Effect of the Basicity on Mineralogical Phases and Micro-Structure of Dephosphorization Slag in the New Double Slag Converter Steelmaking Process
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Decarburization Slag and Pig Iron
2.2. Experimental Procedure and Analysis Method
3. Results
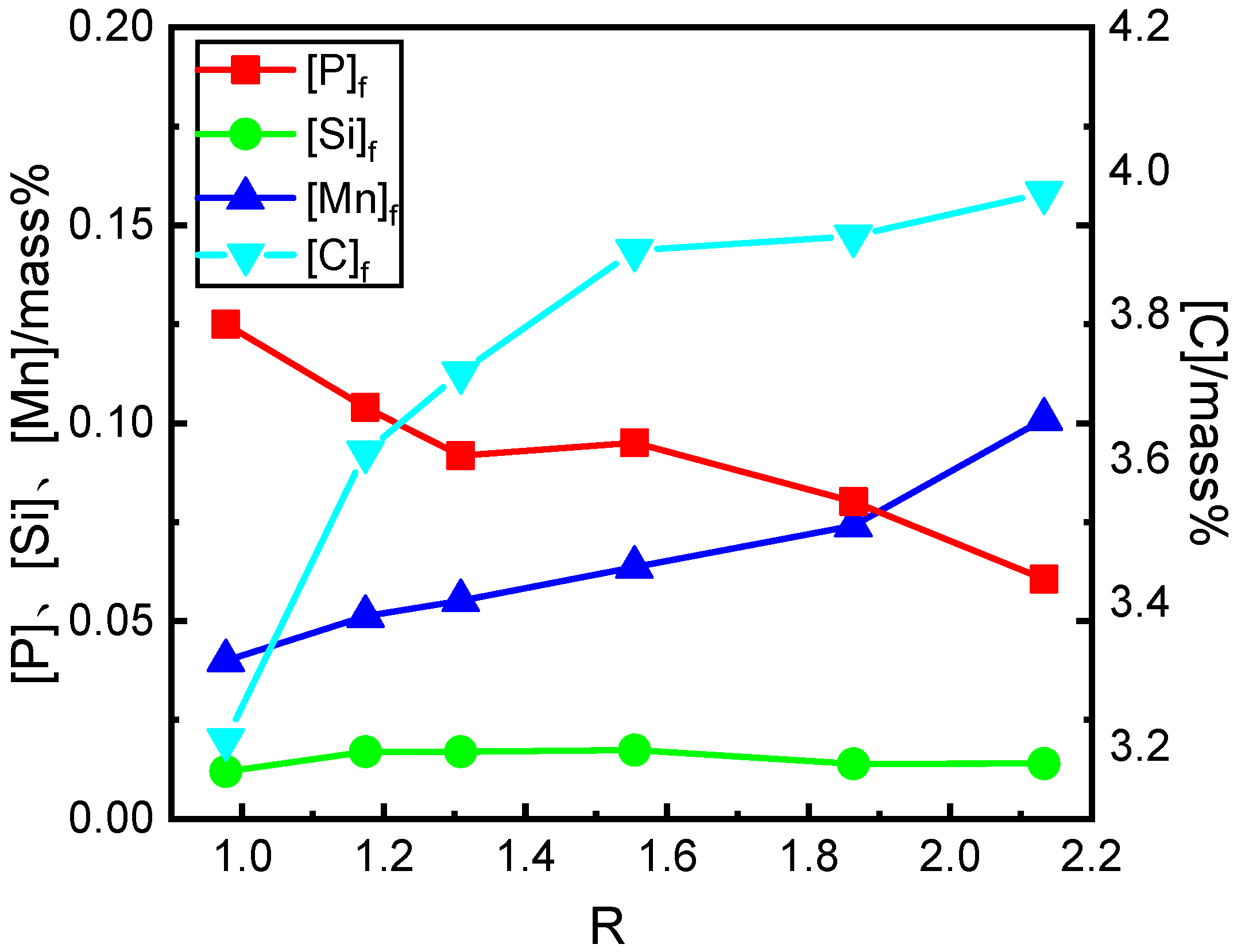
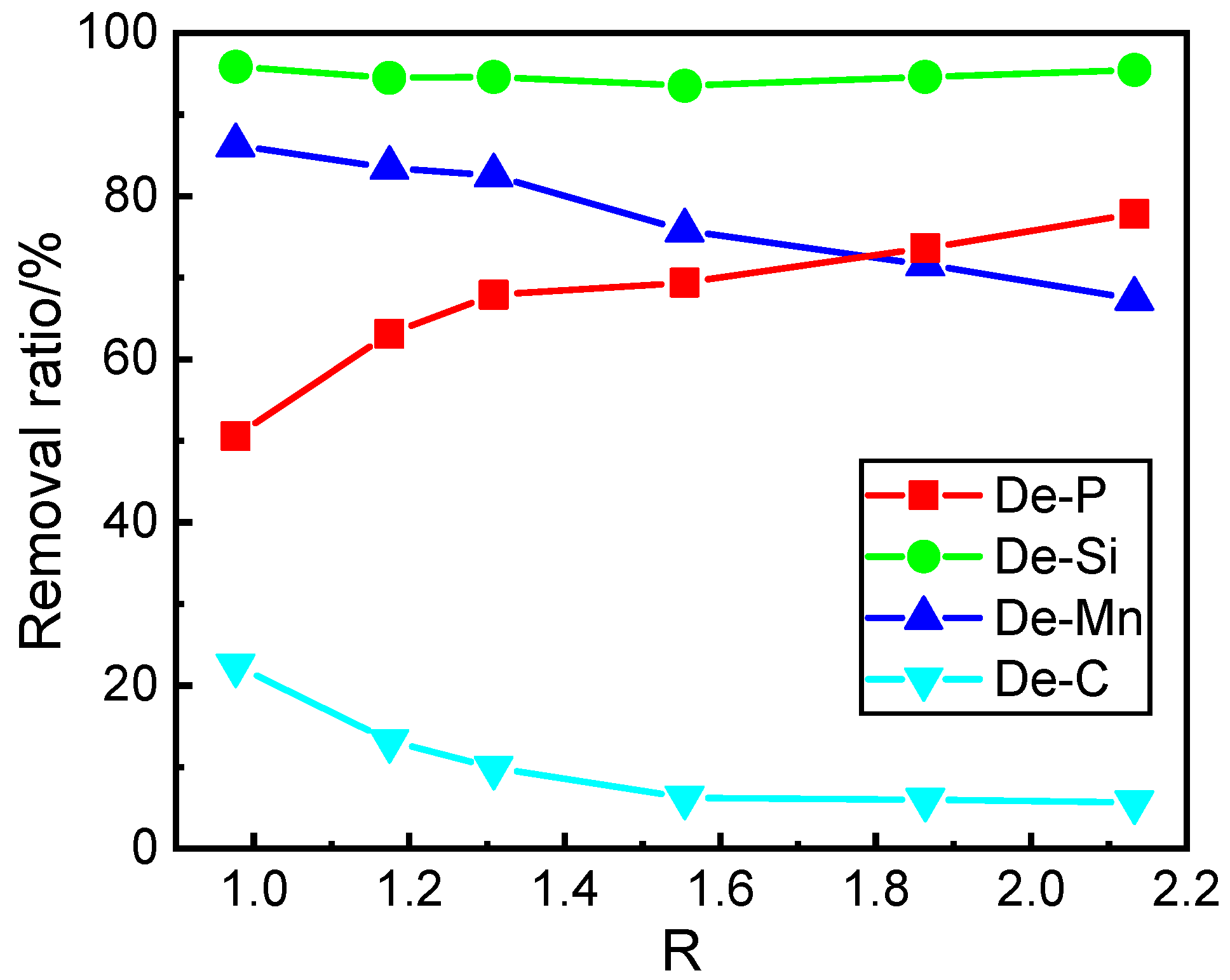
3.1. Effect of Basicity on the Contents and Removal Ratios of Elements in the Hot Metal after Dephosphorization
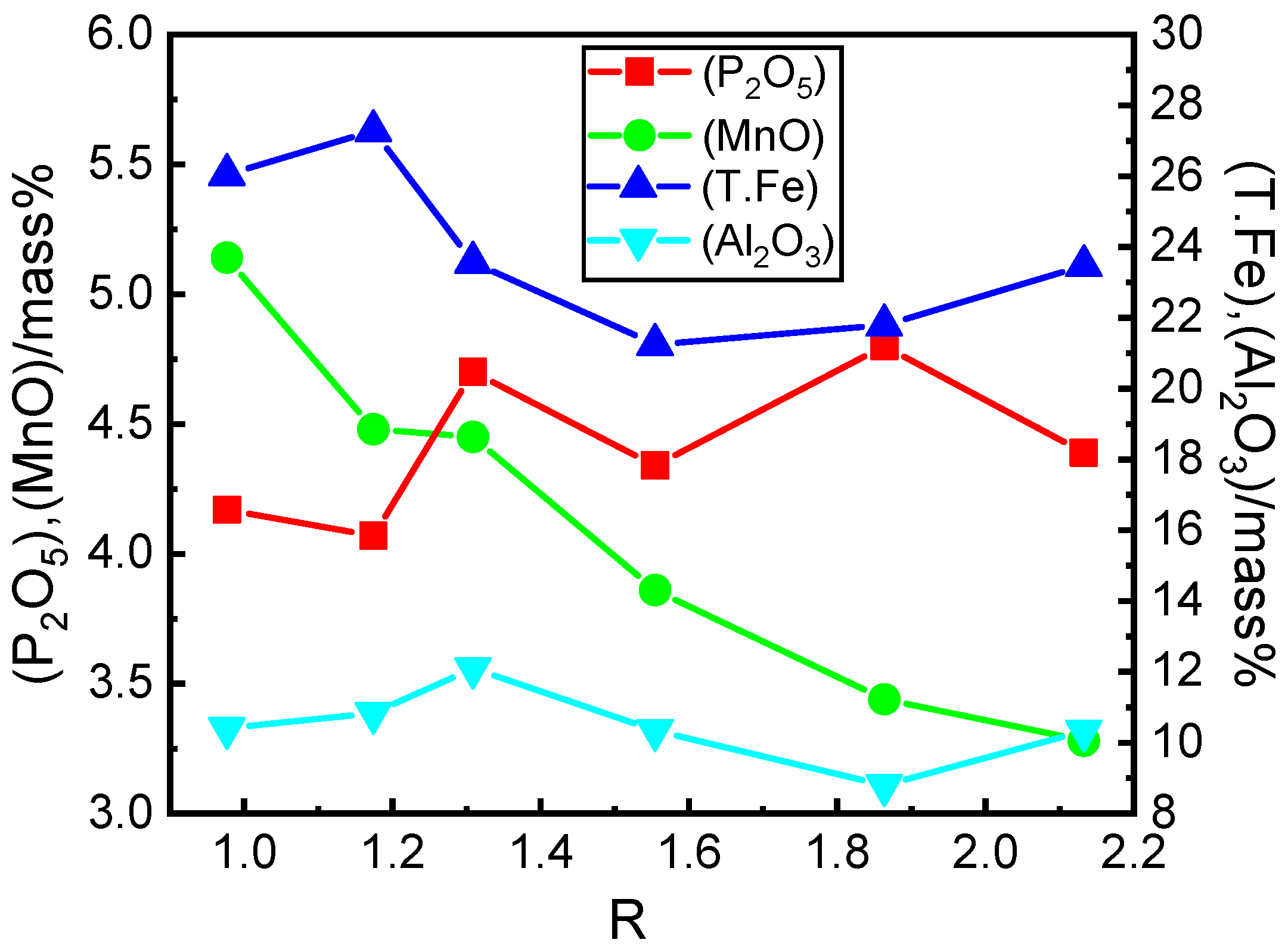
3.2. Effect of Basicity on the Oxide Contents in Slag
