The Effect of Solution Treatment on the Si Particles’ Morphology Evolution and the Thermal Conductivity and Tensile Properties of Sb-Modified Al-8Si-0.6Mg Alloys
Abstract
:1. Introduction
2. Material and Experimental Details
2.1. Materials
2.2. Microstructure Characterization and Test Methods
3. Results and Discussion
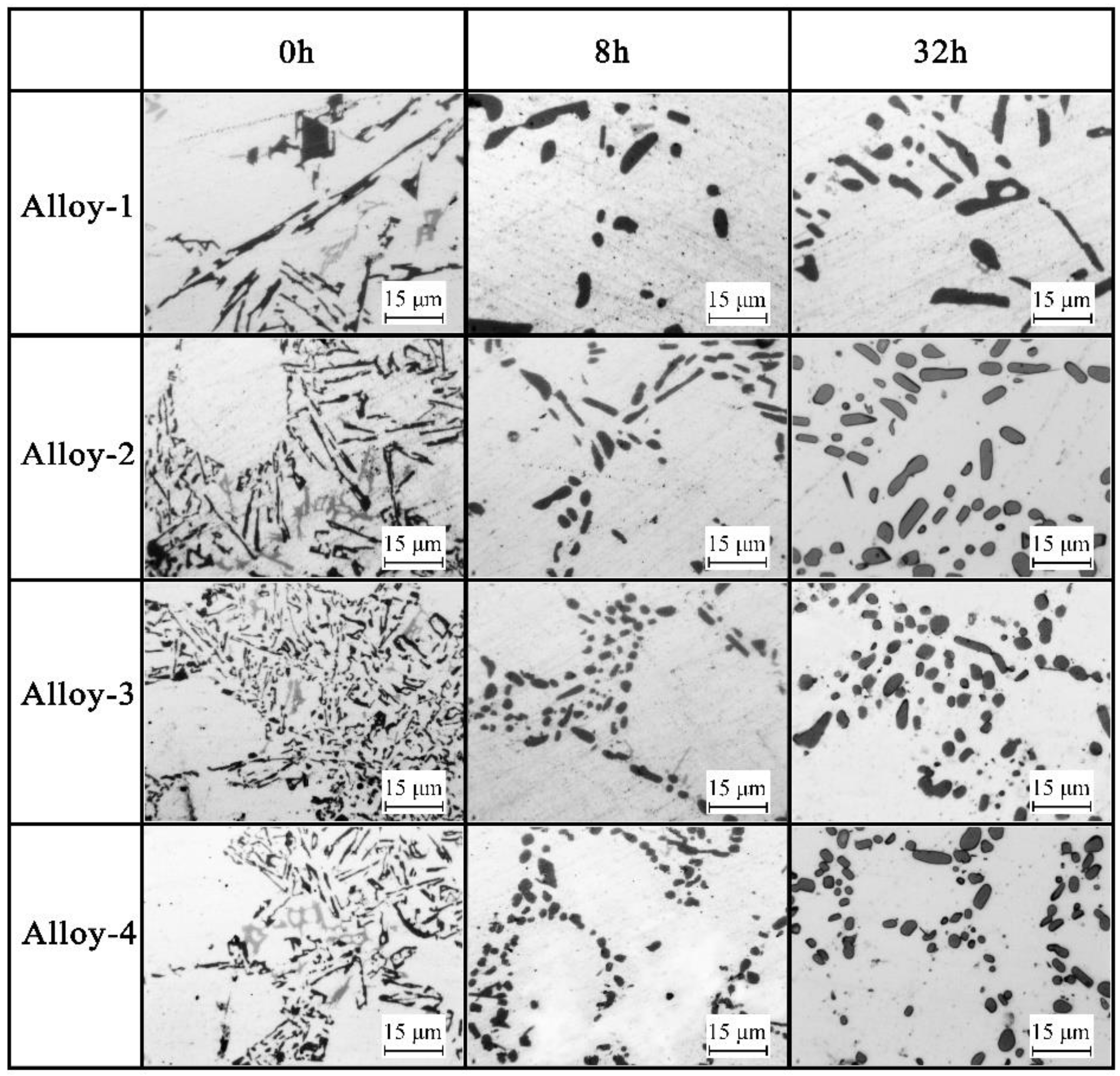
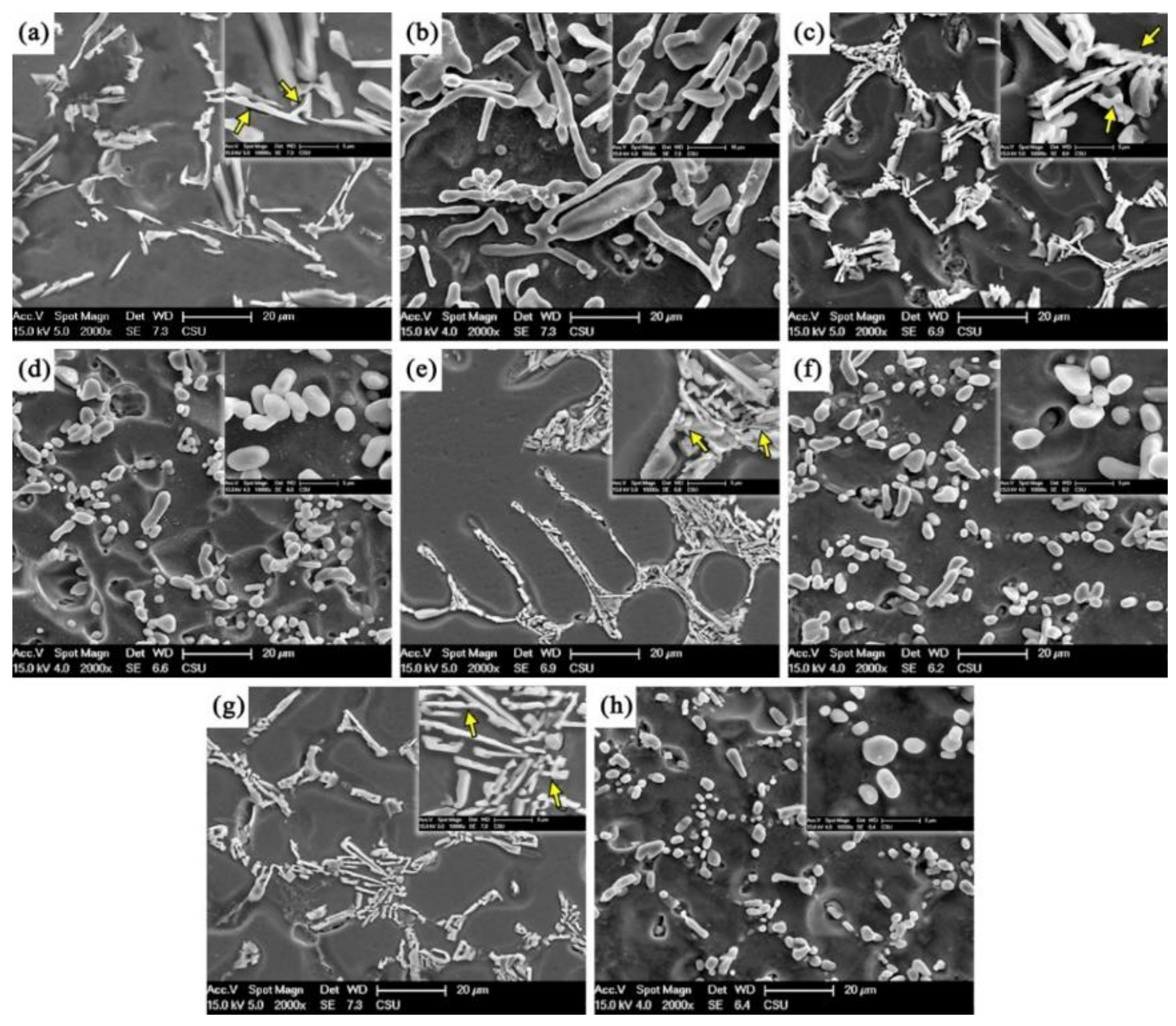
