The Effect of Mn and Ti Ratio on Microstructure and Mechanical and Machinability Properties of 316 L Stainless Steel Used in Biomedical Applications
Abstract
:1. Introduction
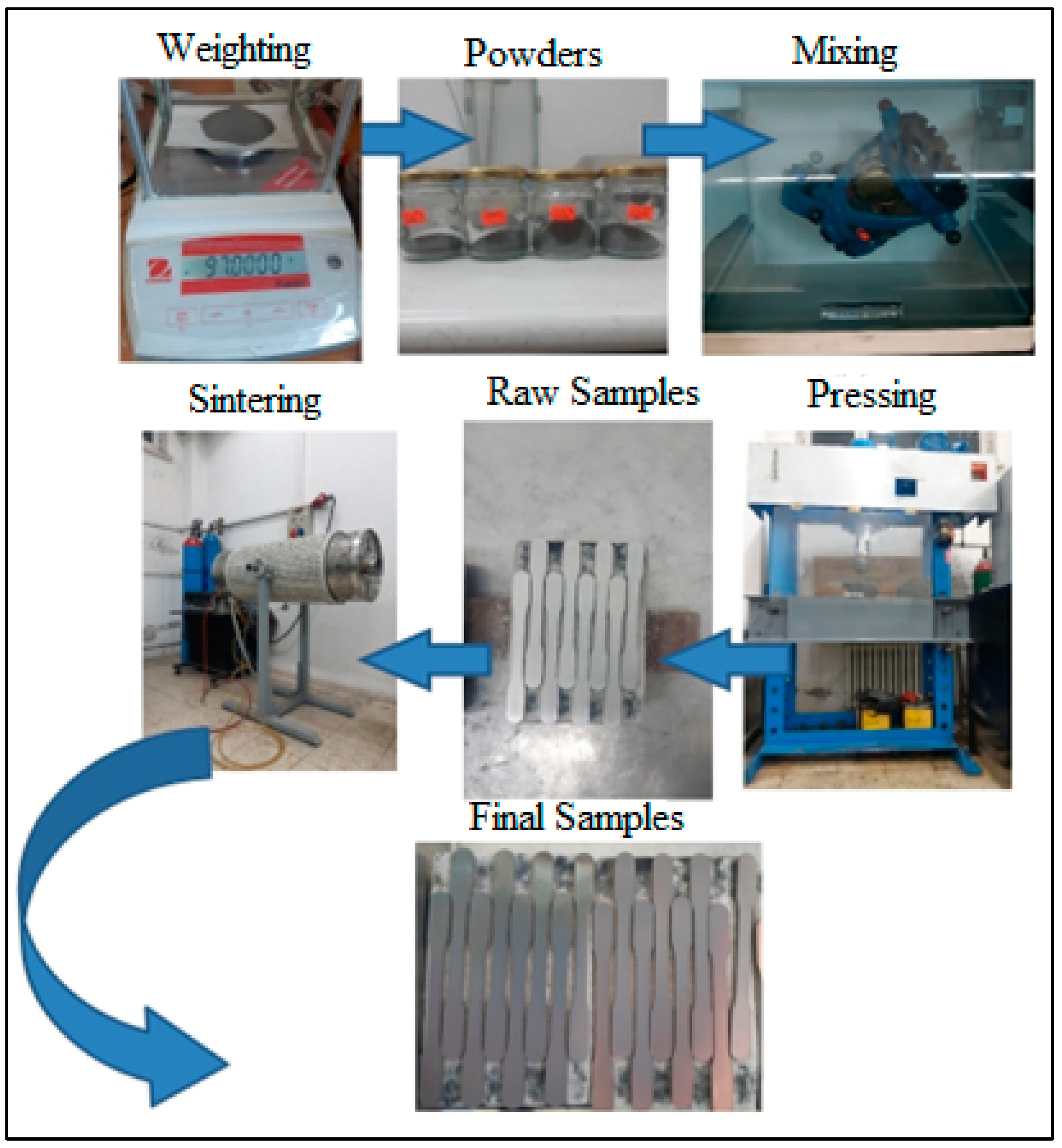
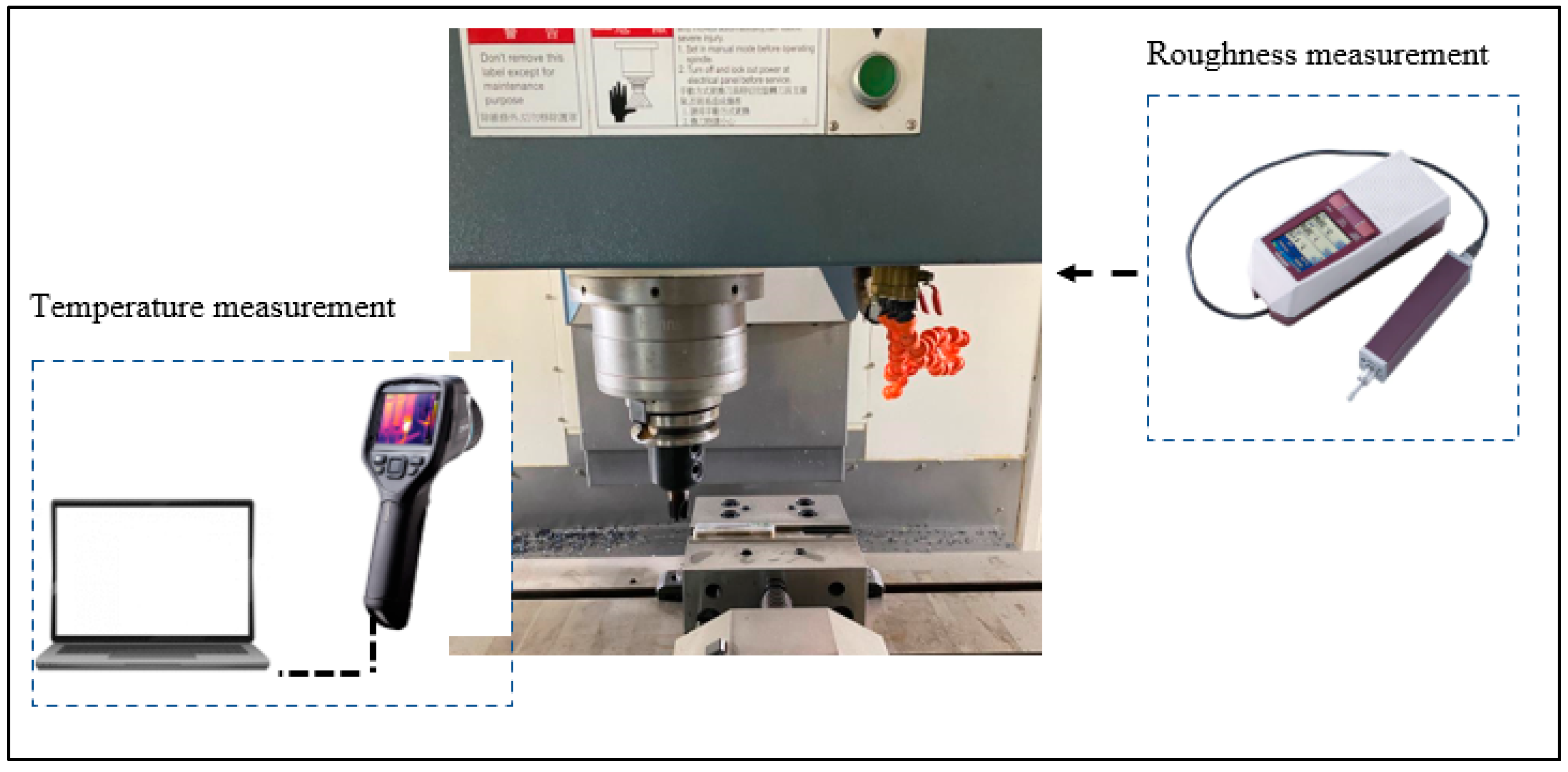
