The Precipitation Behavior in Al0.3CoCrFeNi High-Entropy Alloy Affected by Deformation and Annealing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
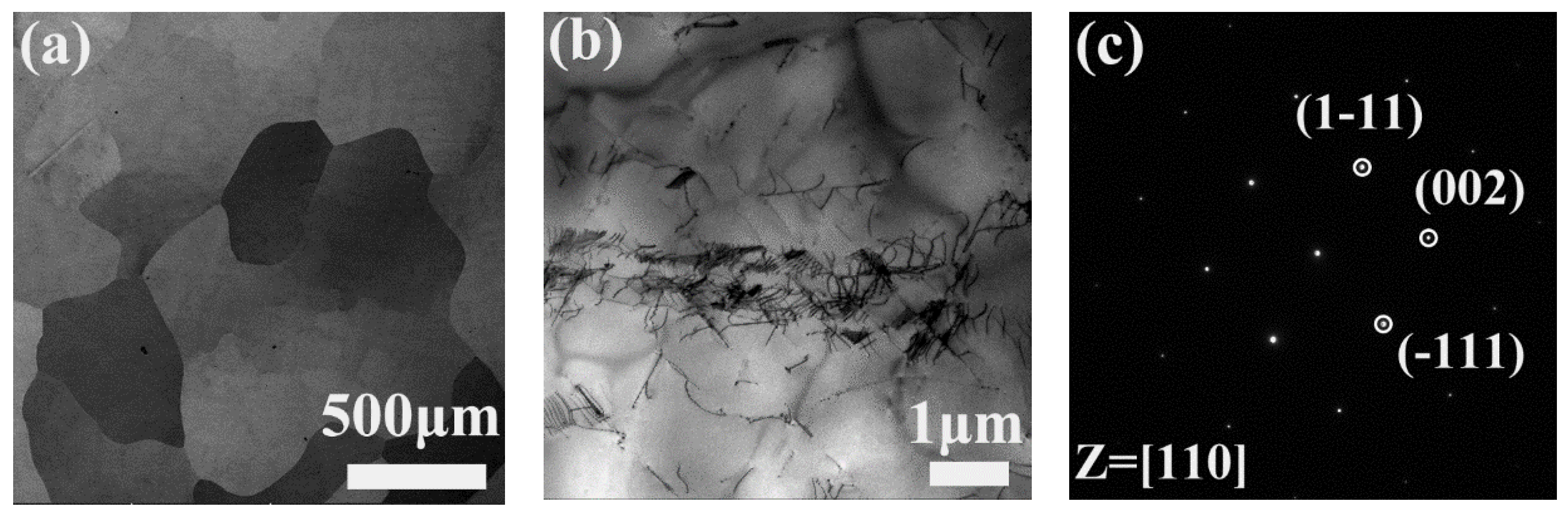
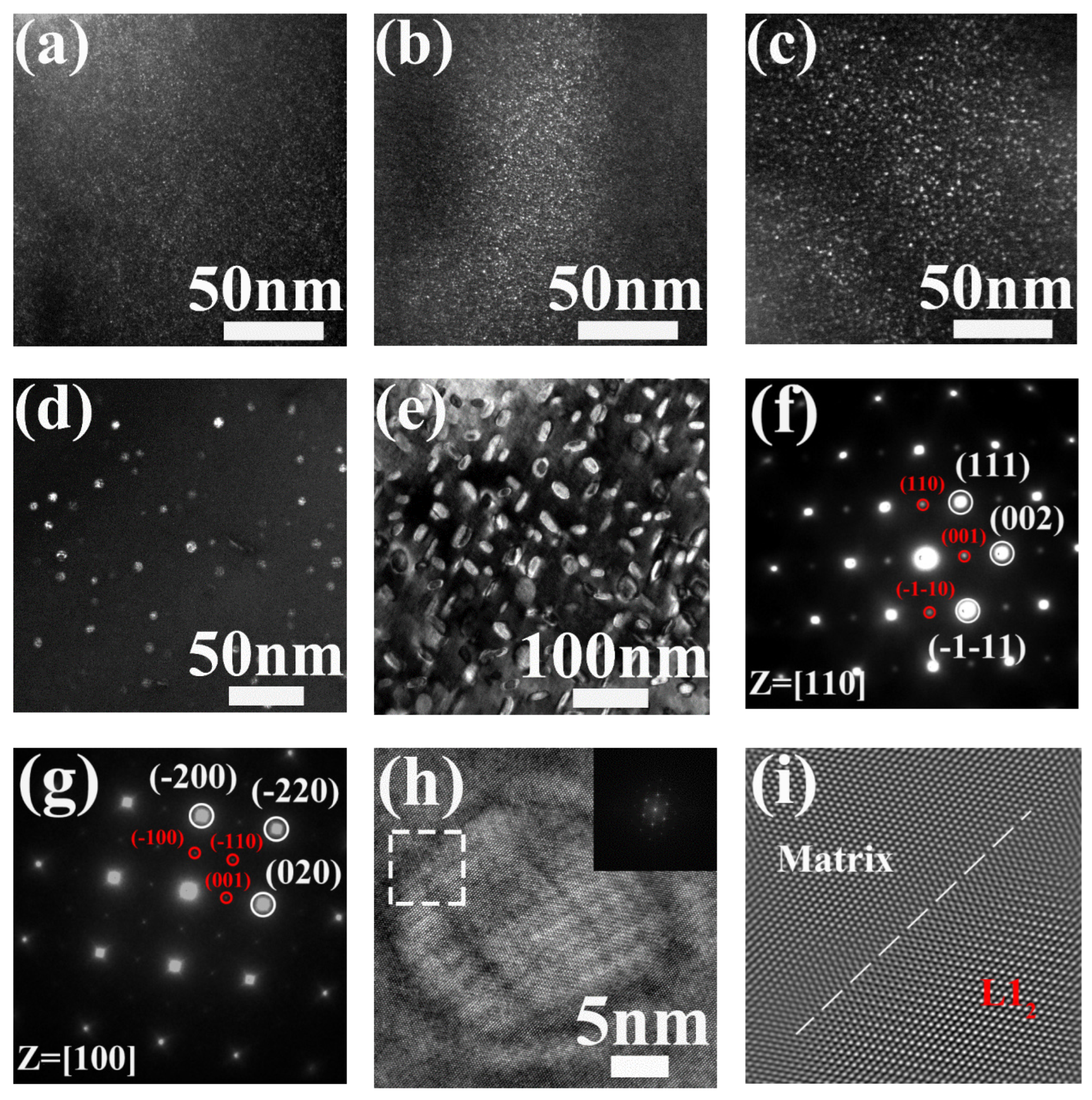
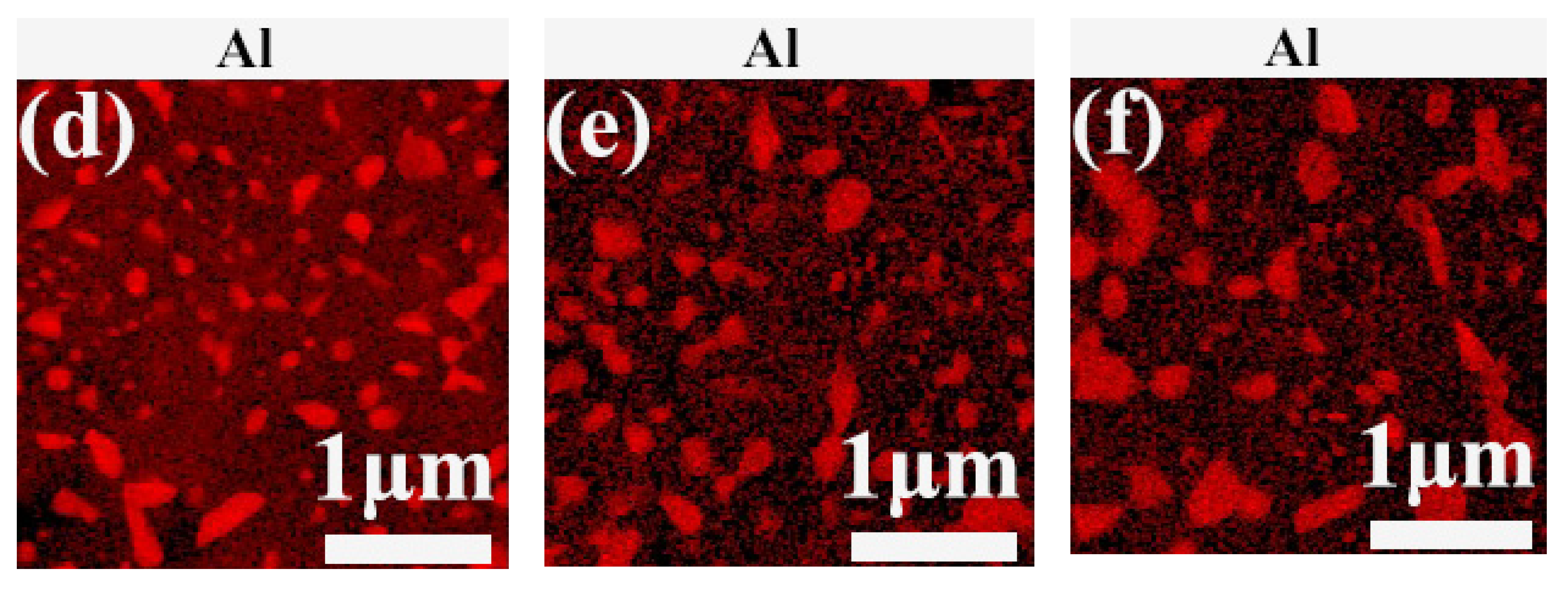
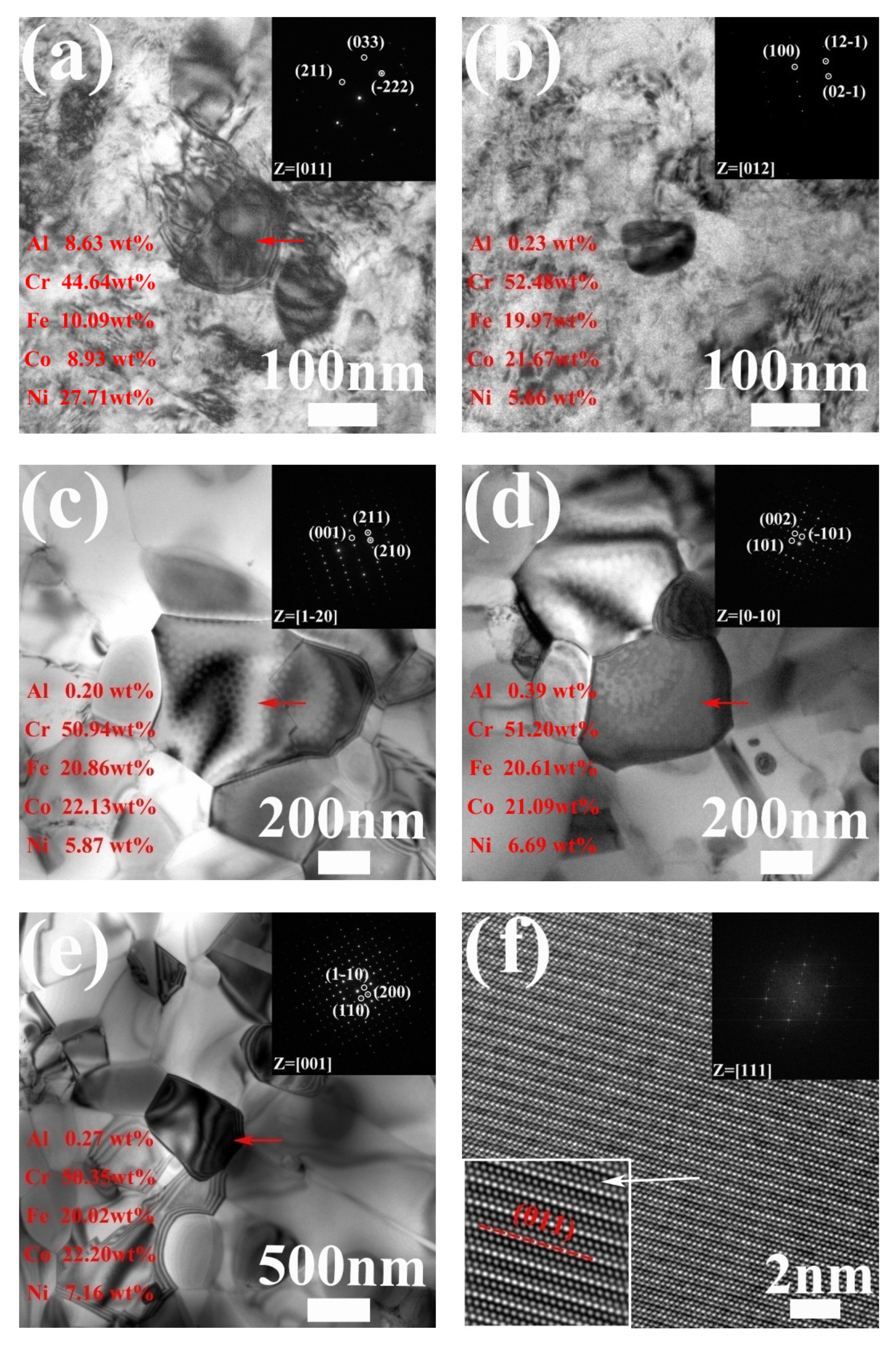
3.1. Precipitation in AH Samples
3.2. Precipitation in AR Samples
4. Conclusions
- (1)
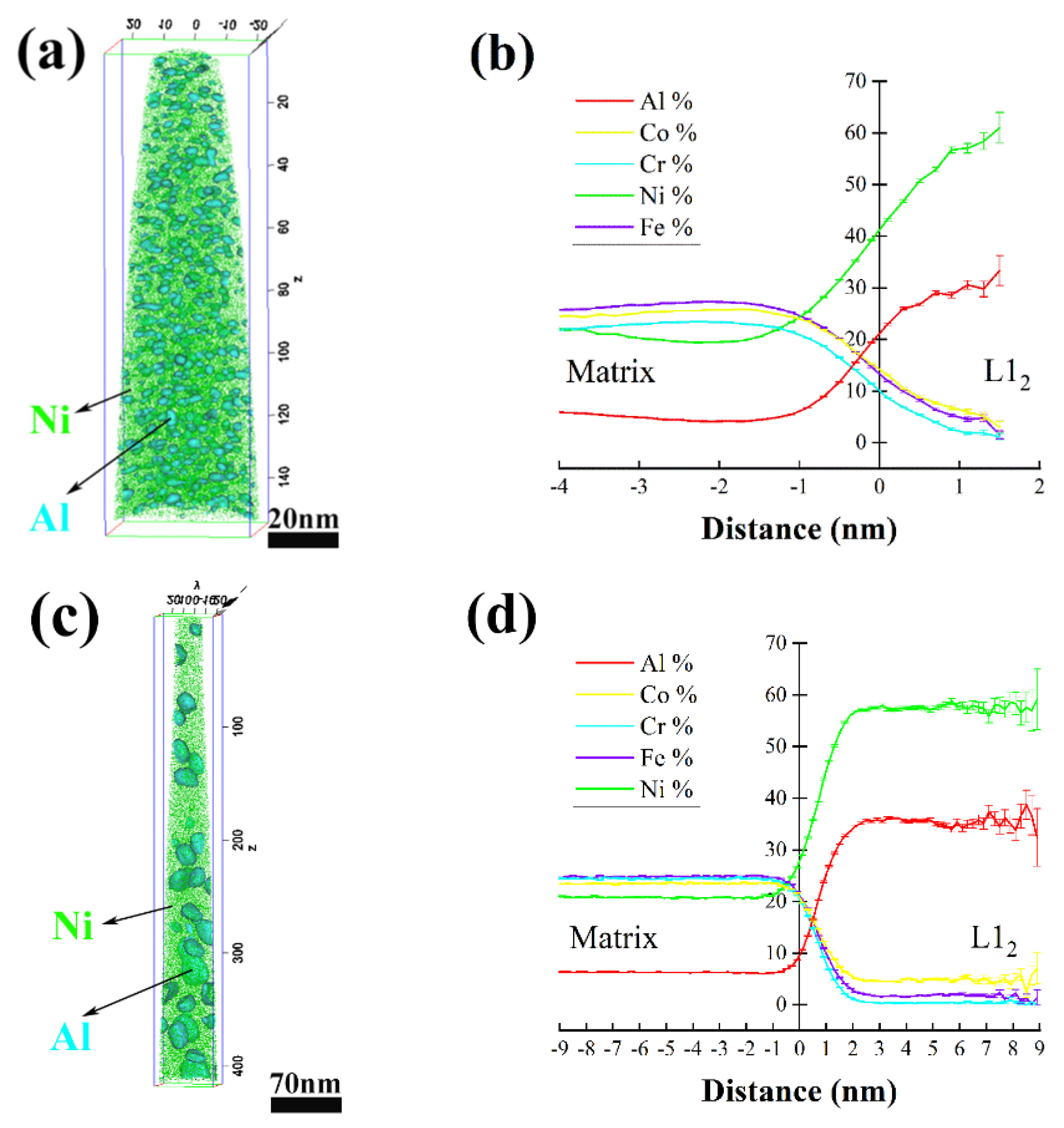
- The L12 phase precipitated in the AH samples heated at 500 °C, 540 °C, 590 °C, 660 °C and 700 °C was of the ordered FCC structure and coordinated with the FCC matrix. As analyzed from the 3DAP results, as the annealing temperature was raised from 590 °C to 700 °C, the size of the L12 phase increased from 2 nm to 9 nm, while the chemical composition was almost invariable.
- (2)
- The B2 phase primarily composed of Al and Ni was of the BCC structure with a lattice diameter a = 0.294 nm, which was discovered in AH800 and all annealed AR samples. The BCC phase enriched in Cr and produced in the AR samples annealed at 500 °C, 540 °C and 590 °C was of the BCC structure with a lattice diameter a = 0.296 nm. The σ phase enriched in Cr, Fe and Co and observed in the 540 °C, 590 °C, 660 °C, 700 °C and 800 °C annealed AR samples was of a tetragonal structure.
- (3)
- The B2 and σ phases, with complementary chemical compositions, promoted the precipitation behavior reciprocally.
Author Contributions
Funding
Conflicts of Interest
References