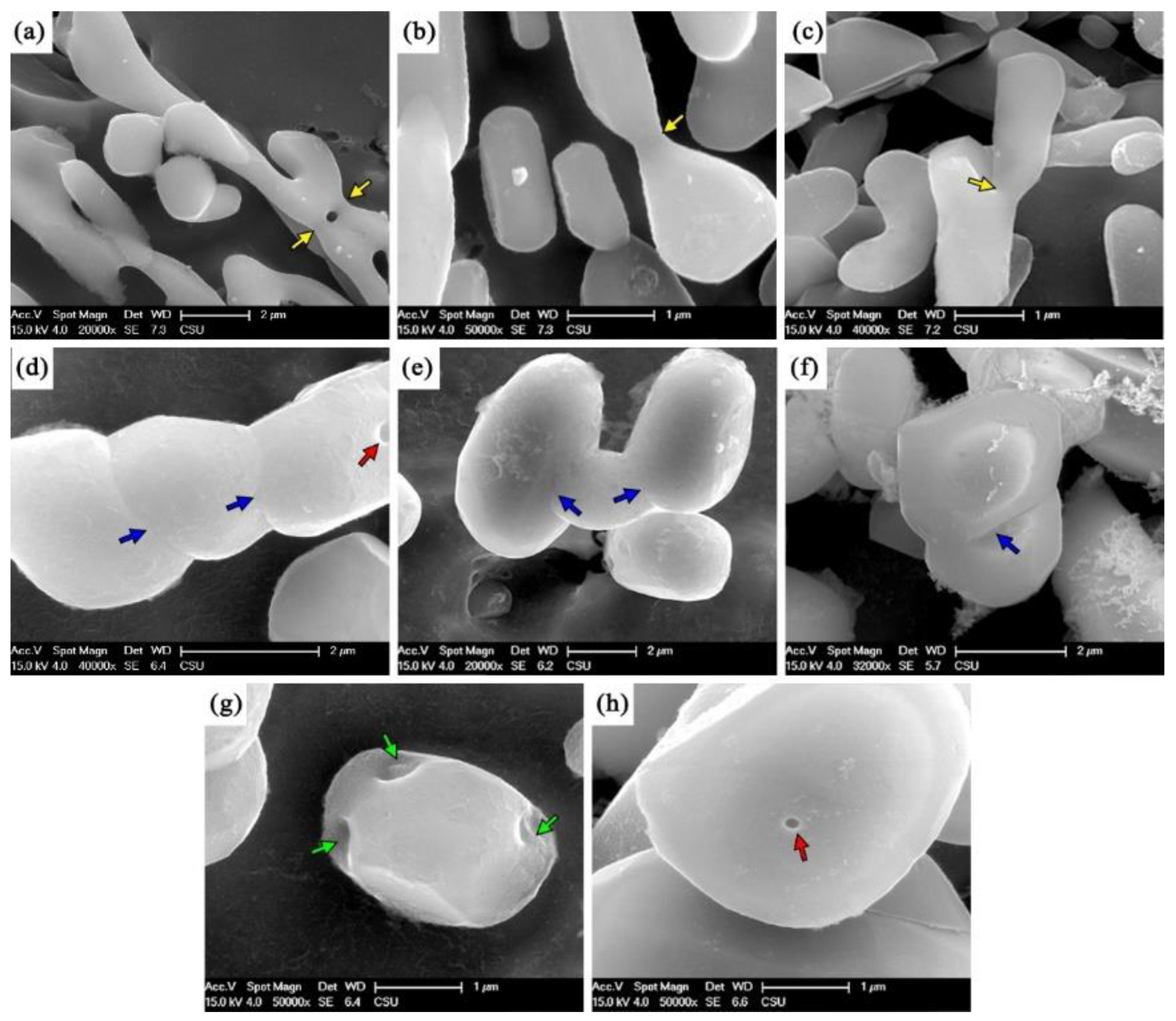
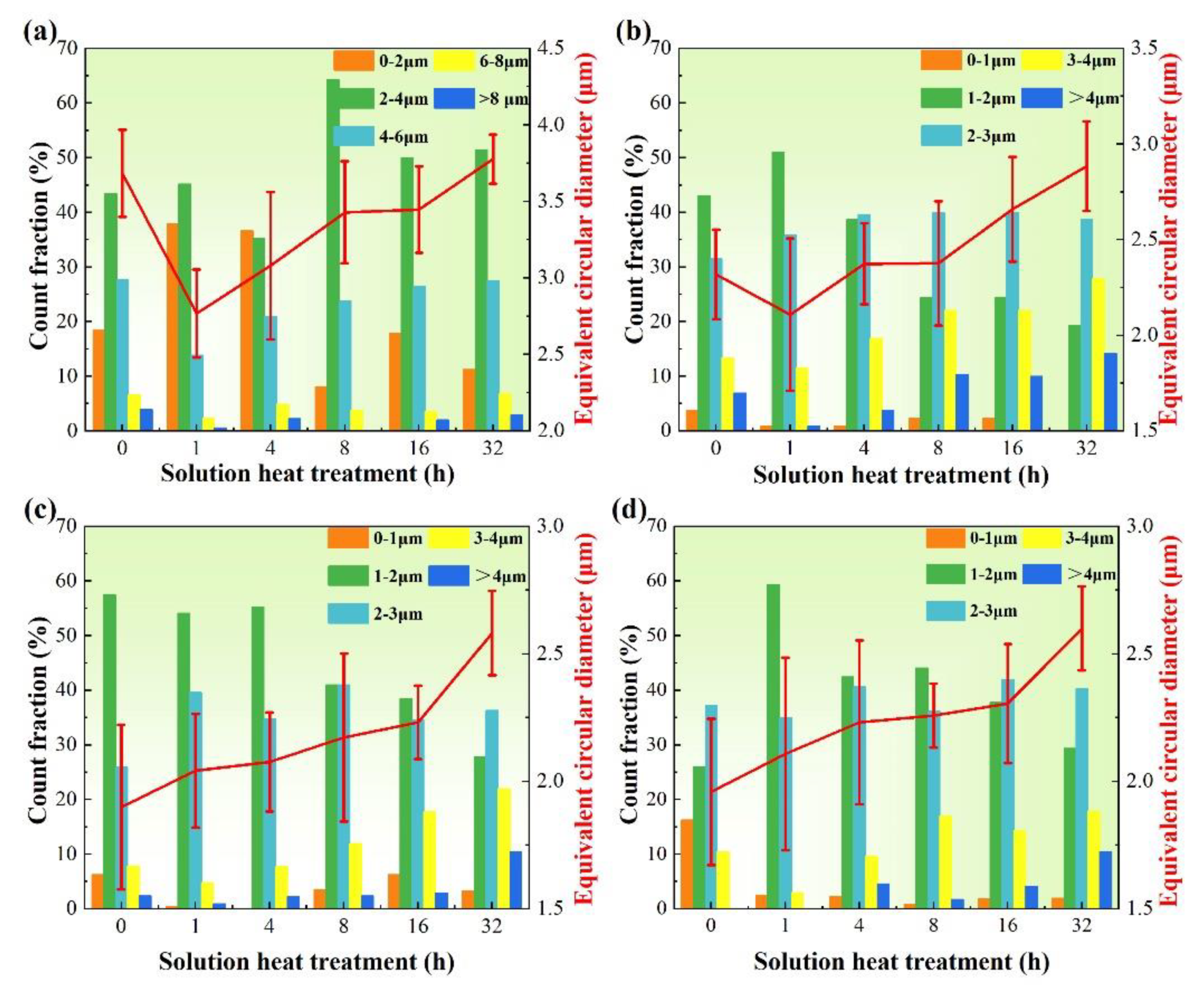
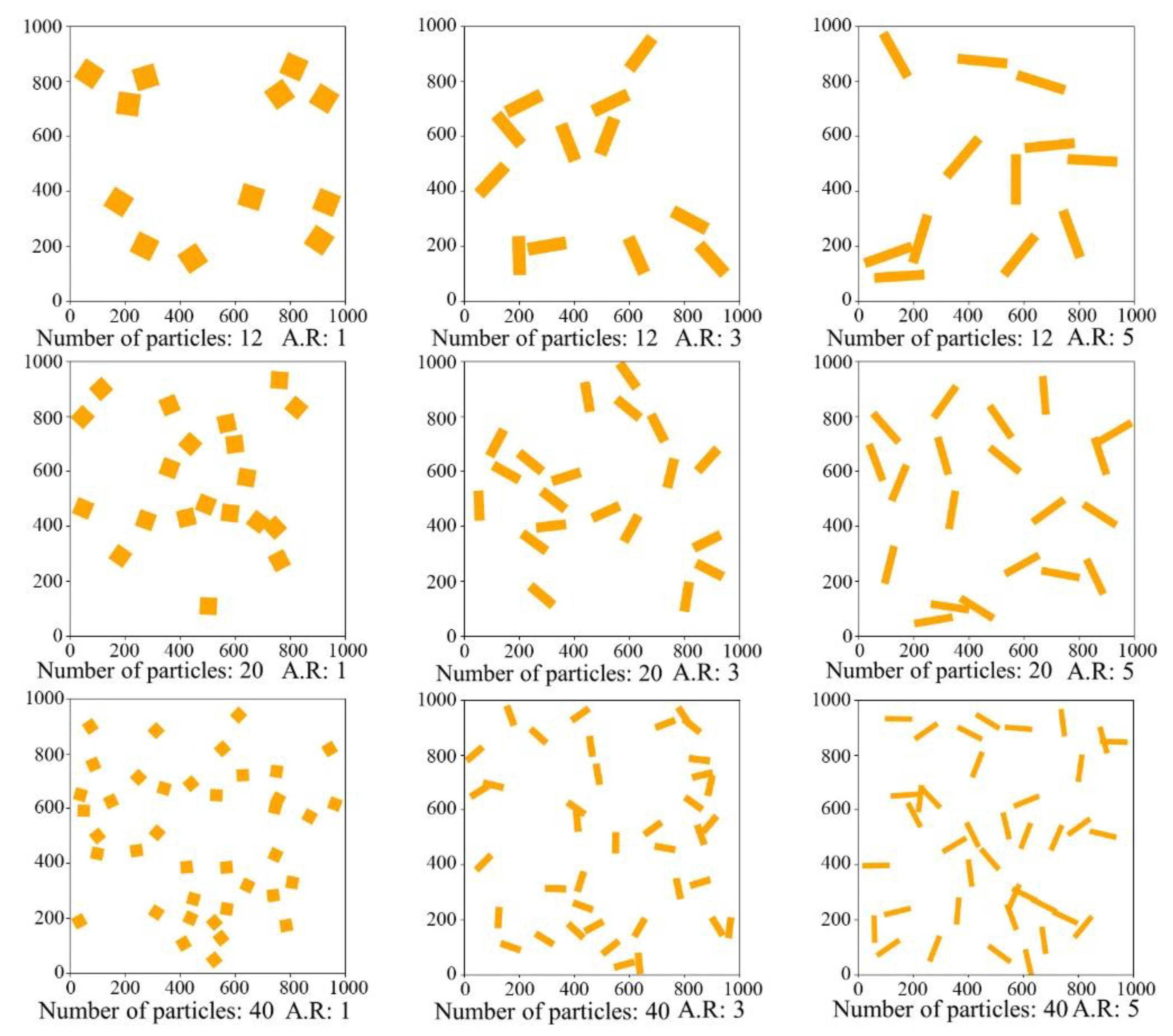
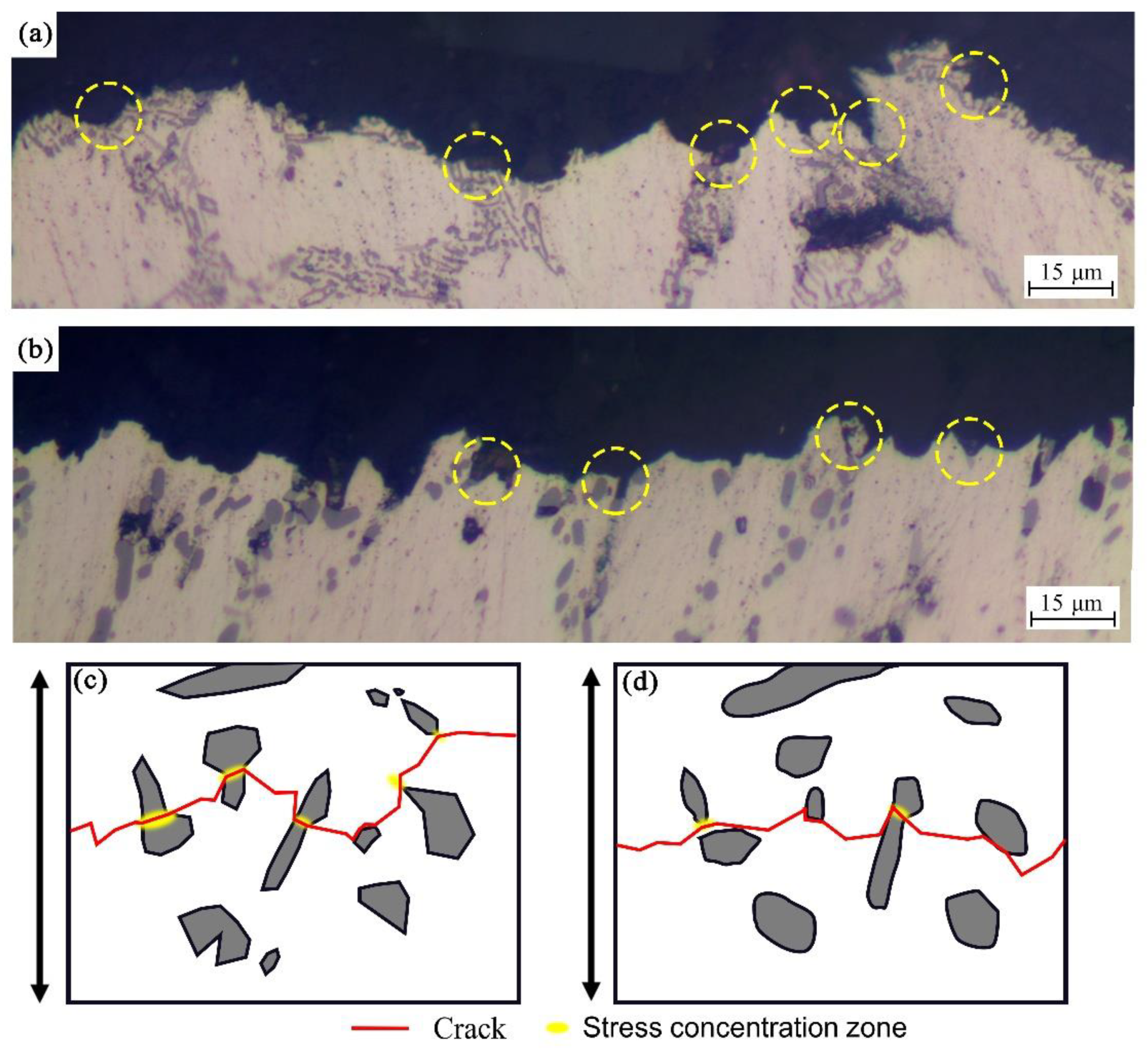
3.1. Microstructure
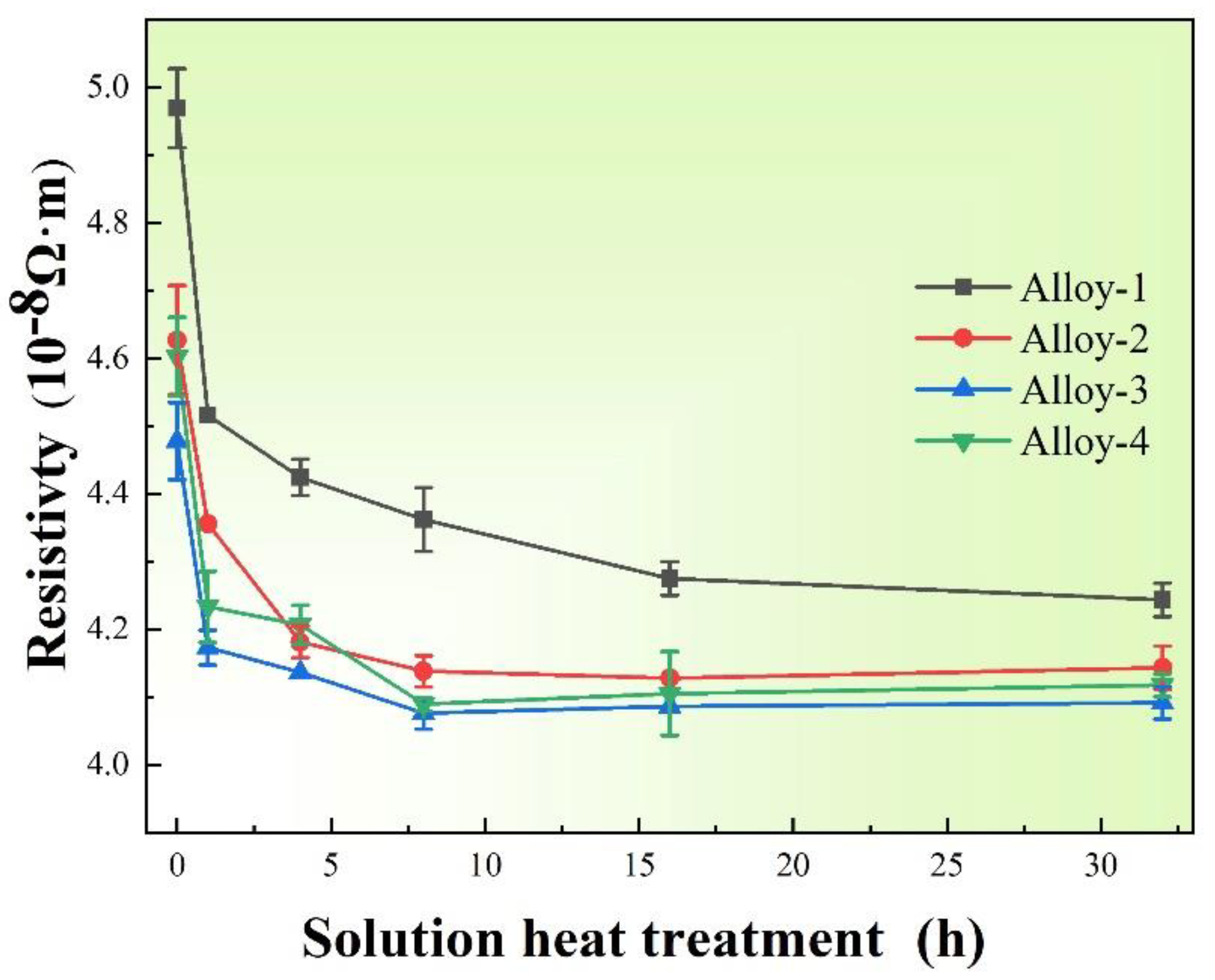