2. Materials and Methods
3. Results
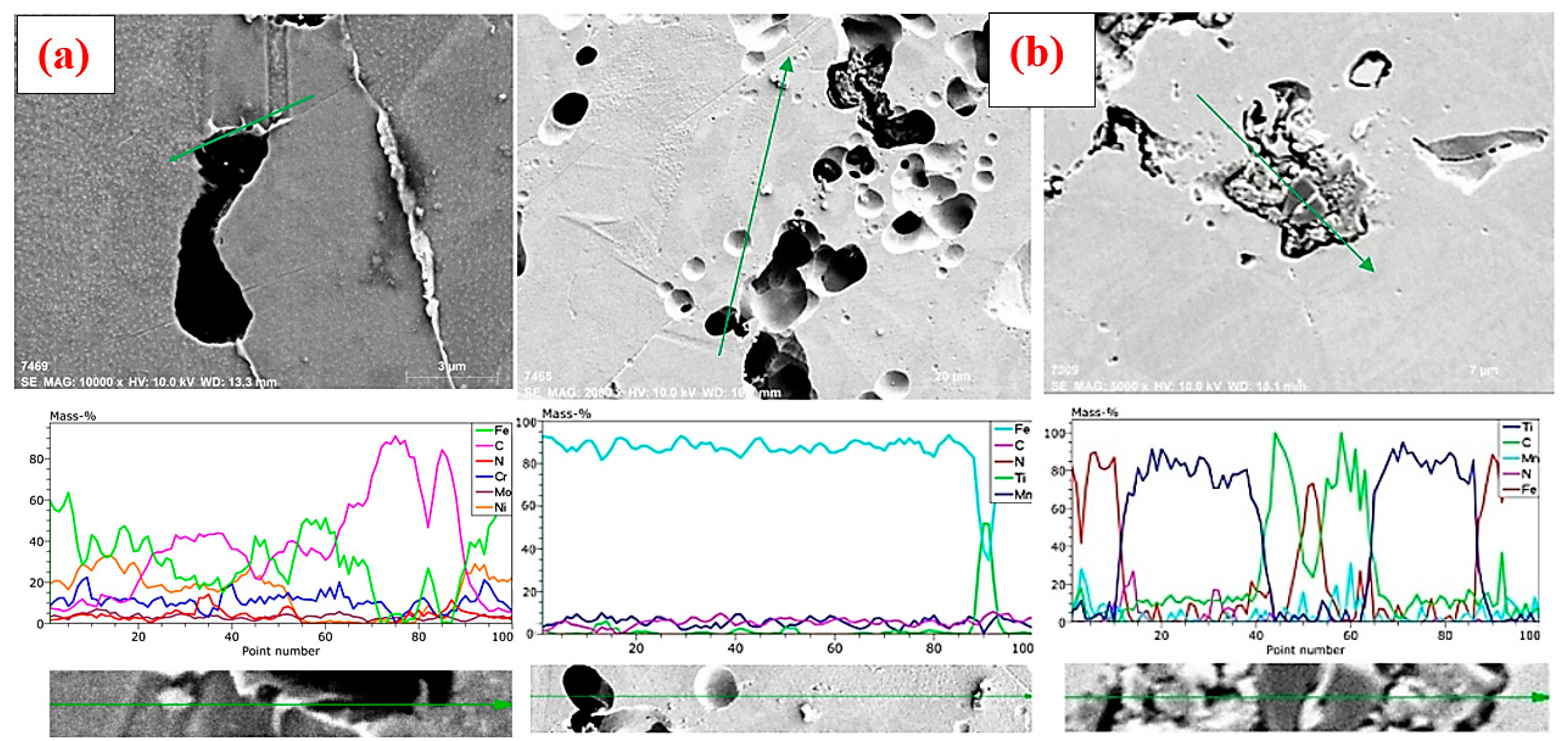
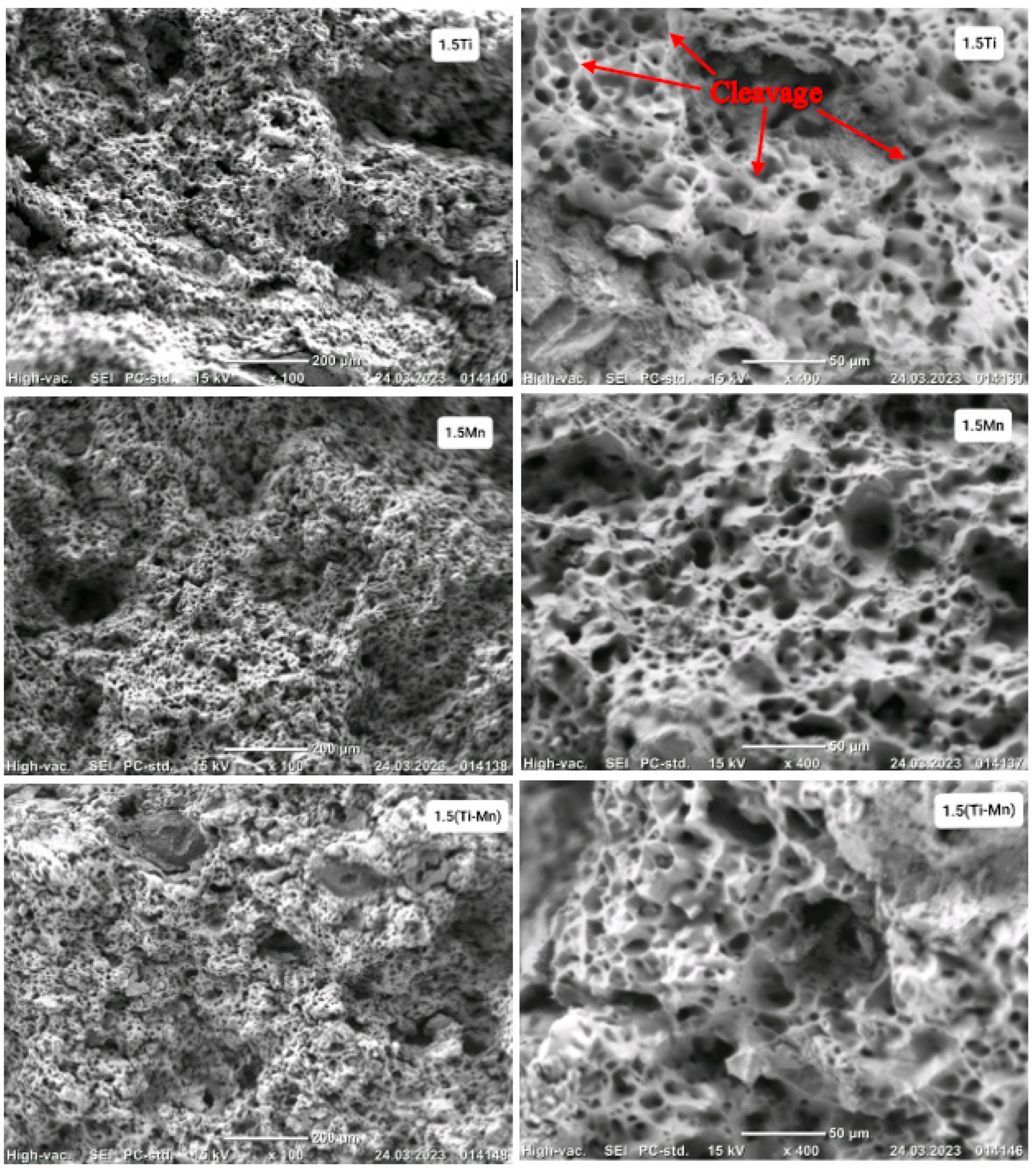
3.1. Microstructure Results and Evaluation
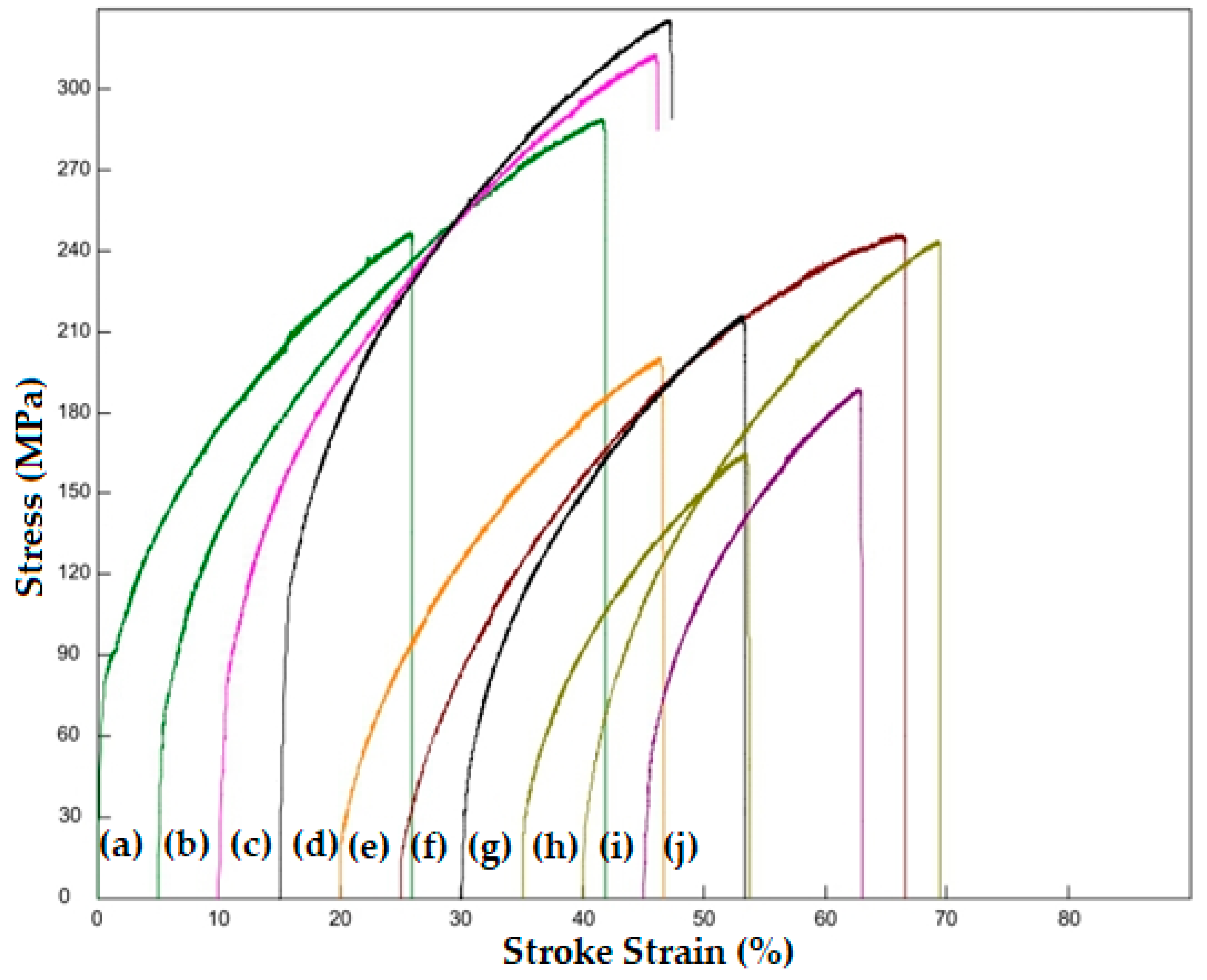
3.2. Evaluation of Mechanical Test Results