4. Discussion
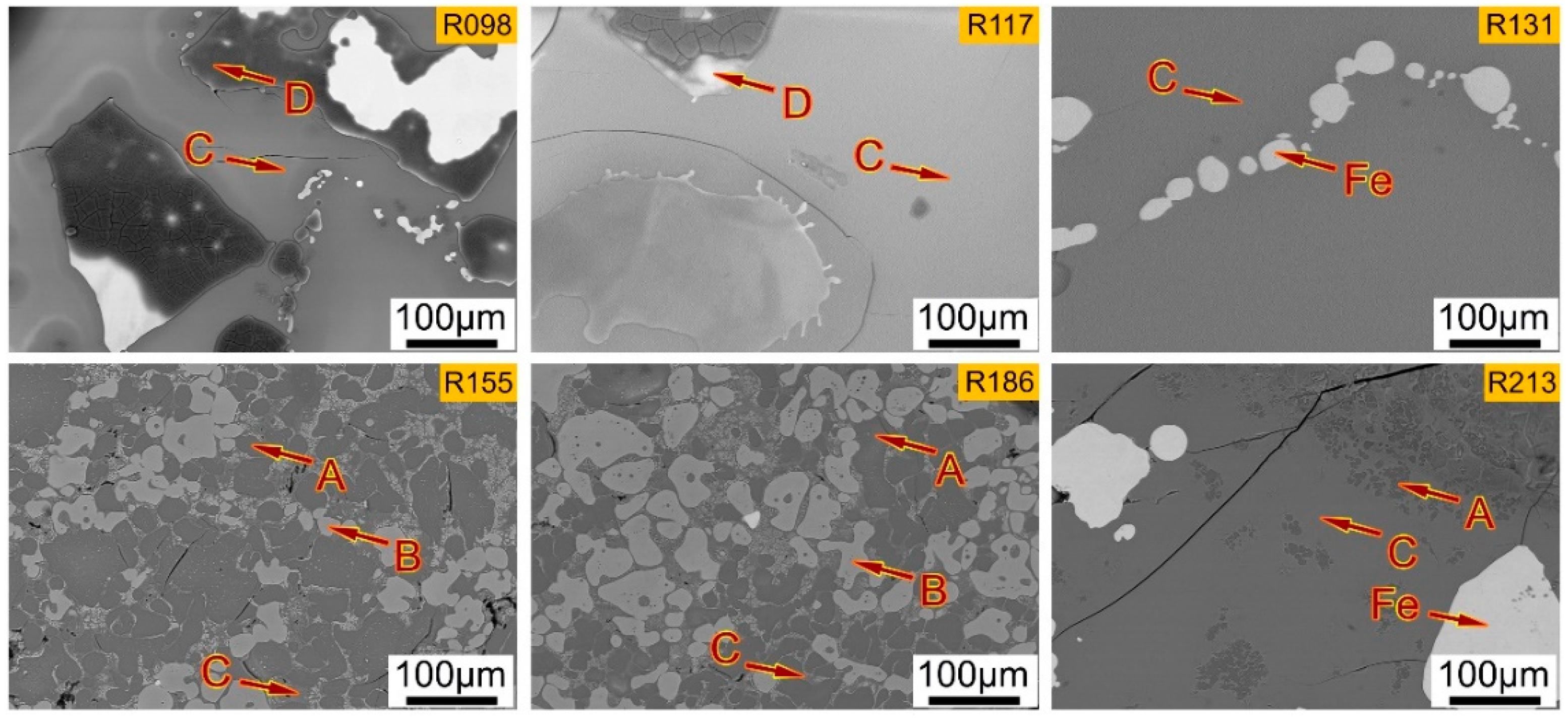
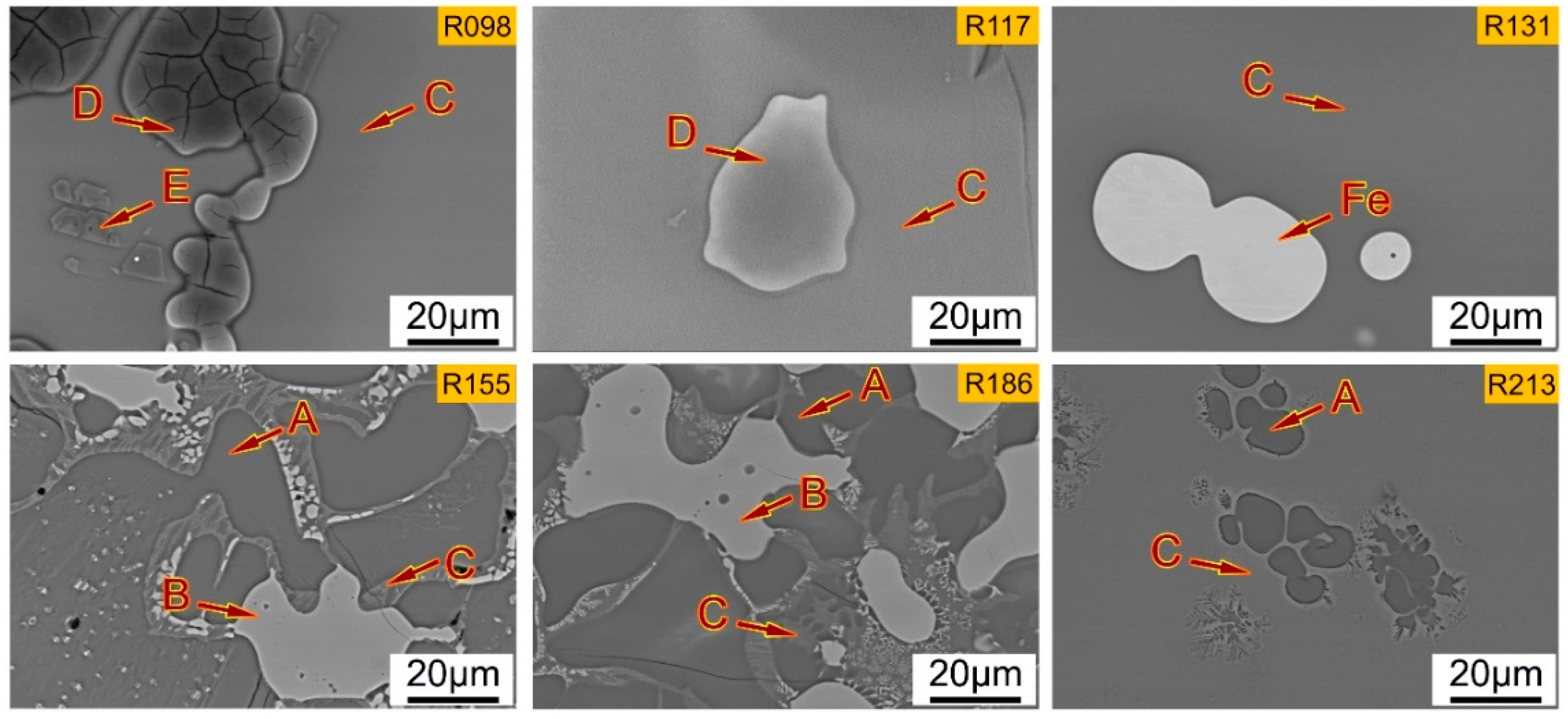
4.1. Influence of Basicity on the Micro-Morphologies of Dephosphorization Slag with SEM-EDS Analysis
4.2. Effect of Basicity on Mineralogical Phases of Dephosphorization Slag with XRD Analysis
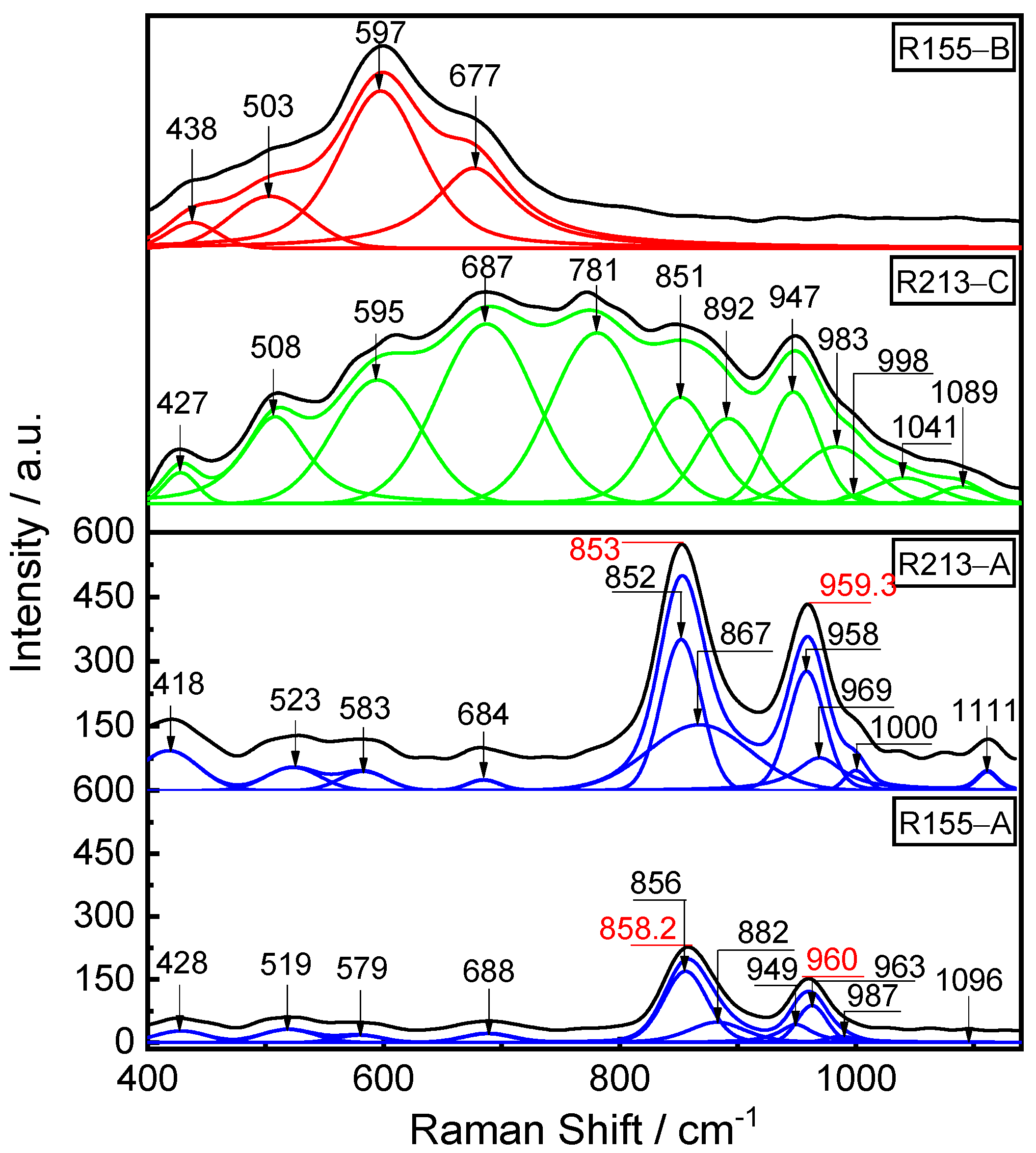
4.3. Analysis of Micro-Structure of Dephosphorization Slag by Raman Spectroscopy
4.4. Effect of Basicity on Activity Coefficient of P2O5 and the Basicity in the Matrix Phase of Dephosphorization Slag
5. Conclusions
- (1)
