One Step Production of Silver-Copper (AgCu) Nanoparticles
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
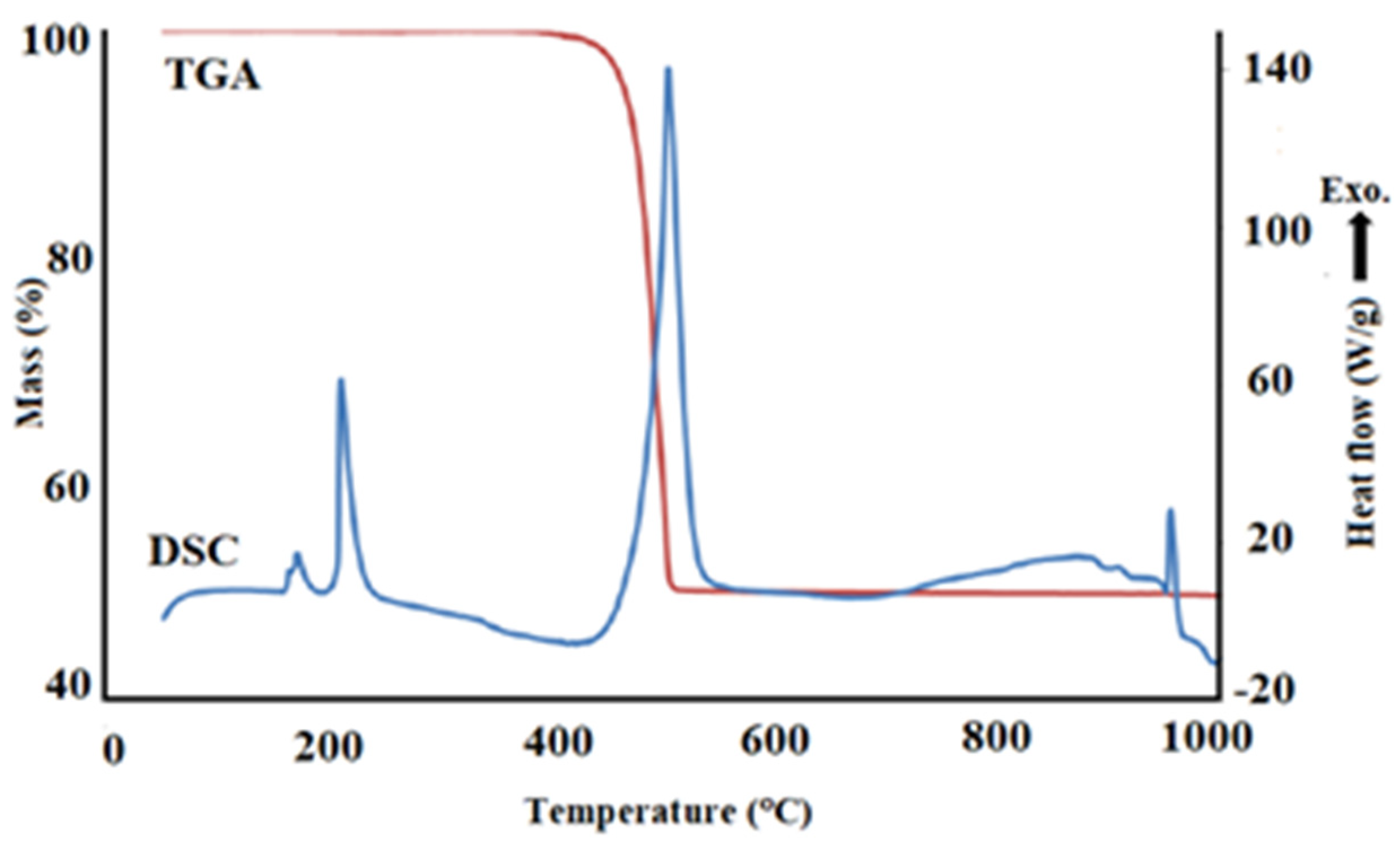