- Miracle, D.; Senkov, O.A. critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B.; Chang, I.; Knight, P.; Vincenta, J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Shen, J.; Agrawal, P.; Rodrigues, T.; Lopes, J.; Schell, N.; Zeng, Z.; MiShra, R.; Oliverira, J.P. Gas tungsten arc welding of as-cast AlCoCrFeNi2.1 eutectic high entropy alloy. Mater. Des. 2022, 223, 111176. [Google Scholar] [CrossRef]
- Martin, A.; Oliveira, J.; Fink, C. Elemental Effects on Weld Cracking Susceptibility in AlxCoCrCuyFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2020, 51, 778–787. [Google Scholar] [CrossRef]
- Yasuda, H.Y.; Shigeno, K.; Nagase, T. Dynamic strain aging of Al0.3CoCrFeNi high entropy alloy single crystals. Scr. Mater. 2015, 108, 80–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.; Dahmen, K.A.; Liaw, P.K.; Lu, Z. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Gao, Z. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Tian, X.; Wei, Y.; Zhuang, Y. Effect of heat treatment on microstructure and mechanical properties of Al0.3CoCrFeNi high entropy alloy with cold rolling. Trans. Mater. Heat Treat. 2017, 18, 2409–2414. [Google Scholar]
- Huang, Y.; Tang, Q.; Dai, P. Effects of Rolling Deformation on Microstructure and Properties of Al0.3CoCrFeNi High-Entropy Alloy. Mater. Mech. Eng. 2015, 39, 51–54. [Google Scholar]
- Gwalani, B.; Soni, V.; Lee, M.; Mantri, S.; Banerjee, R. Optimizing the coupled effects of Hall-Petch and precipitation strengthening in a Al0.3CoCrFeNi high entropy alloy. Mater. Des. 2017, 121, 254–260. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Mishra, R. Grain Size Dependence of Strain Rate Sensitivity in a single phase FCC High Entropy Alloy Al0.3CoCrFeNi. Mater. Sci. Eng. A 2018, 736, 344–348. [Google Scholar] [CrossRef]