3.2. Thermal Conductivity
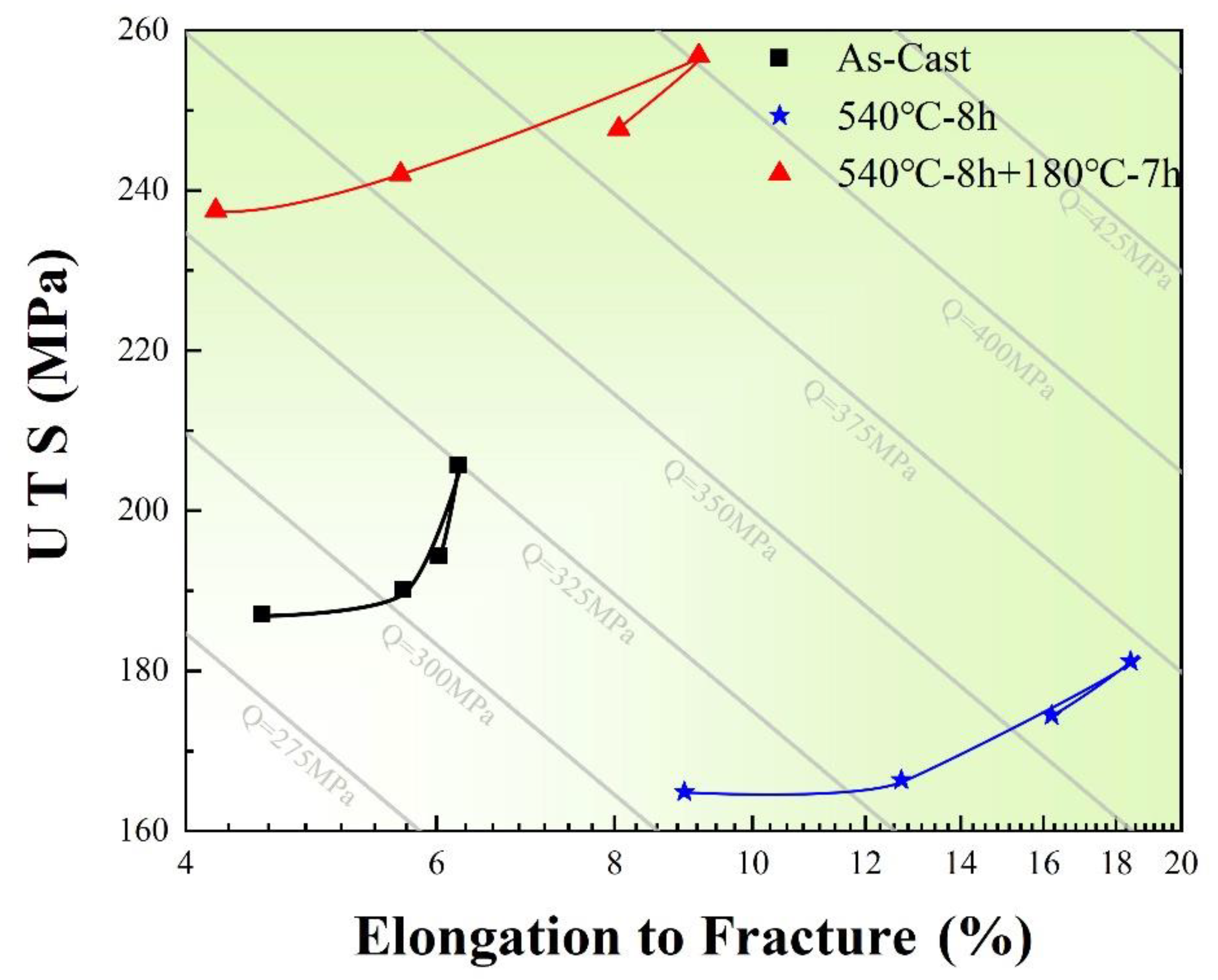
3.3. Mechanical Properties
4. Conclusions
- (1)
- There are four evolution mechanisms of Si particles during the solution treatment: spheroidization, splitting, coalescence, and coarsening. Spheroidization, fusion, and coalescence are active at the early stage. The growth of Si particles is a coarsening process controlled by diffusion.