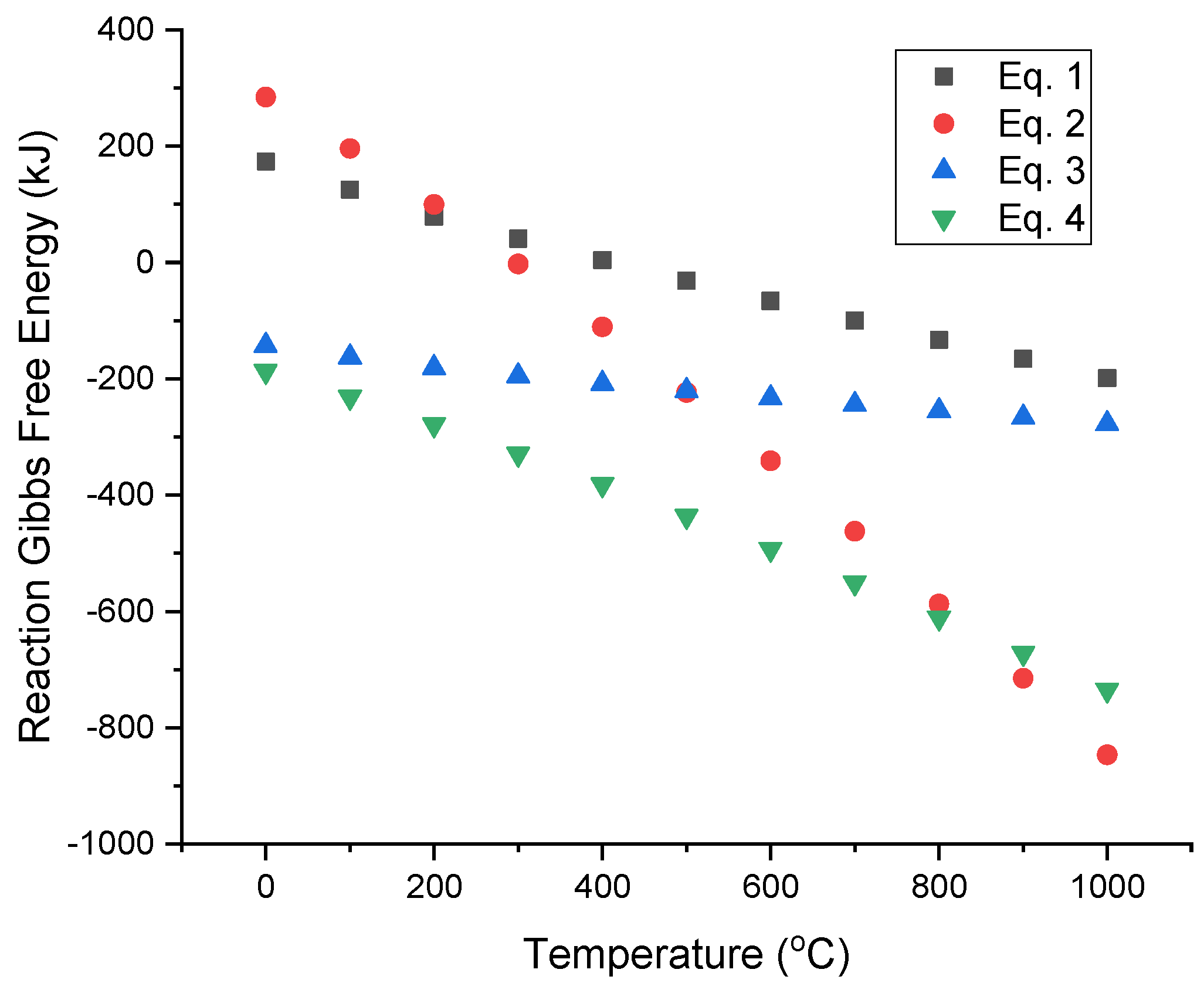
3.1. Thermodynamic Analysis of Ag and Cu Nitrate Salts