- Ma, L.; Gao, Z.; Hu, S.; Zeng, Z. Effect of cooling rate on microstructure and mechanical properties of Al0.3CoCrFeNi high-entropy alloy. Mater. Res. Express 2019, 6, 056540. [Google Scholar] [CrossRef]
- Kireeva, I.; Chumlyakov, Y.; Pobedennaya, Z.; Vyrodova, A.; Kuksgauzen, I.V.; Kuksgauzen, D.A. Orientation and temperature dependence of a planar slip and twinning in single crystals of Al0.3CoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2018, 737, 47–60. [Google Scholar] [CrossRef]
- Annasamy, M.; Haghdadi, N.; Taylor, A.; Hodgson, P.; Fabijanic, D. Dynamic recrystallization behaviour of AlxCoCrFeNi high entropy alloys during high-temperature plane strain compression. Mater. Sci. Eng. A 2018, 745, 90–106. [Google Scholar] [CrossRef]
- Jiao, Z.; Ma, S.; Yuan, G.; Wang, H.; Qiao, J. Plastic Deformation of Al0.3CoCrFeNi and AlCoCrFeNi High-Entropy Alloys Under Nanoindentation. J. Mater. Eng. Perform. 2015, 24, 3077–3083. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Wang, J.; Wang, Y.; Kou, H.; Beaugnon, E. Temperature dependent deformation mechanisms of Al0.3CoCrFeNi high-entropy alloy, starting from serrated flow behavior. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid-State Chem. Phys. 2018, 757, 39–43. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, P.; Cheng, H.; Zhang, H.; Diao, H.Y.; Shi, Y.; Chen, B.L.; Chen, P.; Feng, R.; Bai, J.; et al. Nanoindentation Creep Behavior of an Al0.3CoCrFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2016, 47, 5871–5875. