Microstructural Transformations in Solid-State Annealed Al/Ag/Al Diffusion Couples Examined via High-Voltage Electron Microscopy (HVEM)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Diffusion Couples and Equilibrated Alloy Specimens
2.2. Observation and Identification of Phases at the Interface
3. Results
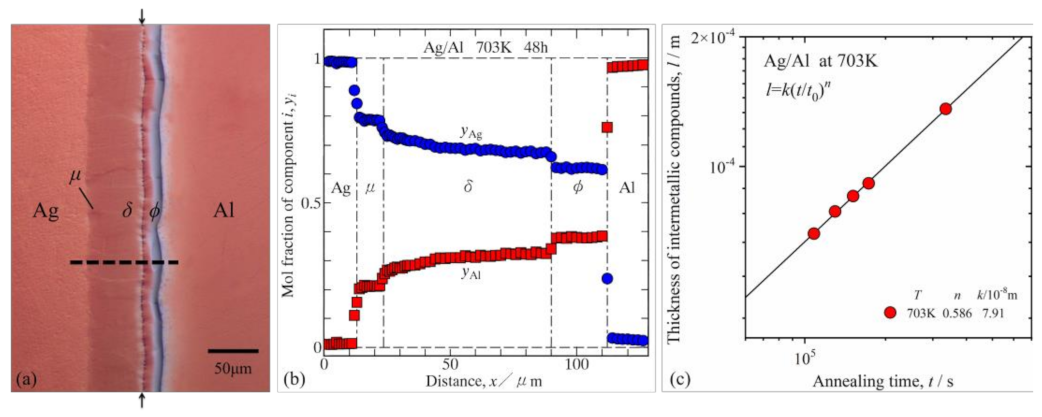
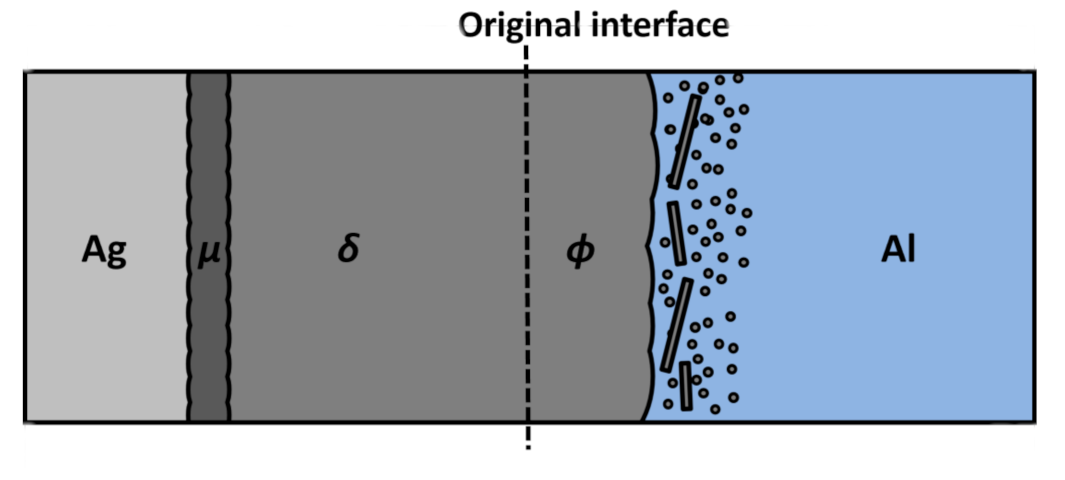
3.1. Microstructure and Compound Growth at the Interface
3.2. Direct Observation of the Ag/Al Diffusion Couple Using HVEM
3.2.1. δ Phase
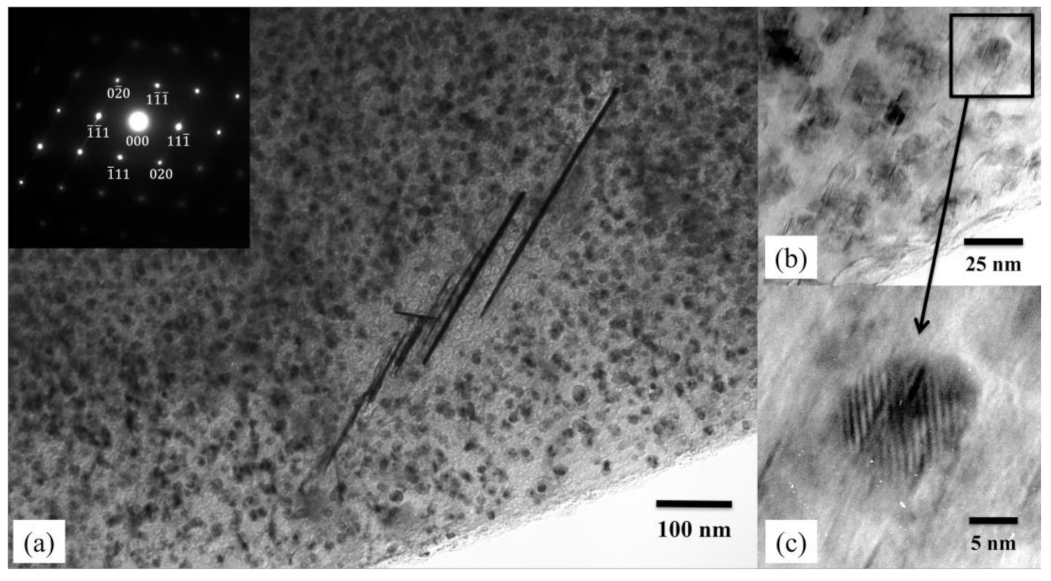
3.2.2. Intermediate Phase between δ Phase and Al Matrix
3.2.3. Intermediate Phases in the Al Side within 40 µm from the Initial Ag/Al Interface
3.3. Crystal Structure Analysis of Each Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, S.W.; Lee, C.C. A Study on Intermetallic Compound Formation in Ag–Al System and Evaluation of Its Mechanical Properties by Micro-Indentation. J. Mater. Sci. Mater. Electron. 2018, 29, 3985–3991. [Google Scholar] [CrossRef]
- Chang, L.P.; Huang, S.Y.; Chang, T.C.; Ouyang, F.Y. Low Temperature Ag-Ag Direct Bonding under Air Atmosphere. J. Alloys Compd. 2021, 862, 158587. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Tarnishing (Ag2S) Layer on Silver-Plated Electrical Contacts: Its Influence on Electrical Contact Resistance. IEEE Trans. Compon. Packag. Manuf. Technol. 2023, 13, 45–58. [Google Scholar] [CrossRef]
- Meguro, K.; O, M.; Kajihara, M. Growth Behavior of Compounds Due to Solid-State Reactive Diffusion between Cu and Al. J. Mater. Sci. 2012, 47, 4955–4964. [Google Scholar] [CrossRef]
- O, M.; Kajihara, M. Kinetics of Solid-State Reactive Diffusion between Au and Al. Mater. Trans. 2011, 52, 677–684. [Google Scholar] [CrossRef]
- Odashima, N.; O, M.; Kajihara, M. Formation of Intermetallic Compounds and Microstructure Evolution Due to Isothermal Reactive Diffusion at the Interface Between Solid Co and Liquid Sn. J. Electron. Mater. 2020, 49, 1568–1576. [Google Scholar] [CrossRef]
- Zarkevich, N.A.; Johnson, D.D. Predicted Hcp Ag-Al Metastable Phase Diagram, Equilibrium Ground States, and Precipitate Structure. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 064104. [Google Scholar] [CrossRef]
- McAlister, A.J. The Ag-Al (Silver-Aluminum) System. Bull. Alloy Phase Diagr. 1987, 8, 526–533. [Google Scholar] [CrossRef]
- Ben, F.; Olubambi, P.A. Phase and Properties Prediction of Al–Ag Binary System Using Thermo-Calc. MRS Adv. 2023, 1–6. [Google Scholar] [CrossRef]