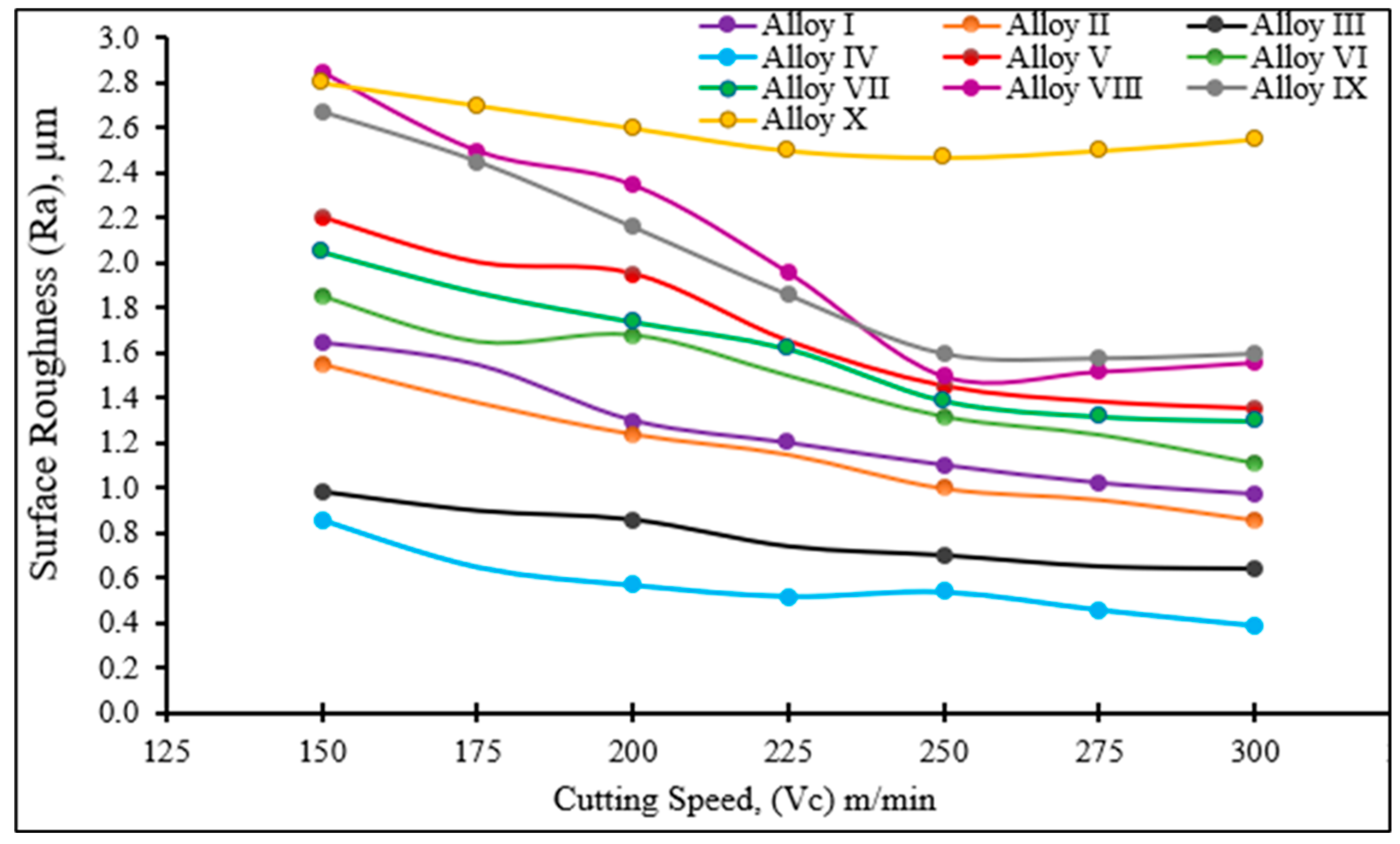
3.3. Evaluation of Milling Test Results
4. Conclusions
- When Ti and Mn (0.35 wt %) are added by weight to the original steel alloy without additives, the tensile strength, yield strength, and hardness of the steel alloy increase because the elements precipitate formation, and these precipitates prevent grain growth.
- When Ti and Mn are added together at the same weight percentage (0.35 wt %), better properties are obtained than when Ti or Mn are added via monotherapy, because when the two elements TiC(N) are added together, all kinds of precipitates are formed, namely, MnC(N) and TiMnC(N), which prevents the expansion of boundaries during recrystallization and sintering.