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.D.; Sha, G.; Lewandowski, J.; Liaw, P.K.; Zhang, Y. High-entropy Al0.3CoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Zhang, M.; Zhao, Y.; Liaw, P.K.; Qiao, J. Effects of Ni-P amorphous films on mechanical and corrosion properties of Al0.3CoCrFeNi high-entropy alloys. Intermetallics 2018, 94, 65–72. [Google Scholar] [CrossRef]
- Tong, Y.; Qiao, J.; Yao, Y. The constitutive model and threshold stress for characterizing the deformation mechanism of Al0.3CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2018, 730, 137–146. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Y.; Cheng, H.; Liao, X.Z.; Langdon, T.G. The effect of grain size on the annealing-induced phase transformation in an Al0.3CoCrFeNi high entropy alloy. Mater. Des. 2016, 105, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Gao, M.C.; Hawk, J.A.; Zhang, Y. Annealing effect for the Al0.3CoCrFeNi high-entropy alloy fibers. J. Alloys Compd. 2019, 778, 23–29. [Google Scholar] [CrossRef]
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Zhang, Y.; Mishra, R.S.; Banerjeeab, R. Tensile yield strength of a single bulk Al0.3CoCrFeNi high entropy alloy can be tuned from 160 MPa to 1800 MPa. Scr. Mater. 2018, 162, 18–23. [Google Scholar] [CrossRef]
- Shun, T.; Du, Y. Microstructure and tensile behaviors of FCC Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2009, 479, 157–160. [Google Scholar] [CrossRef]
- Choudhuri, D.; Gwalani, B.; Gorsse, S.; Mikler, C.V.; Ramanujan, R.V.; Gibson, M.A.; Bannerjee, R. Change in the primary solidification phase from fcc to bcc-based B2 in high entropy or complex concentrated alloys. Scr. Mater. 2017, 127, 186–190. [Google Scholar] [CrossRef]