- Fouracre, R.A. Electron Microscope Observations and Measurements in the Al/Ag Thin Film System. Thin Solid Film. 1987, 146, 83–92. [Google Scholar] [CrossRef]
- Roy, R.; Sen, S.K. Calorimetric and Other Studies of Intermetallic Phase Formation in Ag/Al Bilayer Thin Films. J. Mater. Sci. 1992, 27, 6098–6104. [Google Scholar] [CrossRef]
- Schleiwies, J.; Schmitz, G. Thin Film Interreaction of Al/Ag Analyzed by Tomographic Atom Probe. Mater. Sci. Eng. A 2002, 327, 94–100. [Google Scholar] [CrossRef]
- Pang, M.; Zhan, Y.; Yang, W.; Li, C.; Wang, H.; Jiang, W.; Du, Y. First-Principles Calculations on the Crystal, Electronic Structures and Elastic Properties of Ag-Rich Γ′ Phase Approximates in Al-Ag Alloys. Comput. Mater. Sci. 2012, 51, 415–421. [Google Scholar] [CrossRef]
- Moore, K.T.; Howe, J.M. Characterization of γ Plate-Shaped Precipitates in an Al-4.2 at.% Ag Alloy-Growth Kinetics, Solute Field, Composition and Modeling. Acta Mater. 2000, 48, 4083–4098. [Google Scholar] [CrossRef]
- Howe, J.M.; Aaronson, H.I.; Gronsky, R. Atomic Mechanisms of Precipitate Plate Growth in the AlAg System-II. High-Resolution Transmission Electron Microscopy. Acta Metall. 1985, 33, 649–658. [Google Scholar] [CrossRef]
- Zhang, Z.; Rosalie, J.M.; Medhekar, N.V.; Bourgeois, L. Resolving the FCC/HCP Interfaces of the γ′ (Ag2Al) Precipitate Phase in Aluminium. Acta Mater. 2019, 174, 116–130. [Google Scholar] [CrossRef]
- Nicholson, R.B.; Nutting, J. The Metallography of Precipitation in an Al-16% Ag Alloy. Acta Metall. 1961, 9, 332–343. [Google Scholar] [CrossRef]
- Zarkevich, N.A.; Johnson, D.D.; Smirnov, A.V. Structure and Stability of Hcp Bulk and Nano-Precipitated Ag2Al. In Acta Materialia; Elsevier: Amsterdam, The Netherlands, 2002; Volume 50. [Google Scholar]
- Neumann, J.P. Determination of the Ordering in the Intermetallic Compound Ag2Al. Acta Metall. 1966, 14, 505–511. [Google Scholar] [CrossRef]
- Takamatsu, Y.; O, M.; Kajihara, M. Kinetics of Reactive Diffusion in the Co/Zn System at Solid-State Temperatures. Mater. Trans. 2017, 58, 567–573. [Google Scholar] [CrossRef]
- Nakayama, M.; O, M.; Kajihara, M. Experimental Observation of Diffusion Reaction in the (Sn-Ag)/Cu System at Solid-State Temperatures. J. Electron. Mater. 2019, 48, 1766. [Google Scholar] [CrossRef]