- When the weight percent of Ti and Mn is increased above (0.35 wt %), a decrease in mechanical properties is observed, which is thought to cause the fragility of the microstructure and weak mechanical properties due to the excessive deposition of carbide and nitride in the grain boundaries of alloying elements. The higher the percentage of Ti and Mn added, the more noticeable the increase in relative porosity due to the deposition of carbides and nitrides of titanium and manganese at the grain boundaries and the formation of microscopic voids in the crystalline structure.
- In order to precipitate all carbide and nitrate forms of TiC(N) alloying elements, when Ti and Mn are added at the rate of 0.35 wt % in the alloy where Ti and Mn are added together, the grain size decreases. Such precipitates inhibit the dislocation movement and cause an increase in mechanical properties such as yield strength, tensile strength, and hardness.
- When Mn was added at a rate higher than 0.35 wt %, it was noticed that the tensile strength remained almost the same, but the yield strength decreased by almost half compared to the original alloy that did not contain the additive. This is because the percentage of sediment formed by manganese (Mn) was much smaller than the percentage of sediment formed by titanium (Ti), according to what was observed via EDS analysis, and as large amounts of MnC(N) do not accumulate on the grain boundaries, its negative impact on the mechanical properties is less.
- It was seen that the Ra values decreased with increasing cutting speed.
- When Ti and Mn are added at 0.35 wt % to stainless steel 316 L and the milling process is applied, the average surface roughness (Ra) decreases due to the ease of extracting chips when the tensile strength and yield strength increase and the ductility decreases.
- Ti and Mn were added at percentages higher than 0.35 wt % and the milling process was applied to the alloys; an increase in the average surface roughness (Ra) was noticed because the sediment accumulates at the grain boundaries and forms large pores.
- When the cutting speed was increased in all samples, the cutting temperature increased due to the increase in the contact length between the cutting tool and the chips.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- German, R.M. Powder Metallurgy of Iron and Steel; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- German, R.M. Powder Metallurgy & Particulate Materials Processing; Metal Powder Industry Federation: Princeton, NJ, USA, 2005. [Google Scholar]
- Trent, E.M.; Wright, P.K. Metal Cutting: Theory and Application; Butterwort Heinemann: Auckland, New Zealand, 2000. [Google Scholar]
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials, Processes and Systems; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Black, J.T.; Kohser, R.A. DeGarmo’s Materials and Processes in Manufacturing, 13th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Jawaid, A.; Koksal, S.; Sharif, S. Cutting performance and wear characteristics of PVD coated and uncoated carbide tools in face milling Inconel 718 aerospace alloy. J. Mater. Process. Technol. 2001, 116, 2–9. [Google Scholar] [CrossRef]
- Llewellyn, D.T.; Hudd, R.C. Steels: Metallurgy and Applications, 3rd ed.; Reed Educational and Professional Publishing Ltd.: Oxford, UK, 1998; pp. 15–40. [Google Scholar]
- Iversen, A.; Leffler, B. Aqueous Corrosion of Stainless Steels. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ashraf, M.; Gardner, L.; Nethercot, D.A. Compression strength of stainless steel cross-sections. J. Constr. Steel Res. 2006, 62, 105–115. [Google Scholar] [CrossRef]
- Novak, C.J. Handbook of Stainless Steels; Peckner, D., Bernstein, I.M., Eds.; McGraw-Hill: New York, NY, USA, 1977. [Google Scholar]
- Ertenli, M.F.; Erdal, E.; Buyukkaragoz, A.; Kalkan, I.; Aksoylu, C.; Özkılıç, Y.O. Lateral torsional buckling of doubly-symmetric steel cellular I-Beams. Steel Compos. Struct. 2023, 46, 709–718. [Google Scholar] [CrossRef]
- Gulsoy, H.O.; Pazarlioglu, S.; Gulsoy, N.; Gundede, B.; Mutlu, O. Effect of Zr, Nb and Ti addition on injection molded 316L stainless steel for bio-applications: Mechanical, electrochemical and biocompatibility properties. J. Mech. Behav. Biomed. Mater. 2015, 51, 215–224. [Google Scholar] [CrossRef]
- Ali, S.; Irfan, M.; Niazi, U.M.; Rani, A.M.A.; Rashedi, A.; Rahman, S.; Trefil, A. Microstructure and mechanical properties of modified 316L stainless steel alloy for biomedical applications using powder metallurgy. Materials 2022, 15, 2822. [Google Scholar] [CrossRef] [PubMed]
- Albahlol, O.A.A.; Elkilani, R.; Çuğ, H.; Erden, M.A.; Özmen, R.; Esen, I. Investigation of microstructure and mechanical properties of layered material produced by adding Al2O3 to 316L stainless steel. Metals 2023, 13, 1226. [Google Scholar] [CrossRef]
- ASTM E8/E8M; Standard Test Methods for Tension Testing of Metallic Materials ASTM E8/E8M. American Society for Testing and Material: West Conshohocken, PA, USA, 2013.
- ISO 4287; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 1982.