- Dasari, S.; Sarkar, A.; Gwalanib, C.; Choudhuri, D.; Soni, V.; Manda, S.; Samajdar, I.; Bannerjee, R. Recovery of cold-worked Al0.3CoCrFeNi complex concentrated alloy through twinning assisted B2 precipitation. Acta Mater. 2021, 202, 448–462. [Google Scholar] [CrossRef]
- Gwalani, B.; Torgerson, T.; Dasari, S.; Jajetia, A.; Nartu, M.; Gangireddy, S.; Pole, M.; Wang, T.; Scharf, T.W. Influence of fine-scale B2 precipitation on dynamic compression and wear properties in hypo-eutectic Al0.5CoCrFeNi high-entropy alloy. J. Alloys Compd. 2020, 853, 157126. [Google Scholar] [CrossRef]
- Yasuda, H.; Miyamoto, H.; Cho, K.; Nagase, T. Formation of ultrafine-grained microstructure in Al0.3CoCrFeNi high entropy alloys with grain boundary precipitates. Sci. Mater. Lett. 2017, 199, 120–123. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; ChoudHuri, D.; Lee, M.; Hwang, J.; Nam, S.; Ryu, H.; Hong, S.; Banerjee, R. Stability of ordered L12 and B2 precipitates in face centered cubic based high entropy alloys Al0.3CoFeCrNi and Al0.3CuFeCrNi2. Scr. Mater. 2016, 123, 130–134. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Banerjee, R.; Mishra, R.S. Microstructures with extraordinary dynamic work hardening and strain rate sensitivity in Al0.3CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2018, 734, 42–50. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Zhang, H.; Chen, J.; Luo, L.; Zhao, X.; Guo, F.; Xiao, P. Oxidation behavior of gas-atomized AlCoCrFeNi high-entropy alloy powder at 900–1100 °C. Corros. Sci. 2021, 181, 190257. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. The effects of secondary elemental Mo or Ti addition in Al0.3CoCrFeNi high-entropy alloy on age hardening at 700 °C. J. Alloys Compd. 2010, 495, 55–58. [Google Scholar] [CrossRef]