- O, M.; Tanaka, Y.; Kobayashi, E. Microstructure Evolution at the Interface between Cu and Eutectic Sn-Bi Alloy with the Addition of Ag or Ni. J. Mater. Res. Technol. 2023, 26, 8165–8180. [Google Scholar] [CrossRef]
- O, M.; Tanaka, Y.; Kobayashi, E. Growth Behavior of Intermetallic Layers at the Interface between Cu and Eutectic Sn–Bi by Grain Boundary Diffusion with the Grain Growth at Solid-State Temperatures. Intermetallics 2023, 161, 107986. [Google Scholar] [CrossRef]
- Inada, H.; Kakibayashi, H.; Isakozawa, S.; Hashimoto, T.; Yaguchi, T.; Nakamura, K. Hitachi’s Development of Cold-Field Emission Scanning Transmission Electron Microscopes. Adv. Imaging Electron Phys. 2009, 159, 123–186. [Google Scholar]
- Matsumoto, S.; Sato, A.; Mori, T. Formation of h.c.p. and f.c.c. Twins in an FeMnCrSiNi Alloy. Acta Metall. Mater. 1994, 42, 1207–1213. [Google Scholar] [CrossRef]
- Li, J.; Malis, T.; Dionne, S. Recent Advances in FIB-TEM Specimen Preparation Techniques. Mater. Charact. 2006, 57, 64–70. [Google Scholar] [CrossRef]
- Michalcová, A.; Marek, I.; Knaislová, A.; Sofer, Z.; Vojtěch, D. Phase Transformation Induced Self-Healing Behavior of Al-Ag Alloy. Materials 2018, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- O, M.; Vakanas, G.; Moelans, N.; Kajihara, M.; Zhang, W. Formation of Compounds and Kirkendall Vacancy in the Cu-Sn System. Microelectron. Eng. 2014, 120, 133–137. [Google Scholar] [CrossRef]
- Vakanas, G.; O, M.; Dimcic, B.; Vanstreels, K.; Vandecasteele, B.; De Preter, I.; Derakhshandeh, J.; Rebibis, K.; Kajihara, M.; De Wolf, I.; et al. Formation, Processing and Characterization of Co-Sn Intermetallic Compounds for Potential Integration in 3D Interconnects. Microelectron. Eng. 2015, 140, 72–80. [Google Scholar] [CrossRef]
- O, M.; Suzuki, T.; Kajihara, M. Kinetics of Isothermal Reactive Diffusion Between Solid Cu and Liquid Sn. J. Electron. Mater. 2018, 47, 18–26. [Google Scholar] [CrossRef]
- Murakami, S.; O, M.; Kajihara, M. Growth Behavior of Compounds during Reactive Diffusion in the Solid-Cu/Liquid-Sn System. Mater. Trans. 2018, 59, 198–203. [Google Scholar] [CrossRef]
- Kizaki, T.; O, M.; Kajihara, M. Rate-Controlling Process of Compound Growth in Cu-Clad Al Wire during Isothermal Annealing at 483-543K. Mater. Trans. 2020, 61, 188–194. [Google Scholar] [CrossRef]
- Atkinson, H.V. Overview No. 65. Theories of Normal Grain Growth in Pure Single Phase Systems. Acta Metall. 1988, 36, 469–491. [Google Scholar] [CrossRef]