- With the increase of the basicity from 0.98 to 1.31, the P and C contents in hot metal rapidly decrease and increase at first, respectively. With the increase of the basicity from 1.31 to 2.13, the P and C contents gradually decrease and increase, respectively. With the increase of the basicity, the Mn contents in the hot metal increase gradually. In the slag, the MnO content decreases gradually, and the P2O5 content in slag only increases slightly. The T.Fe content firstly decreases and then increases slightly.
- (2)
- From the SEM-EDS and XRD results, when the basicities are 0.98 and 1.17, the slag is mainly composed of matrix phase rich in P, Fe2SiO4 phase, and spinel phase rich in Al, Mg, Fe, and Mn. With the increase of the basicity to 1.31, the slag is mainly composed of matrix phase and a small amount of pure iron. With the increase of the basicity to 1.55 and 1.86, the slag is mainly composed of P-rich phase containing nC2S-C3P solid solution, Fe-rich phase, and matrix phase. With further increase of the basicity to 2.13, the slag is mainly composed of P-rich phase and matrix phase.
- (3)
- With the increase of the basicity, the phase containing the high P content changes from the matrix phase into the P-rich phase. Therefore, under the present experimental conditions, the P-rich phase can only be precipitated from the liquid slag when the basicity is higher than 1.55, which is a benefit to the dephosphorization.
- (4)
- The Raman intensity of the P-O-Ca structure unit in the P-rich phase is significantly higher than that of the P-O-Si structure unit, indicating that most of the phosphorus in P-rich phase exists in the P-O-Ca structure unit along with a small amount of phosphorus in the P-O-Si structure unit.