- (2)
- Sb changes the contribution of each mechanism Si particle, resulting in the thermal modification of the Sb-modified alloy being more effective. This is because the modified Si particles have smaller aspect ratios and more branches and faults, leading to more effective spheroidization and more active fusion and coalescence.
- (3)
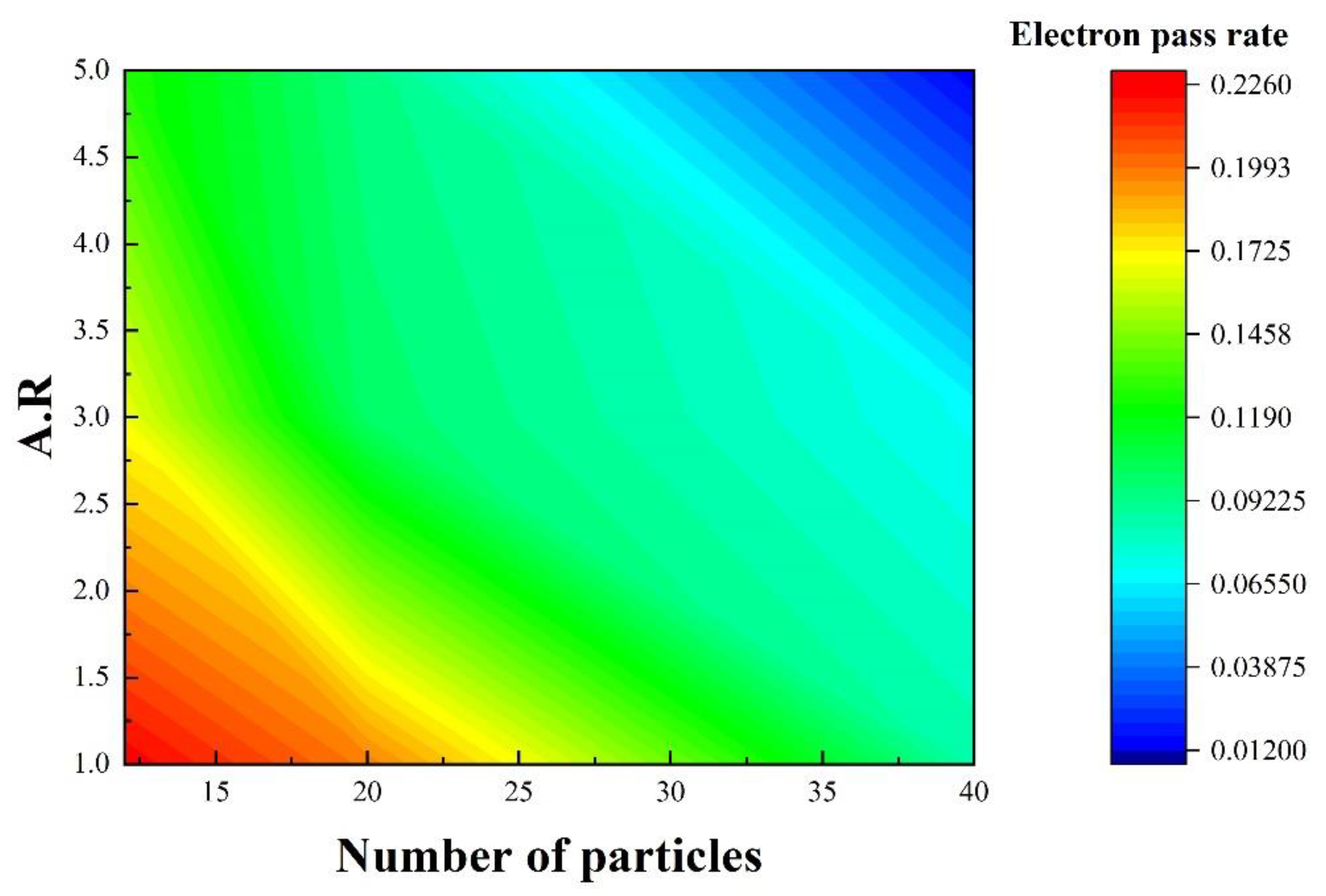
- The improved thermal conductivity of the alloy due to thermal modification benefits from the effect of Si-particle evolution on phonon propagation and electron transport. After solution treatment, the interface length of Si particles decreases, which reduces the anharmonicity of lattice vibration and lattice wave scattering. In addition, the more spheroidized Si particles also provide more channels for electron transport.