- Gwalani, B.; Gangireddy, S.; Zheng, Y.; Song, V.; Mishra, R.S.; Banerjee, R. Influence of ordered L12 precipitation on strain-rate dependent mechanical behavior in a eutectic high entropy alloy. Nature 2019, 9, 6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwalani, B.; Pohan, R.; Waseem, O.A.; Alam, T.; Hong, S.; Ryu, H.J. Strengthening of Al0.3CoCrFeMnNi-based ODS high entropy alloys with incremental changes in the concentration of Y2O3. Scr. Mater. 2019, 162, 477–481. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Alam, T.; Dasari, S.; Zheng, Y.; Senkov, O.N.; Miracle, D.; Banerjee, R. Phase Inversion in a Two-phase, BCC+B2, Refractory High Entropy Alloy. Acta Mater. 2019, 185, 89–97. [Google Scholar] [CrossRef]
- Anber, E.; Lang, A.; Lass, E.; Suri, P.K.; Hart, J.L.; Dantuono, D.S.; Diao, H.Y.; Feng, R.; Doherty, R.; Liaw, P.K. Insight into the Kinetic Stabilization of Al0.3CoCrFeNi High-Entropy Alloys. Materialia 2020, 14, 100872. [Google Scholar] [CrossRef]
- Kao, Y.; Chen, S.; Chen, T.; Chu, P.C.; Yew, J.W.; Lin, S.J. Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Strumza, E.; Hayun, S. Comprehensive study of phase transitions in equiatomic AlCoCrFeNi high-entropy alloy. J. Alloys Compd. 2020, 856, 158220. [Google Scholar] [CrossRef]
- Dasari, S.; Jagetia, A.; Soni, B.; Gwalani, B.; Gorsse, S. Engineering transformation pathways in an Al0.3CoFeNi complex concentrated alloy leads to excellent strength-ductility combination. Mater. Res. Lett. 2020, 8, 399–407. [Google Scholar] [CrossRef]
