.L.; Liu, X.; Wang, Y.F.; Yang, K.J.; Fu, E.G. Ab-initio study on electronic, mechanical and dynamical properties of maraging steel containing coherent B2 type (Fe, Al)Ni nanoprecipitates. Mater. Today Commun. 2023, 36, 106564. [Google Scholar] [CrossRef]
- Liang, H.; He, R.; Liu, L.; Zhang, W.; Fang, L. Investigating the elastic, mechanical, and thermal properties of polycrystalline Mo2C under high pressure and high temperature. Ceram. Int. 2023, 49, 7341–7349. [Google Scholar] [CrossRef]
- Feng, X.; Xue, F.; Zhao, P.; Lu, Y. The electronic structure, elastic properties, dynamical stability and thermoelectric properties of rock-salt and orthorhombic phases of CdS: First-principles calculations. Solid State Commun. 2022, 353, 114878. [Google Scholar] [CrossRef]
- Zhang, F.; Luan, B.; Chu, L.; Wen, S.; Zhang, S.; Wang, Y.; Wu, L.; Murty, K.L. Effects of composition on phase stabilities and elastic properties in TiZrAlV alloys: Experiments and first-principles calculations. J. Alloys Compd. 2021, 863, 158054. [Google Scholar] [CrossRef]
- Mizushima, A.; Isikawa, Y.; Maeda, A.; Oyabe, K.; Mori, K.; Sato, K.; Kamigaki, K. A new dense-kondo compound CeNiAl4. J. Phys. Soc. Jpn. 1991, 60, 753–756. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Ce-Ni (Aluminum-Cerium-Nickel). J. Phase Equilb. Diff. 2009, 30, 265–267. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Oliver, G.L.; Perdew, J.P. Spin-density gradient expansion for the kinetic energy. Phys. Rev. A 1979, 20, 397–403. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, 1133–1137. [Google Scholar] [CrossRef]
- Cava, R.J.; Ramirez, A.P.; Takagi, H.; Krajewski, J.J.; Peck, W.F. Physical properties of some ternary ce intermetallics with the transition metals Ni and Pd. J. Magn. Magn. Mater. 1993, 128, 124–128. [Google Scholar] [CrossRef]