- (4)
- The particles treated by chemical modification and thermal modification have a smaller size and better morphology, which reduce the stress in its center and the shear stress in the matrix. Therefore, the crack germination is slowed down, and the alloy achieves higher mechanical properties.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Zhang, J.; Chen, X.; Yin, Y.; Guan, R. Microstructure evolution of eutectic Si in Al-7Si binary alloy by heat treatment and its effect on enhancing thermal conductivity. J. Mater. Res. Technol. 2020, 9, 8780–8786. [Google Scholar] [CrossRef]
- Wislei, R.O.; Noé, C.; José, E.S.; Goulart, P.R.; Garcia, A. The effects of a eutectic modifier on microstructure and surface corrosion behavior of Al-Si hypoeutectic alloys. J. Solid State Electrochem. 2007, 11, 1421–1427. [Google Scholar]
- Fat-Halla, N. Structural modification of Al-Si eutectic alloy by Sr and its effect on tensile and fracture characteristics. J. Mater. Sci. 1989, 24, 2488–2492. [Google Scholar] [CrossRef]
- Reyes, R.V.; Bello, T.S.; Kakitani, R.; Costa, T.A.; Garcia, A.; Cheung, N.; Spinelli, J.E. Tensile properties and related microstructural aspects of hypereutectic Al-Si alloys directionally solidified under different melt superheats and transient heat flow conditions. Mater. Sci. Eng. A 2017, 685, 235–243. [Google Scholar] [CrossRef]
- Natori, K.; Utsunomiya, H.; Tanaka, T. Improvement in formability of semi-solid cast hypoeutectic Al-Si alloys by equal-channel angular pressing. J. Mater. Process. Tech. 2017, 240, 240–248. [Google Scholar] [CrossRef]
- Gursoy, O.; Timelli, G. Lanthanides: A focused review of eutectic modification in hypoeutectic Al-Si alloys. J. Mater. Res. Technol. 2020, 9, 8652–8666. [Google Scholar] [CrossRef]
- Barrirero, J.; Li, J.; Engstler, M.; Ghafoor, N.; Schumacher, P.; Odén, M.; Mücklich, F. Cluster formation at the Si/liquid interface in Sr and Na modified Al-Si alloys. Scripta Mater. 2016, 117, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Elgallad, E.M.; Doty, H.W.; Alkahtani, S.A.; Samuel, F.H. Effects of La and Ce addition on the modification of Al-Si based alloys. Adv. Mater. Sci. Eng. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Farahany, S.; Ourdjini, A.; Bakhsheshi-Rad, H.R. Microstructure, mechanical properties and corrosion behavior of Al-Si-Cu-Zn-X (X=Bi, Sb, Sr) die cast alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 28–38. [Google Scholar] [CrossRef]
- Nampoothiri, J.; Balasundar, I.; Raj, B.; Murty, B.S.; Ravi, K.R. Porosity alleviation and mechanical property improvement of strontium modified A356 alloy by ultrasonic treatment. Mater. Sci. Eng. A 2018, 724, 586–593. [Google Scholar] [CrossRef]
- Mao, G.; Yan, H.; Zhu, C.; Wu, Z.; Gao, W. The varied mechanisms of yttrium (Y) modifying a hypoeutectic Al-Si alloy under conditions of different cooling rates. J. Alloys Compd. 2019, 806, 909–916. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Mao, F.; Feng, J.; Kang, Y. Effect of cooling rate on eutectic Si in Al-7.0Si-0.3Mg alloys modified by La additions. J. Alloys Compd. 2020, 826, 154206. [Google Scholar] [CrossRef]
- Lin, Y.C.; Luo, S.; Huang, J.; Yin, L.; Jiang, X. Effects of solution treatment on microstructures and micro-hardness of a Sr-modified Al-Si-Mg alloy. Mater. Sci. Eng. A 2018, 725, 530–540. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Jia, Z.; Liu, B. Precipitation behavior and hardening effects of Si-containing dispersoids in Al-7Si-Mg alloy during solution treatment. Mater. Des. 2016, 90, 1059–1068. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effect of extended thermal exposure and alloying elements on the morphology of eutectic Si in Al-Si cast alloys. Int. J. Metalcast. 2020, 14, 1013–1024. [Google Scholar] [CrossRef]
- Lee, C.; Shin, K.; Kim, Y. Dependence of tensile ductility on damage evolution of eutectic Si-particles and pre-existing micro-voids in Al-Si casting alloy. Eng. Fract. Mech. 2017, 175, 339–356. [Google Scholar] [CrossRef]
- Osorio, W.R.; Garcia, L.R.; Goulart, P.R.; Garcia, A. Effects of eutectic modification and T4 heat treatment on mechanical properties and corrosion resistance of an Al-9wt%Si casting alloy. Mater. Chem. Phys. 2007, 106, 343–349. [Google Scholar] [CrossRef]
- Mulazimoglu, M.H.; Drew, R.A.L.; Gruzileski, J.E. Solution treatment study of cast Al-Si alloys by electrical conductivity. Can. Metall. Quart. 1989, 28, 251–258. [Google Scholar] [CrossRef]
- Haghayeghi, R.; Timelli, G. An investigation on primary Si refinement by Sr and Sb additions in a hypereutectic Al-Si alloy. Mater. Lett. 2020, 283, 128779. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xue, J.; Zhu, C.; Li, X.; Wang, Z. High energy efficiency of Al-based anodes for Al-air battery by simultaneous addition of Mn and Sb. Chem. Eng. J. 2020, 417, 128006. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Liu, Q.Y. Kinetics of granulation of discontinuous phase in eutectic structures. Metar. Sci. Tech-Lond. 1986, 2, 500–507. [Google Scholar] [CrossRef]
- Wan, G.; Sahm, P.R. Particle growth by coalescence and Ostwald ripening in rheocasting of PbSn. Acta Metall. Mater. 1990, 38, 2367–2372. [Google Scholar] [CrossRef]