- (5)
- With the increase of the basicity of the dephosphorization slag, the activity coefficient of P2O5, , in the liquid phase decreases, while the basicity in the liquid phase increases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogawa, Y.; Yano, M.; Kitamura, S.; Hirata, H. Development of the continuous dephosphorization and decarburization process using BOF. Tetsu-to-Hagané 2001, 87, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Fix, W.; Heymann, H.; Heinke, R. Subsolidus relations in the system 2CaO·SiO2-3CaO·P2O5. J. Am. Ceram. Soc. 1969, 52, 346–347. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, W.; Wu, W.; Meng, H.; Gao, Q.; Guo, Z. Study on the occurrence of phosphorus in the slag of hot metal dephosphorization for stainless steel production. Steel Res. Int. 2020, 91, 2000021. [Google Scholar] [CrossRef]
- Zhou, J.; Bi, X.; Yue, R.; Yang, F. Phosphorus distribution ratio between multi phase CaO-FeOt-SiO2-P2O5 (6%–13%) slags with MP near hot metal temperature and C-saturated molten iron at 1 573 K. ISIJ Int. 2017, 57, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, S.Y.; Yonezawa, K.; Ogawa, Y.; Sasaki, N. Improvement of reaction efficiency in hot metal dephosphorisation. Ironmak. Steelmak. 2002, 29, 121–124. [Google Scholar] [CrossRef]
- Liu, F.; Wang, G.; Zhao, Y.; Tan, J.; Zhao, C.; Wang, Q. Hot metal dephosphorisation by low basicity slag in the early stage of converting process. Ironmak. Steelmak. 2019, 46, 392–403. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakano, S.; Serizawa, H.; Umesaki, N. In-situ phase identification of crystallized compound from 2CaO·SiO2-3CaO·P2O5 liquid. ISIJ Int. 2020, 60, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.F.; Liu, Y. Effects of basicity, MgO and MnO on mineralogical phases of CaO-FeOx-SiO2-P2O5 slag. Ironmak. Steelmak. 2017, 45, 363–370. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Chou, K.; Shu, Q. Effects of oxygen atmosphere, FeOx and basicity on mineralogical phases of CaO-SiO2-MgO-Al2O3-FetO-P2O5 steelmaking slag. Ironmak. Steelmak. 2019, 46, 987–997. [Google Scholar] [CrossRef]
- Uchida, Y.I.; Sasaki, N.; Miki, Y. Change of phosphorus-concentrated phase in low basicity steelmaking slag. ISIJ Int. 2018, 58, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.F.; Yang, J.; Zhang, R.H.; Yang, W.K.; Sun, H. Behavior of phosphorus enrichment in dephosphorization slag at low temperature and low basicity. Int. J. Min. Met. Mater. 2021, 28, 66–75. [Google Scholar] [CrossRef]
- Yang, W.K.; Yang, J.; Zhang, R.H.; Sun, H. Effect of the initial P content on dephosphorization of hot metal with low basicity slag at 1623 K. Steel Res. Int. 2021, 92, 2100066. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, S.; Zhang, M.; Guo, M.; Zhang, Z. Structural investigation of phosphorus in CaO-SiO2-P2O5 ternary glass. Metall. Mater. Trans. B 2017, 48, 1139–1148. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shu, Q.F.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z.T. Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metall. Mater. Trans. B 2015, 46, 758–765. [Google Scholar] [CrossRef]
- Yang, W.K.; Yang, J.; Shi, Y.Q.; Yang, Z.J.; Gao, F.B.; Zhang, R.H.; Ye, G.F. Effect of temperature on dephosphorization of hot metal in double slag converter steelmaking process by high-temperature laboratorial experiments. Steel Res. Int. 2021, 92, 2000438. [Google Scholar] [CrossRef]
- Yang, W.K.; Yang, J.; Shi, Y.Q.; Yang, Z.J.; Gao, F.B.; Zhang, R.H.; Sun, H. Effect of the Fe2O3 addition amount on dephosphorization of hot metal with low basicity slag by high-temperature laboratorial experiments. Metals 2021, 11, 417. [Google Scholar] [CrossRef]