- Isikawa, Y.; Mizushima, T.; Sakurai, J.; Mori, K.; Munoz, A.; Givord, F.; Boucherle, J.; Voiron, J.; Oliveira, I.S.; Flouquet, J. Magnetic properties and neutron diffraction measurements of dense-kondo compound CeNi2Al5. J. Phys. Soc. Jpn. 1994, 63, 2349–2358. [Google Scholar] [CrossRef]
- Oesterreicher, H. Structural and magnetic studies on rare-earth compounds RNiAl and RCuAl. J. Less-Common Met. 1973, 30, 225–236. [Google Scholar] [CrossRef]
- Gout, D.; Benbow, E.; Gourdon, O.; Miller, G.J. Crystallographic, electronic and magnetic studies of Ce4Ni6Al23: A new intermetallic compound in the cerium-nickel-aluminum phase diagram. J. Solid State Chem. 2003, 174, 471–481. [Google Scholar] [CrossRef]
- Fang, C.M.; Huis, M.A.; Zandbergen, H.W. Stability and structures of the CFCC-TmC phases: A first-principles study. Comput. Mater. Sci. 2012, 51, 146–150. [Google Scholar] [CrossRef]
- Rougab, M.; Gueddouh, A. First-principles insights into structural stability, elastic anisotropies, mechanical and thermodynamic properties of the Hf2GeX (X = C, N, and B) 211 MAX phases. J. Phys. Chem. Solids 2023, 176, 111251. [Google Scholar] [CrossRef]
- Tang, C.; Du, Y.; Zhou, H. The phase equilibria of the Al–Ce–Ni system at 500 °C. J. Alloys Compd. 2009, 470, 222–227. [Google Scholar] [CrossRef]
- Yang, K.; Wan, R.D.; Zhang, Z.F.; Lei, Y.; Tian, G.C. First-principles investigation on the thermoelectric and electronic properties of HfCoX (X = As, Sb, Bi) half-Heusler compounds. J. Solid State Chem. 2022, 314, 123386. [Google Scholar] [CrossRef]
- Beckstein, O.; Klepeis, J.E.; Hart, G.L.; Pankratov, O. First-principles elastic constants and electronic structure of α − Pt2Si and PtSi. Phys. Rev. B 2001, 63, 134112. [Google Scholar] [CrossRef]
- Watt, J.P.; Peselnick, L. Elastic properties of polycrystalline minerals: Comparison of theory and experiment. J. Appl. Phys. 1980, 51, 1525. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Ling, Y.; Liu, W.; Zou, X.; Yan, H. Phase stability and elastic properties of Al–Pr intermetallic compounds from first principles calculations. Physica B 2023, 663, 415001. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, F. First-principles study on the lattice vibration, anisotropy, tensile strength and electronic properties of CuxHfySiz intermetallics. Chem. Phys. Lett. 2023, 830, 140811. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. Ser. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Zhang, F.; Xue, D. Electronegativity Identification of Novel Superhard Materials. Phys. Rev. Lett. 2008, 100, 235504. [Google Scholar] [CrossRef] [PubMed]
- KÖSTER, W.; Franz, H. Poisson’s ratio for metals and alloys. Int. Mater. Rev. 1961, 6, 1–56. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, G.; Ren, X.; Wang, M.; Xu, H.; Fu, Y.; Tang, Y.; Li, D. First-principles studies on phase stability, anisotropic elastic and electronic properties of Al-La binary system intermetallic compounds. Mater. Today Commun. 2020, 24, 101101. [Google Scholar] [CrossRef]
- Chen, H.; Yang, L.; Long, J. First-principles investigation of the elastic, Vickers hardness and thermodynamic properties of Al–Cu intermetallic compounds. Superlattices Microstruct. 2015, 79, 156–165. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Dong, Q.; Chen, X.; Tan, J. First-principles study on the thermodynamic, electronic and mechanical properties of Mg-Al-Si ternary compounds. J. Mater. Res. Technol. 2022, 19, 2848–2862. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Cui, P. Modeling of alloying effect on elastic properties in BCC Nb-Ti-V-Zr solid solution: From unary to quaternary. Comput. Mater. Sci. 2020, 172, 109289. [Google Scholar] [CrossRef]
- Özer, T. Investigation of pressure dependence of anisotropy and elastic modulus of SbSI compound in ferroelectric phase by Ab initio method. Mater. Sci. Eng. B 2023, 297, 116787. [Google Scholar] [CrossRef]
- Gaillac, R.; Pullumbi, P.; Coudert, F.X. ELATE: An open-source online application for analysis and visualization of elastic tensors. J. Phys. Condens. Matter. 2016, 28, 275201. [Google Scholar] [CrossRef] [PubMed]