- O, M.; Sato, K.; Kobayashi, E. Investigation of the Rate-Controlling Process of Intermetallic Layer Growth at the Interface between Ferrous Metal and Molten Al–Mg–Si Alloy. Intermetallics 2023, 163, 108069. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Mixed Surface Reaction and Diffusion-Controlled Kinetic Model for Adsorption at the Solid/Solution Interface. J. Phys. Chem. C 2013, 117, 8310–8317. [Google Scholar] [CrossRef]
- Sun, W. Kinetics for Coarsening Co-Controlled by Diffusion and a Reversible Interface Reaction. Acta Mater. 2007, 55, 313–320. [Google Scholar] [CrossRef]
- O, M.; Takamatsu, Y.; Kajihara, M. Kinetics of Solid-State Reactive Diffusion between Co and Sn. Mater. Trans. 2014, 55, 1058–1064. [Google Scholar] [CrossRef]
- Chen, W.H.; Yu, C.F.; Cheng, H.C.; Tsai, Y.M.; Lu, S.T. IMC Growth Reaction and Its Effects on Solder Joint Thermal Cycling Reliability of 3D Chip Stacking Packaging. Microelectron. Reliab. 2013, 53, 30–40. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Su, R.; Neffati, D.; Zhang, Y.; Cho, J.; Li, J.; Wang, H.; Kulkarni, Y.; Zhang, X. The Influence of Stacking Faults on Mechanical Behavior of Advanced Materials. Mater. Sci. Eng. A 2021, 803, 140696. [Google Scholar] [CrossRef]
- Jomni, S.; Mliki, N.; Belhi, R.; Abdelmoula, K.; Ayadi, M.; Nihoul, G. Face Centered Cubic Cobalt Ultrathin-Layers in Au/Co(111) Multilayers: A Study by Electron Diffraction and by HREM. Thin Solid Film. 2000, 370, 186–191. [Google Scholar] [CrossRef]
- Chen, X.; O, M.; Kobayashi, E. Enhanced Mechanical Properties in an Al-Mg-Cu Alloy Processed by the Combination of Cyclic Deformation and Aging Heat Treatment. J. Alloy. Compd. 2022, 911, 165070. [Google Scholar] [CrossRef]
- Kirekawa, N.; Saito, K.; O, M.; Kobayashi, E. Effect of Cold Rolling on Cluster(1) Dissolvability during Artificial Aging and Formability during Natural Aging in Al-0.6Mg-1.0Si-0.5Cu Alloy. Metals 2022, 12, 92. [Google Scholar] [CrossRef]
- Chen, X.; Mørtsell, E.A.; Sunde, J.K.; O, M.; Marioara, C.D.; Holmestad, R.; Kobayashi, E. Enhanced Mechanical Properties in 6082 Aluminum Alloy Processed by Cyclic Deformation. Metals 2021, 11, 1735. [Google Scholar] [CrossRef]
- Bell, W.L. 2 1/2D Electron Microscopy: Through-Focus Dark-Field Image Shifts. J. Appl. Phys. 1976, 47, 1676–1682. [Google Scholar] [CrossRef]