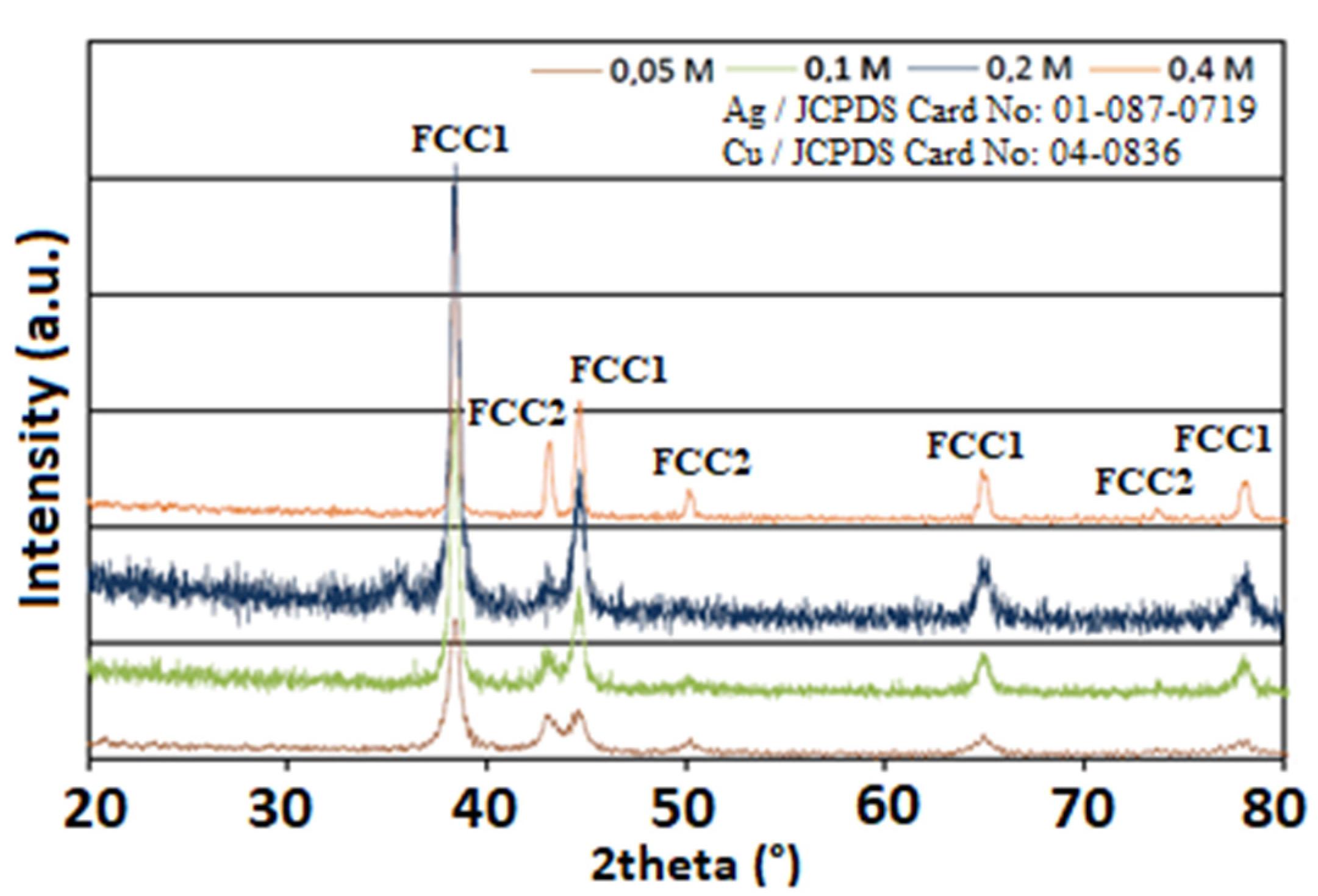
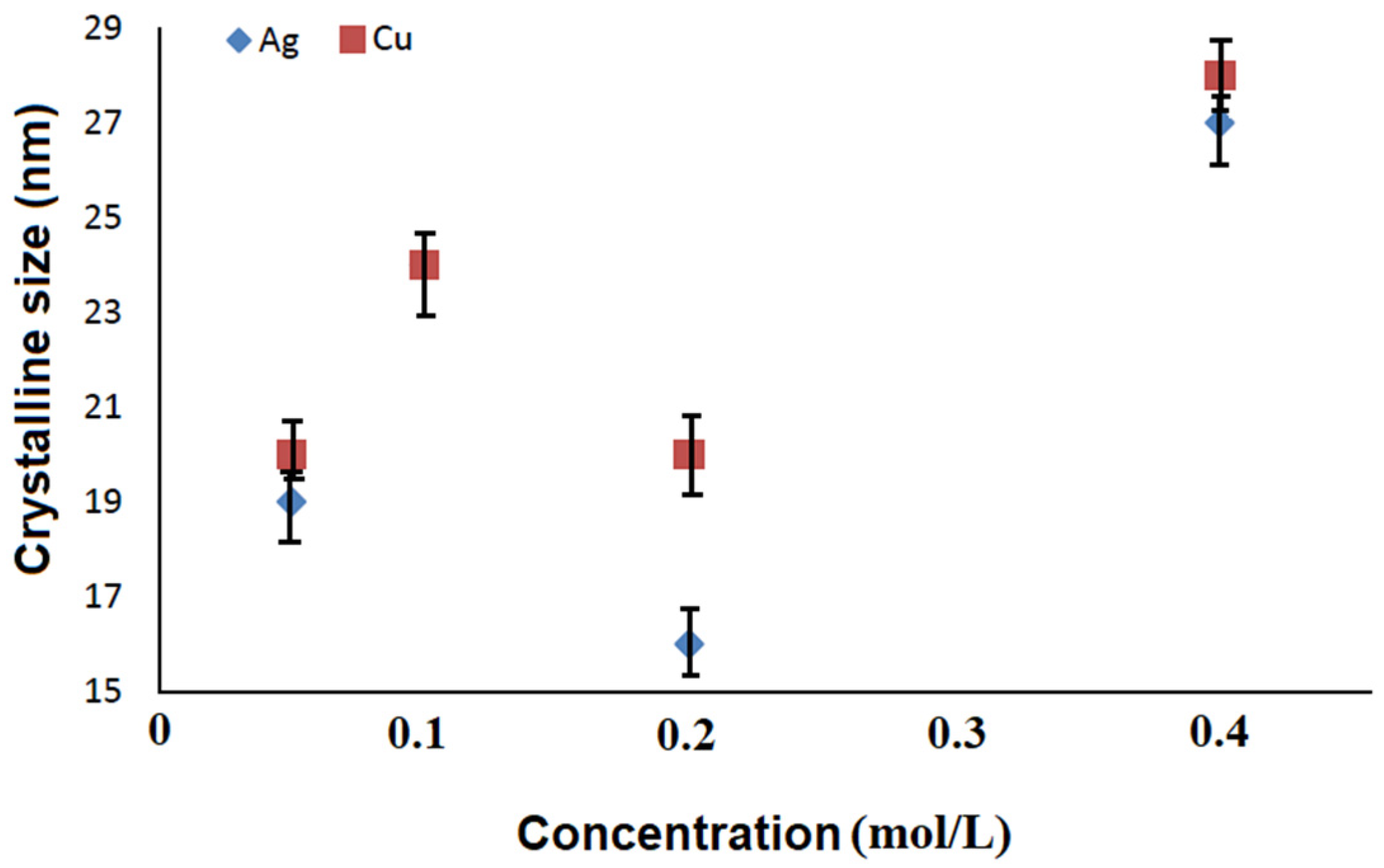