- Schramm, R.E.; Reed, R.P. Stacking fault energies of seven commercial austenitic stainless steels. Metall. Trans. A 1975, 6, 1345–1351. [Google Scholar] [CrossRef]
- Chowdhury, S.G.; Das, S.; De, P.K. Cold rolling behaviour and textural evolution in AISI 316L austenitic stainless steel. Acta Mater. 2005, 53, 3951–3959. [Google Scholar] [CrossRef]
- Fang, K.; Luo, K.; Wang, L. Effect of microstructure on mechanical properties of 316 LN austenitic stainless steel. Coatings 2022, 12, 1461. [Google Scholar] [CrossRef]
- Ertugrul, O.; Park, H.S.; Onel, K.; Willert-Porada, M. Effect of particle size and heating rate in microwave sintering of 316L stainless steel. Powder Technol. 2014, 253, 703–709. [Google Scholar] [CrossRef]
- Erden, M.A.; Uzun, F.G.; Akgün, M.; Gökçe, H. Influence of Ti and Nb addition on the microstructure, mechanical, and machinability properties of 316L stainless steel fabricated by powder metallurgy. Mater. Test. 2023, 65, 1237–1253. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Wettability and kinetics of hydroxyapatite precipitation on a laser-textured Ca–P bioceramic coating. Acta Biomater. 2009, 5, 2763–2772. [Google Scholar] [CrossRef]
- Becker, B.S.; Bolton, J.D.; Eagles, A.M. Sintering of 316L stainless steels to high density via the addition of chromium—Molybdenum dibromide powders Part 1: Sintering performance and mechanical properties. Proc. Inst. Mech. Eng. Part L 2000, 214, 139–152. [Google Scholar] [CrossRef]
- Pandya, S.; Ramakrishna, K.S.; Annamalai, A.R.; Upadhyaya, A. Effect of sintering temperature on the mechanical and electrochemical properties of austenitic stainless steel. Mater. Sci. Eng. A 2012, 556, 271–277. [Google Scholar] [CrossRef]
- Uzunsoy, D. Investigation of dry sliding wear properties of boron doped powder metallurgy 316L stainless steel. Mater. Des. 2010, 31, 3896–3900. [Google Scholar] [CrossRef]
- Dewidar, M. Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel for possible hard-tissue applications. Int. J. Mech. Mech. Eng. 2012, 12, 10–24. [Google Scholar]
- Rawers, J.; Croydon, F.; Krabbe, R.; Duttlinger, N. Tensile characteristics of nitrogen enhanced powder injection moulded 316L stainless steel. Powder Metall. 1996, 39, 125–129. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L: Experimental and modeling results. Mater. Sci. Eng. A 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Schaeffler, A. Constitution diagram for stainless steel weld metal. Met. Prog. 1949, 56, 680. [Google Scholar]
- Kurt, B.; Özdoğan, L.; Güney, B.; Bölükbaşı, Ö.S.; Günen, A. Characterization and wear behavior of TiBC coatings formed by thermo-reactive diffusion technique on AISI D6 steel. Surf. Coat. Technol. 2020, 385, 497–508. [Google Scholar] [CrossRef]
- Guldibi, A.S.; Demir, H. Aging effect on microstructure and machinability of Corrax steel. Eng. Technol. Appl. Sci. Res. 2020, 10, 5168–5174. [Google Scholar] [CrossRef]
- Shanmugasundaram, D.; Chandramouli, R. Tensile and impact behaviour of sinter-forged Cr, Ni and Mo alloyed PM steels. Mater. Des. 2009, 30, 3444–3449. [Google Scholar] [CrossRef]
- de Campos Carolo, L.; Ordoñez, R.E.C. A review on the influence of process variables on the surface roughness of Ti-6Al-4V by electron beam powder bed fusion. Addit. Manuf. 2022, 59, 103103. [Google Scholar] [CrossRef]
- Arbizu, I.P.; Pérez, C.J.L. Surface roughness prediction by factorial design of experiments in turning processes. J. Mater. Process. Technol. 2003, 143–144, 390–396. [Google Scholar] [CrossRef]