- Yang, W.K.; Yang, J.; Zhang, R.H.; Sun, H. Microstructure and viscosity of dephosphorization slag in new double slag converter steelmaking process. ISIJ Int. 2021, 61. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Fan, D.; Hou, G. Effects of interfacial oxygen potential and slag phase changing during slag formation process on dephosphorization behavior. ISIJ Int. 2019, 59, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.K.; Yang, J.; Shi, Y.Q.; Yang, Z.J.; Gao, F.B.; Zhang, R.H.; Ye, G.F. Effect of basicity on dephosphorization of hot metal with a low basicity slag at 1653 K. Ironmak. Steelmak. 2021, 48, 69–77. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Li, G.Q.; Chen, Z.P.; Ma, G.J.; Liu, J. Manganese distribution equilibrium between CaO-FetO-SiO2-MnO-P2O5-(Al2O3) slags and carbon saturated iron. ISIJ Int. 2008, 48, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.K.; Zhang, R.H.; Sun, H.; Yang, J. Dephosphorization in new double slag converter steelmaking process with high temperature laboratorial experiments. Steel Res. Int. 2021. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Thermodynamic assessment of hot metal and steel dephosphorization with MnO-containing BOF slags. ISIJ Int. 1995, 35, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.S.; Sohn, I. Crystallization control for remediation of an FetO-rich CaO-SiO2-Al2O3-MgO EAF waste slag. Environ. Sci. Technol. 2014, 48, 1886–1892. [Google Scholar] [CrossRef]
- Hass, M. Raman spectra of vitreous silica, germania and sodium silicate glasses. J. Phys. Chem. Solids. 1970, 31, 415–422. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.; Ventura, J.; Kannan, S.; Saranti, A.; Karakassides, M.; Ferreira, J. Structural analysis and devitrification of glasses based on the CaO-MgO-SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J. Non-Cryst. Solids. 2006, 352, 322–328. [Google Scholar] [CrossRef]
- McKeown, D.; Galeener, F.; Brown, G., Jr. Raman studies of Al coordination in silica-rich sodium aluminosilicate glasses and some related minerals. J. Non-Cryst. Solids. 1984, 68, 361–378. [Google Scholar] [CrossRef]
- Mohri, M.; Sasaki, Y.; Ishii, K. Comparative roles of Al3+ and Fe3+ ions for network construction in sodium silicate melts. ISIJ Int. 2001, 41, 410–415. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.; Liu, L.; Wang, X. Investigation of the viscosity and structural properties of CaO-SiO2-TiO2 slags. Metall. Mater. Trans. B 2014, 45, 1389–1397. [Google Scholar] [CrossRef]
- Tarte, P. Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra. Spectrochim. Acta Part A: Mol. Spectrosc. 1967, 23, 2127–2143. [Google Scholar] [CrossRef]
- Leekes, G.; Nowack, N.; Schlegelmilch, F. Dissolution of water vapour in ESR-slags of the systems CaO-Al2O3 and CaF2-CaO-Al2O3 by application of Fourier-Transform-Infrared-Spectroscopy. Steel Res. 1988, 59, 406–416. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Structural investigation of CaO-Al2O3 and CaO-Al2O3-CaF2 slags via Fourier transform infrared spectra. ISIJ Int. 2002, 42, 38–43. [Google Scholar] [CrossRef]
- Mysen, B.O.; Ryerson, F.J.; Virgo, D. The structural role of phosphorus in silicate melts. Am. Mineral. 1981, 66, 106–117. [Google Scholar]
- Wong, J. Vibrational spectra of vapor-deposited binary phosphosilicate glasses. J. Non-Cryst. Solids 1976, 20, 83–100. [Google Scholar] [CrossRef]
- Ban, Y.S. Mathematical expression of slag-metal reactions in steelmaking process by quadratic formalism based on the regular solution model. ISIJ Int. 1993, 33, 2–11. [Google Scholar] [CrossRef] [Green Version]



| Sample | CaO | MgO | MnO | P2O5 | SiO2 | FeO | R |
|---|---|---|---|---|---|---|---|
| Target | 46.5 | 8.20 | 3.73 | 3.00 | 15.6 | 22.0 | 2.98 |
| Actual | 43.4 | 8.58 | 4.57 | 3.01 | 16.2 | 21.8 | 2.69 |
| Sample No. | [C]i | [Si]i | [Mn]i | [P]i | [C]f | [Si]f | [Mn]f | [P]f |
|---|---|---|---|---|---|---|---|---|
| R098 | 4.14 | 0.533 | 0.291 | 0.253 | 3.21 | 0.012 | 0.040 | 0.125 |
| R117 | 4.16 | 0.587 | 0.310 | 0.282 | 3.61 | 0.017 | 0.051 | 0.104 |
| R131 | 4.13 | 0.592 | 0.316 | 0.286 | 3.72 | 0.017 | 0.055 | 0.092 |