- Mueller, M.G.; Fornabaio, M.; Mortensen, A. Silicon particle pinhole defects in aluminium-silicon alloys. J. Mater. Sci. 2017, 52, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effect of morphological changes of eutectic Si particles on the ambient and high temperature tensile properties of Zr containing Al-Si alloys. J. Mater. Res. Technol. 2020, 9, 5962–5981. [Google Scholar] [CrossRef]
- Tong, Z.; Li, S.; Ruan, X.; Bao, H. A comprehensive first-principles analysis of phonon thermal conductivity and electron-phonon coupling in different metals. Phys. Rev. B 2019, 100, 144306. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, H.; Yang, M.; Sun, W.; Yin, G.; Zhang, Q.; Ren, Z. Thermal reliability of Al-Si eutectic alloy for thermal energy storage. Mater. Res. Bull. 2017, 95, 300–306. [Google Scholar] [CrossRef]
- Sauvage, X.; Bobruk, E.V.; Murashkin, M.Y.; Nasedkina, Y.; Enikeev, N.A.; Valiev, R.Z. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al-Mg-Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Jiang, H.; Li, S.; Zhang, L.; He, J.; Zheng, Q.; Song, Y.; Li, Y.; Zhao, J. The influence of rare earth element lanthanum on the microstructures and properties of as-cast 8176 (Al-0.5Fe) aluminum alloy. J. Alloys Compd. 2021, 859, 157804. [Google Scholar] [CrossRef]
- Costa, T.A.; Dias, M.; Gomes, L.G.; Rocha, O.L.; Garcia, A. Effect of solution time in T6 heat treatment on microstructure and hardness of a directionally solidified Al-Si-Cu alloy. J. Alloys Compd. 2016, 683, 485–494. [Google Scholar] [CrossRef]
- Miyajima, Y.; Komatsu, S.Y.; Mitsuhara, M.; Hata, S.; Nakashima, H.; Tsuji, N. Change in electrical resistivity of commercial purity aluminium severely plastic deformed. Phil. Mag. 2010, 90, 4475–4488. [Google Scholar] [CrossRef]
- Evans, H.E. Stress effects in high temperature oxidation of metals. Int. Mater. Rev. 1995, 40, 1–40. [Google Scholar] [CrossRef]
- Caceres, C.H. A phenomenological approach to the quality index of Al-Si-Mg casting alloys. Int. J. Cast Metal. Res. 2000, 12, 367–375. [Google Scholar] [CrossRef]
- Ragab, K.A.; Bouazara, M.; Chen, X.G. Quality index charts of Al-Si-Mg semi solid alloys subjected to multiple temperatures aging treatments and different quenching media. Materials 2019, 12, 1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.; Tyson, W.R. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–338. [Google Scholar] [CrossRef]
- Yang, J.; Cady, C.; Hu, M.S.; Zok, F.; Mehrabian, R.; Evans, A.G. Effects of damage on the flow strength and ductility of a ductile Al alloy reinforced with SiC particulates. Acta Metall. Mater. 1990, 38, 2613–2619. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, F.; Chen, L.; Zha, M.; Liu, G.J.; Jiang, Q.C. The effect of Sb addition on microstructures and tensile properties of extruded Al-20Mg2Si-4Cu alloy. Mater. Sci. Eng. A 2016, 657, 331–338. [Google Scholar] [CrossRef]
- Tong, X.; Ghosh, A. Fabrication of in situ TiC reinforced aluminum matrix composites. J. Mater. Sci. 2001, 36, 4059–4069. [Google Scholar] [CrossRef]


| Code | Alloy | Si | Mg | Sb | Cu | Fe | Al |
|---|---|---|---|---|---|---|---|
| Alloy-1 | Al-8Si-0.6Mg-0Sb | 7.90 | 0.65 | 0 | 0.12 | 0.18 | Bal. |
| Alloy-2 | Al-8Si-0.6Mg-0.2Sb | 7.92 | 0.63 | 0.18 | 0.10 | 0.22 | Bal. |
| Alloy-3 | Al-8Si-0.6Mg-0.4Sb | 7.86 | 0.64 | 0.41 | 0.11 | 0.21 | Bal. |
| Alloy-4 | Al-8Si-0.6Mg-0.6Sb | 7.89 | 0.60 | 0.54 | 0.10 | 0.19 | Bal. |
| Code | Alloy | KLSW-1 | KLSW-2 |
|---|---|---|---|
| Alloy-1 | Al-8Si-0.6Mg-0Sb | 0.3347 | 0.0961 |
| Alloy-2 | Al-8Si-0.6Mg-0.2Sb | 0.1252 | 0.0489 |
| Alloy-3 | Al-8Si-0.6Mg-0.4Sb | 0.2079 | 0.0347 |
| Alloy-4 | Al-8Si-0.6Mg-0.6Sb | 0.2107 | 0.0304 |
| Code | ||
|---|---|---|
| Alloy-1 | 1.13 | 0.50 |
| Alloy-2 | 1.24 | 0.47 |
| Alloy-3 | 1.82 | 0.69 |
| Alloy-4 | 1.24 | 0.47 |
| Condition | Alloy | YS (MPa) | UTS (MPa) | EL (%) |
|---|---|---|---|---|
| As-cast | Alloy-1 | 117.3 ± 10.9 | 187.1 ± 15.3 | 4.5 ± 0.6 |
| Alloy-2 | 120.6 ± 9.6 | 190.2 ± 17.6 | 5.7 ± 0.4 | |
| Alloy-3 | 123.1 ± 5.4 | 205.7 ± 18.1 | 6.2 ± 0.6 | |
| Alloy-4 | 119.4 ± 6.7 | 194.4 ± 12.4 | 6.0 ± 0.8 | |
| T4 | Alloy-1 | 78.2 ± 8.3 | 164.9 ± 13.2 | 8.9 ± 1.2 |
| Alloy-2 | 82.2 ± 4.6 | 166.4 ± 9.6 | 12.7 ± 1.6 | |
| Alloy-3 | 87.5 ± 5.1 | 181.2 ± 14.3 | 18.4 ± 0.8 | |
| Alloy-4 | 80.4 ± 6.0 | 174.4 ± 11.0 | 16.2 ± 1.4 | |
| T6 | Alloy-1 | 179.9 ± 11.8 | 237.5 ± 17.3 | 4.2 ± 0.7 |
| Alloy-2 | 169.6 ± 7.5 | 242.0 ± 10.7 | 5.6 ± 0.6 | |
| Alloy-3 | 174.7 ± 6.2 | 256.8 ± 12.9 | 9.1 ± 1.2 | |
| Alloy-4 | 178.8 ± 5.7 | 247.7 ± 14.7 | 8.1 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, Y.; Wang, L.; Guo, X.; Zhang, L.; Li, H. The Effect of Solution Treatment on the Si Particles’ Morphology Evolution and the Thermal Conductivity and Tensile Properties of Sb-Modified Al-8Si-0.6Mg Alloys. Metals 2022, 12, 377. https://doi.org/10.3390/met12030377
Liang X, Wang Y, Wang L, Guo X, Zhang L, Li H. The Effect of Solution Treatment on the Si Particles’ Morphology Evolution and the Thermal Conductivity and Tensile Properties of Sb-Modified Al-8Si-0.6Mg Alloys. Metals. 2022; 12(3):377. https://doi.org/10.3390/met12030377
Chicago/Turabian StyleLiang, Xiaopeng, Yihao Wang, Li Wang, Xinming Guo, Liangjie Zhang, and Huizhong Li. 2022. "The Effect of Solution Treatment on the Si Particles’ Morphology Evolution and the Thermal Conductivity and Tensile Properties of Sb-Modified Al-8Si-0.6Mg Alloys" Metals 12, no. 3: 377. https://doi.org/10.3390/met12030377