- Abbas, A.T.; Pimenov, D.Y.; Erdakov, I.N.; Mikolajczyk, T.; El Danaf, E.A.; Taha, M.A. Minimization of turning time for high strength steel with a given surface roughness using the Edgeworth–Pareto optimization method. Int. J. Adv. Manuf. Technol. 2017, 93, 2375–2392. [Google Scholar] [CrossRef]
- Kıvak, T.; Sarıkaya, M.; Yıldırım, Ç.V.; Şirin, Ş. Study on turning performance of PVD TiN coated Al2O3+ TiCN ceramic tool under cutting fluid reinforced by nano sized solid particles. J. Manuf. Process. 2020, 56, 522–539. [Google Scholar] [CrossRef]







| Elemental Powders | Powder Size (µm) | Density (g/cm3) | Purity Value % | Company |
|---|---|---|---|---|
| 316 L | <149 | 7.95 | 99.9 | Höganas |
| Ti | 45 | 4.54 | 99.9 | Aldrich |
| Mn | 45 | 7.43 | 99.9 | Aldrich |
| No. | Alloy Name | Ti (wt %) | Mn (wt %) | 316 L (wt %) |
|---|---|---|---|---|
| Alloy I | 316 L | 0.17 | 100 | |
| Alloy II | 316 L + 0.35 Ti | 0.35 | 99.65 | |
| Alloy III | 316 L + 0.35 Mn | 0.35 | 99.65 | |
| Alloy IV | 316 L + 0.35 (Ti + Mn) | 0.35 | 0.35 | 99.30 |
| Alloy V | 316 L + 0.75 Ti | 0.75 | 99.25 | |
| Alloy VI | 316 L + 0.75 Mn | 0.75 | 99.25 | |
| Alloy VII | 316 L + 0.75 (Ti + Mn) | 0.75 | 0.75 | 98.50 |
| Alloy VIII | 316 + 1.5 Ti | 1.5 | 98.50 | |
| Alloy IX | 316 + 1.5 Mn | 1.5 | 98.5 | |
| Alloy X | 316 L + 1.5 (Ti + Mn) | 1.5 | 1.5 | 97 |
| No. | Alloy | Theoretical Density (g/cm3) | Density after Sintering (g/cm3) | Relative Density % | Relative Porosity % | Average Grain Dimensions µm |
|---|---|---|---|---|---|---|
| I | 316 L | 7.95 | 7.309 | 91.9372 | 8.0628 | 21.25 |
| II | 316 L + 0.35 Ti | 7.9378 | 7.2069 | 90.7922 | 9.2078 | 20.45 |
| III | 316 L + 0.35 Mn | 7.9481 | 7.3179 | 92.0711 | 7.9289 | 19.46 |
| IV | 316 L + 0.35 (Mn + Ti) | 7.93619 | 7.2127 | 90.8836 | 9.1164 | 19.01 |
| V | 316 L + 0.75 Ti | 7.92442 | 7.1183 | 89.8274 | 10.1726 | 23.66 |
| VI | 316 L + 0.75 Mn | 7.9461 | 7.2140 | 90.7866 | 9.2133 | 23.3 |
| VII | 316 L + 0.75 (Mn + Ti) | 7.9204 | 7.1450 | 90.2101 | 9.7899 | 24.12 |
| VIII | 316 L + 1.5 Ti | 7.8985 | 6.9596 | 88.1129 | 11.8871 | 31.51 |
| IX | 316 L + 1.5 Mn | 7.9422 | 7.0654 | 88.9602 | 11.0397 | 23.43 |
| X | 316 L + 1.5 (Mn + Ti) | 7.89105 | 6.9428 | 87.9832 | 12.0168 | 28.38 |
| No. | Alloy | Maximum Tensile Strength (MPa) | Elongation (%) | Hardness (Hv) |
|---|---|---|---|---|
| I | 316 L | 248 | 25.79 | 97 |
| II | 316 L + 0.35 Ti | 290 | 36.57 | 107 |
| III | 316 L + 0.35 Mn | 314 | 35.91 | 127 |
| IV | 316 L + 0.35 (Mn + Ti) | 327 | 32.11 | 133 |
| V | 316 L + 0.75 Ti | 201 | 26.30 | 88 |
| VI | 316 L + 0.75 Mn | 247 | 40.79 | 95 |
| VII | 316 L + 0.75 (Mn + Ti) | 217 | 23.10 | 91 |
| VIII | 316 L + 1.5 Ti | 166 | 18.38 | 73 |
| IX | 316 L + 1.5 Mn | 245 | 29.15 | 95 |
| X | 316 L + 1.5 (Mn + Ti) | 190 | 17.72 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türkmen, M.; Tanouz, A.M.; Akgün, M.; Erden, M.A. The Effect of Mn and Ti Ratio on Microstructure and Mechanical and Machinability Properties of 316 L Stainless Steel Used in Biomedical Applications. Metals 2023, 13, 1804. https://doi.org/10.3390/met13111804
Türkmen M, Tanouz AM, Akgün M, Erden MA. The Effect of Mn and Ti Ratio on Microstructure and Mechanical and Machinability Properties of 316 L Stainless Steel Used in Biomedical Applications. Metals. 2023; 13(11):1804. https://doi.org/10.3390/met13111804
Chicago/Turabian StyleTürkmen, Mustafa, Alla Muhammed Tanouz, Mahir Akgün, and Mehmet Akif Erden. 2023. "The Effect of Mn and Ti Ratio on Microstructure and Mechanical and Machinability Properties of 316 L Stainless Steel Used in Biomedical Applications" Metals 13, no. 11: 1804. https://doi.org/10.3390/met13111804