- Dasari, S.; Jagetia, A.; Soni, B.; Gwalani, B.; Gorsse, S.; Banerjee, R. Engineering multi-scale B2 precipitation in a heterogeneous FCC based microstructure to enhance the mechanical properties of a Al0.5Co1.5CrFeNi1.5 high entropy alloy. J. Alloys Compd. 2020, 830, 154707. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.W.; Qiao, J.W.; Ma, S.; Lu, P.; Xu, B.; Wang, Z. Superior tensile properties of Al0.3CoCrFeNi high entropy alloys with B2 precipitated phases at room and cryogenic temperatures. Mater. Sci. Eng. A 2019, 767, 138424. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Chen, X.; Fu, X.; Chen, D.; Chen, S.; Gu, L.; Wei, F.; Bei, H.; Gao, Y.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nature 2019, 574, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kurdjumov, G. SACHS G Über den Mechanis Mus der Stählhartung. Phys. Z 1930, 64, 325–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. J. Miner. Met. Mater. Soc. 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Anomalous solidification microstructures in Co-free AlxCrCuFeNi2 high-entropy alloys. J. Alloys Compd. 2013, 557, 77–81. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, Y.L.; Yeh, J.W.; Lin, S.; Shun, T.T.; Tsau, C.H.; Chang, S. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Zhao, C.D.; Li, J.S.; Liu, Y.D.; Wang, Y.; Kou, H.; Beaugnon, E.; Wang, J. Tailoring mechanical and magnetic properties of AlCoCrFeNi high-entropy alloy via phase transformation. J. Mater. Sci. Technol. 2021, 73, 83–90. [Google Scholar] [CrossRef]