- Nong, Z.S.; Zhu, J.C.; Yang, X.W.; Cao, Y.; Lai, Z.H.; Liu, Y. First-principles investigation of the elastic and electronic properties of the binary intermetallics in the Al–La alloy system. Physica B 2012, 407, 4706–4711. [Google Scholar] [CrossRef]
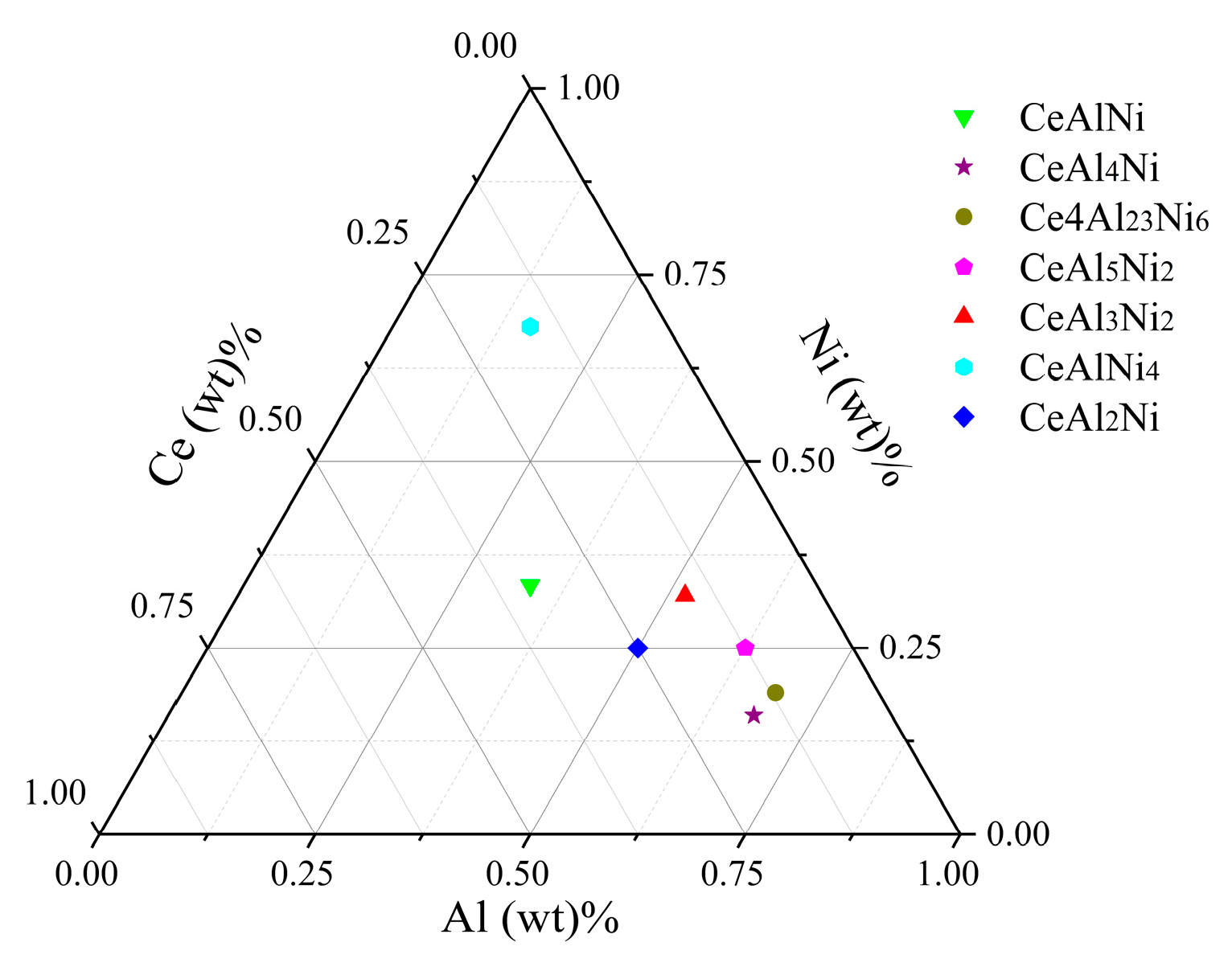

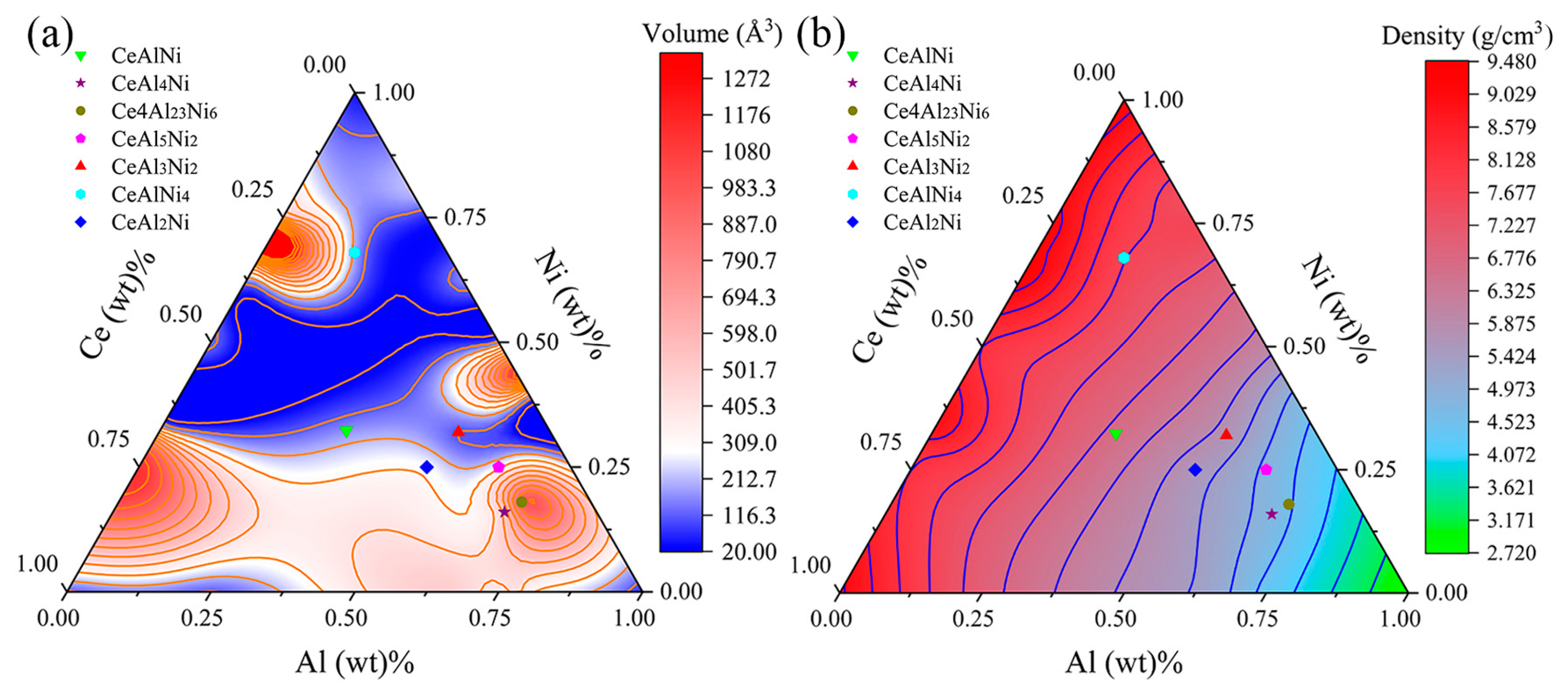

| Crystal | Lattice Constant (Å) | Volume (Å3) | Density (g/cm3) | Reference | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| CeAl2Ni | 4.07 | 10.61 | 6.92 | 299.07 | 5.61 | This work |
| CeAl3Ni2 | 5.29 | 5.29 | 4.04 | 98.24 | 5.72 | This work |
| 5.25 | 5.25 | 4.02 | 95.88 | 5.86 | Exp. [22] | |
| CeAl4Ni | 4.12 | 15.61 | 6.61 | 426.38 | 4.77 | This work |
| 4.10 | 15.57 | 6.61 | 421.86 | 4.83 | Exp. [17] | |
| CeAl5Ni2 | 3.98 | 7.04 | 9.54 | 267.84 | 4.86 | This work |
| 3.98 | 7.00 | 9.52 | 265.20 | 4.91 | Exp. [23] | |
| CeAlNi | 6.96 | 6.96 | 4.01 | 168.50 | 6.67 | This work |
| 6.87 | 6.87 | 4.02 | 164.10 | 6.85 | Exp. [24] | |
| CeAlNi4 | 5.06 | 8.38 | 4.08 | 173.66 | 7.68 | This work |
| Ce4Al23Ni6 | 16.04 | 4.12 | 18.30 | 1113.15 | 4.57 | This work |
| 16.00 | 4.12 | 18.31 | 1107.59 | 4.60 | Exp. [25] | |
| Crystal | Enthalpy of Formation (kJ/mol) | Cohesion Energy (kJ/mol) | |
|---|---|---|---|
| Present Work | Reference [9] | ||
| CeAl2Ni | −52.2 | −55.7 | −4.66 |
| CeAl3Ni2 | −60.52 | −61.5 | −4.77 |
| CeAl4Ni | −52.88 | −53.6 | −4.52 |
| CeAl5Ni2 | −57.06 | −56.6 | −4.62 |
| CeAlNi | −56.16 | −56.2 | −4.84 |
| CeAlNi4 | −51.40 | −5.03 | |
| Ce4Al23Ni6 | −49.57 | −48.3 | −4.47 |
| Crystal | Elastic Stiffness Constants (GPa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CeAl2Ni | 154.57 | 60.98 | 87.28 | 104.54 | 59.92 | 127.28 | 40.50 | 84.35 | 27.40 |
| CeAl3Ni2 | 191.95 | 58.62 | 69.05 | 191.95 | 70.41 | 219.68 | 80.51 | 80.28 | 68.85 |
| CeAl4Ni | 171.92 | 38.14 | 68.39 | 177.88 | 48.02 | 413.69 | 59.63 | 87.25 | 61.29 |