- Zuo, J.M.; Spence, J.C.H. The Geometry of Electron Diffraction Patterns. In Advanced Transmission Electron Microscopy; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kitano, Y.; Komura, Y.; Fujiwara, K.; Iio, A. Short Range Order Diffuse Scattering from Disordered ζ Phase, Ag-Al. J. Phys. Soc. Jpn. 1976, 40, 593–594. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, Y.; Lan, J.; Qiao, Y.; Tan, Y. Self-Standing 3D Nanoporous Ag2Al with Abundant Surface Oxygen Species Facilitating Oxygen Electroreduction for Efficient Hybrid Zn Battery. J. Energy Chem. 2021, 58, 345–354. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Y.; Ma, Q.; Zhang, P.; Zhou, J.; Xue, F.; Sun, Z.M. Intermetallics-Induced Directional Growth of Sn Whiskers in Sn-3.5Ag Coating on Al Substrate. Appl. Surf. Sci. 2021, 539, 148135. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, F.; Han, F.; Ali, M.; Zhang, Y.; Guo, W.; Gu, H.; Li, G. Moiré Fringes in Nanoprecipitates in a Zirconium Alloy. Mater. Lett. 2020, 269, 127678. [Google Scholar] [CrossRef]
- O, M.; Fujita, H.; Kobayashi, E.; Kajihara, M. Kinetics and Thermodynamics of Compound Growth Due to Reactive Diffusion between Solid Cu and Binary Bi-Sn Alloys. J. Mol. Liq. 2022, 348, 118063. [Google Scholar] [CrossRef]
- Cowan, M.J.; Higaki, T.; Jin, R.; Mpourmpakis, G. Understanding the Solubility Behavior of Atomically Precise Gold Nanoclusters. J. Phys. Chem. C 2019, 123, 20006–20012. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Kodentsov, A.; Paul, A. Diffusion Couple Technique: A Research Tool in Materials Science. In Handbook of Solid State Diffusion; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2. [Google Scholar]
- Manjunatheshwara, K.J.; Vinodh, S. Sustainable Electronics Product Design and Manufacturing: State of Art Review. Int. J. Sustain. Eng. 2021, 14, 541–551. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.; Kajihara, M. Microstructural Transformations in Solid-State Annealed Al/Ag/Al Diffusion Couples Examined via High-Voltage Electron Microscopy (HVEM). Metals 2023, 13, 1780. https://doi.org/10.3390/met13101780
Oh M, Kajihara M. Microstructural Transformations in Solid-State Annealed Al/Ag/Al Diffusion Couples Examined via High-Voltage Electron Microscopy (HVEM). Metals. 2023; 13(10):1780. https://doi.org/10.3390/met13101780
Chicago/Turabian StyleOh, Minho, and Masanori Kajihara. 2023. "Microstructural Transformations in Solid-State Annealed Al/Ag/Al Diffusion Couples Examined via High-Voltage Electron Microscopy (HVEM)" Metals 13, no. 10: 1780. https://doi.org/10.3390/met13101780