| R155 | 4.15 | 0.731 | 0.263 | 0.311 | 3.89 | 0.017 | 0.064 | 0.095 |
| R186 | 4.16 | 0.521 | 0.261 | 0.304 | 3.91 | 0.014 | 0.074 | 0.080 |
| R213 | 4.21 | 0.494 | 0.310 | 0.273 | 3.97 | 0.014 | 0.101 | 0.061 |
| Sample No. | T.Fe | FeO | CaO | SiO2 | Al2O3 | MgO | MnO | P2O5 | R |
|---|---|---|---|---|---|---|---|---|---|
| R098 | 26.0 | 32.8 | 20.7 | 21.2 | 10.41 | 3.98 | 5.14 | 4.17 | 0.98 |
| R117 | 27.3 | 34.1 | 22.8 | 19.4 | 10.83 | 3.28 | 4.48 | 4.07 | 1.17 |
| R131 | 23.6 | 29.3 | 26.0 | 19.9 | 12.1 | 3.28 | 4.45 | 4.7 | 1.31 |
| R155 | 21.3 | 26.4 | 31.2 | 20.1 | 10.35 | 3.18 | 3.86 | 4.34 | 1.55 |
| R186 | 21.8 | 27.1 | 33.9 | 18.2 | 8.79 | 3.26 | 3.44 | 4.80 | 1.86 |
| R213 | 23.5 | 29.4 | 33.4 | 15.7 | 10.33 | 2.98 | 3.28 | 4.39 | 2.13 |
| Position | MgO | Al2O3 | SiO2 | P2O5 | CaO | MnO | FeO |
|---|---|---|---|---|---|---|---|
| R098-D | 0.15 | 0.11 | 47.1 | 0.18 | 0.31 | 0.11 | 52.0 |
| R098-E | 13.0 | 41.8 | 1.56 | 0.25 | 1.57 | 9.29 | 32.6 |
| R098-C | 3.74 | 8.61 | 24.6 | 6.46 | 30.4 | 7.00 | 19.2 |
| R117-D | 0.21 | 0.11 | 45.1 | 0.45 | 0.52 | 0.27 | 53.3 |
| R117-C | 3.01 | 10.3 | 24.6 | 5.66 | 32.1 | 6.13 | 18.1 |
| R131-C | 2.46 | 8.47 | 19.6 | 6.08 | 35.9 | 5.12 | 22.4 |
| R155-A | 0.86 | 0.11 | 24.1 | 8.48 | 61.4 | 1.45 | 3.64 |
| R155-B | 10.2 | 0.27 | 0.49 | 0.33 | 0.82 | 9.00 | 78.9 |
| R155-C | 1.39 | 8.59 | 21.38 | 0.97 | 45.6 | 2.98 | 19.1 |
| R186-A | 0.62 | 0.10 | 23.4 | 8.63 | 62.4 | 1.08 | 3.83 |
| R186-B | 10.8 | 0.34 | 0.02 | 0.43 | 0.60 | 10.0 | 77.8 |
| R186-C | 2.60 | 11.3 | 17.0 | 1.30 | 38.0 | 4.86 | 24.9 |
| R213-A | 0.58 | 0.34 | 16.8 | 18.9 | 60.1 | 1.05 | 2.19 |
| R213-C | 2.00 | 15.1 | 11.9 | 2.16 | 34.3 | 5.63 | 29.0 |
| Sample | R155-A | R213-A | R213-C | R155-B | Raman Assignments |
|---|---|---|---|---|---|
| Raman shift (cm−1) | 428 | 418 | 428 | 438 | Si-O-Si [24,25] |
| 519 | 523 | 508 | 503 | Si-O-Al [26,27] | |
| 579 | 583 | 595 | 597 | [FeO6] [23,29,30,31] | |
| 688 | 684 | 687 | 677 | [FeO4] [23,29,30,31] | |
| 781 | [AlO4] [23,29,30,31] | ||||
| 856 | 852 | 851 | Q0 [32] | ||
| 882 | 867 | 892 | Q1 [32] | ||
| 983 | 969 | 983 | Q2 [32] | ||
| 1041 | Q3 [32] | ||||
| 949 | 958 | 947 | P-O-Ca [13] | ||
| 987 | 1000 | 998 | P-O-P [33] | ||
| 1096 | 1111 | 1089 | P-O-Si [13,33] |
| i | j | Fe2+ | Fe3+ | Mn2+ | Ca2+ | Mg2+ | Si4+ | P5+ | Al3+ |
|---|---|---|---|---|---|---|---|---|---|
| Fe2+ | — | −18,660 | 7110 | −31,380 | 33,470 | −41,840 | −31,380 | −41,000 | |
| Fe3+ | −18,660 | — | −56,480 | −95,810 | −2930 | 32,640 | 14,640 | −161,080 | |
| Mn2+ | 7110 | −56,480 | — | −92,050 | 61,920 | −75,310 | −84,940 | −83,680 | |
| Ca2+ | −31,380 | −95,810 | −92,050 | — | −100,420 | −133,890 | −251,040 | −154,810 | |
| Mg2+ | 33,470 | −2930 | 61,920 | −100,420 | — | −66,940 | −37,660 | −71,130 | |
| Si4+ | −41,840 | 32,640 | −75,310 | −133,890 | −66,940 | — | 83,680 | −127,610 | |
| P5+ | −31,380 | 14,640 | −84,940 | −251,040 | −37,660 | 83,680 | — | −261,500 | |
| Al3+ | −41,000 | −161,080 | −83,680 | −154,810 | −71,130 | −127,610 | −261,500 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yang, J.; Zhang, R.; Sun, H.; Qiu, Y. Effect of the Basicity on Mineralogical Phases and Micro-Structure of Dephosphorization Slag in the New Double Slag Converter Steelmaking Process. Metals 2021, 11, 1480. https://doi.org/10.3390/met11091480
Yang W, Yang J, Zhang R, Sun H, Qiu Y. Effect of the Basicity on Mineralogical Phases and Micro-Structure of Dephosphorization Slag in the New Double Slag Converter Steelmaking Process. Metals. 2021; 11(9):1480. https://doi.org/10.3390/met11091480
Chicago/Turabian StyleYang, Wenkui, Jian Yang, Runhao Zhang, Han Sun, and Yunlong Qiu. 2021. "Effect of the Basicity on Mineralogical Phases and Micro-Structure of Dephosphorization Slag in the New Double Slag Converter Steelmaking Process" Metals 11, no. 9: 1480. https://doi.org/10.3390/met11091480