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Styles, M.; Zheng, Y.F.; Mishra, R.S.; Banerjee, R. Modifying transformation pathways in high entropy alloys or complex concentrated alloys via thermo-mechanical processing. Acta Mater. 2018, 153, 169–185. [Google Scholar] [CrossRef]








| Sample | Processing Treatment |
|---|---|
| AH | The as-cast sample |
| AH500 | AH sample aged at 500 °C for 24 h |
| AH540 | AH sample aged at 540 °C for 24 h |
| AH590 | AH sample aged at 590 °C for 24 h |
| AH660 | AH sample aged at 660 °C for 24 h |
| AH700 | AH sample aged at 700 °C for 10 h |
| AH800 | AH sample aged at 800 °C for 10 h |
| AR | AH sample cold-rolled by a thickness reduction of 90% |
| AR500 | AR sample aged at 500 °C for 24 h |
| AR540 | AR sample aged at 540 °C for 24 h |
| AR590 | AR sample aged at 590 °C for 24 h |
| AR660 | AR sample aged at 660 °C for 24 h |
| AR700 | AR sample aged at 700 °C for 10 h |
| AR800 | AR sample aged at 800 °C for 10 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Qiu, R.; Tan, X.; Quan, X.; Song, B.; Liu, Q. The Precipitation Behavior in Al0.3CoCrFeNi High-Entropy Alloy Affected by Deformation and Annealing. Metals 2023, 13, 157. https://doi.org/10.3390/met13010157
Zhang J, Qiu R, Tan X, Quan X, Song B, Liu Q. The Precipitation Behavior in Al0.3CoCrFeNi High-Entropy Alloy Affected by Deformation and Annealing. Metals. 2023; 13(1):157. https://doi.org/10.3390/met13010157
Chicago/Turabian StyleZhang, Jinlong, Risheng Qiu, Xinu Tan, Xuantong Quan, Bo Song, and Qing Liu. 2023. "The Precipitation Behavior in Al0.3CoCrFeNi High-Entropy Alloy Affected by Deformation and Annealing" Metals 13, no. 1: 157. https://doi.org/10.3390/met13010157