| CeAl5Ni2 | 175.27 | 71.30 | 46.44 | 176.63 | 79.34 | 153.36 | 52.36 | 62.71 | 87.54 |
| CeAlNi | 133.20 | 41.68 | 98.22 | 133.20 | 98.22 | 174.58 | 56.90 | 56.90 | 45.75 |
| CeAlNi4 | 196.67 | 81.84 | 88.45 | 198.76 | 79.57 | 241.93 | 40.67 | 85.79 | 47.51 |
| Ce4Al23Ni6 | 175.42 | 52.56 | 48.37 | 180.49 | 63.41 | 166.02 | 70.26 | 61.21 | 77.95 |
| Crystal | Elastic Modulus | ||||
|---|---|---|---|---|---|
| (GPa) | (GPa) | (GPa) | |||
| CeAl2Ni | 100.73 | 86.71 | 38.55 | 0.30 | 2.24 |
| CeAl3Ni2 | 178.15 | 111.08 | 72.26 | 0.23 | 1.53 |
| CeAl4Ni | 152.02 | 89.07 | 62.53 | 0.21 | 1.42 |
| CeAl5Ni2 | 148.16 | 99.12 | 59.22 | 0.25 | 1.67 |
| CeAlNi | 108.44 | 93.79 | 41.47 | 0.30 | 2.26 |
| CeAlNi4 | 151.89 | 125.78 | 58.47 | 0.29 | 2.15 |
| Ce4Al23Ni6 | 158.12 | 94.37 | 64.76 | 0.22 | 1.45 |
| Crystal | Elastic Modulus Anisotropy AX | |||
|---|---|---|---|---|
| Young’s Modulus | Bulk Modulus | Shear Modulus | Poisson’s Ratio | |
| CeAl2Ni | 2.34 | 3.26 | 2.01 | 1.33 |
| CeAl3Ni2 | 1.18 | 1.33 | 1.15 | 1.64 |
| CeAl4Ni | 1.72 | 1.22 | 1.42 | 1.50 |
| CeAl5Ni2 | 1.59 | 1.96 | 1.38 | 2.00 |
| CeAlNi | 2.16 | 639.09 | 1.72 | 3 |
| CeAlNi4 | 1.72 | 1.43 | 1.51 | 1.33 |
| Ce4Al23Ni6 | 1.49 | 1.22 | 1.27 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Hu, W.; Wang, J.; Yan, H. First-Principles Study of Elastic Properties and Electronic Properties of Al-Ni-Ce Ternary Intermetallic Compounds. Metals 2023, 13, 1921. https://doi.org/10.3390/met13121921
Ling Y, Hu W, Wang J, Yan H. First-Principles Study of Elastic Properties and Electronic Properties of Al-Ni-Ce Ternary Intermetallic Compounds. Metals. 2023; 13(12):1921. https://doi.org/10.3390/met13121921
Chicago/Turabian StyleLing, Ying, Wenjie Hu, Jiabin Wang, and Hong Yan. 2023. "First-Principles Study of Elastic Properties and Electronic Properties of Al-Ni-Ce Ternary Intermetallic Compounds" Metals 13, no. 12: 1921. https://doi.org/10.3390/met13121921