3.2. Structural Characterization of AgCu Particles
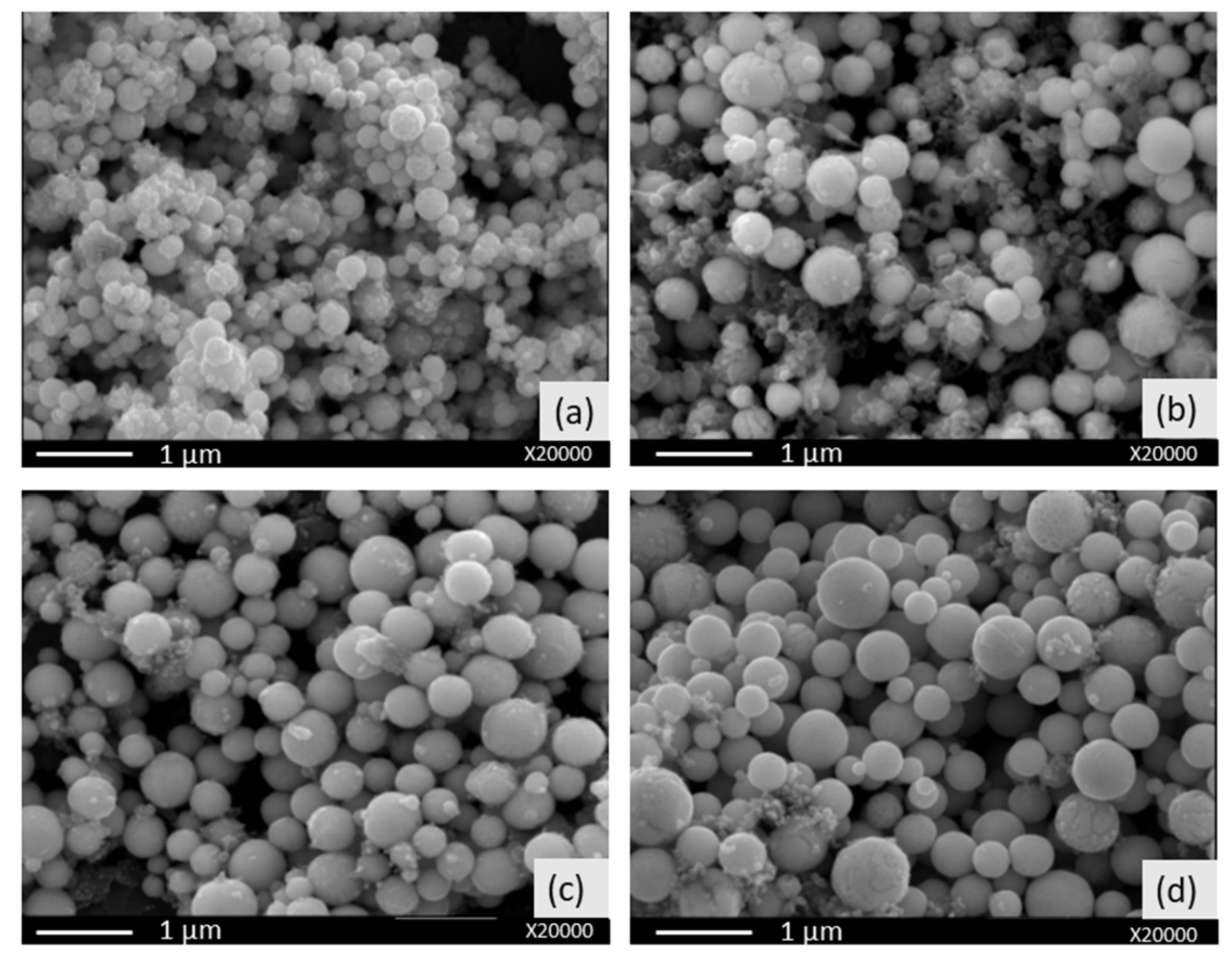
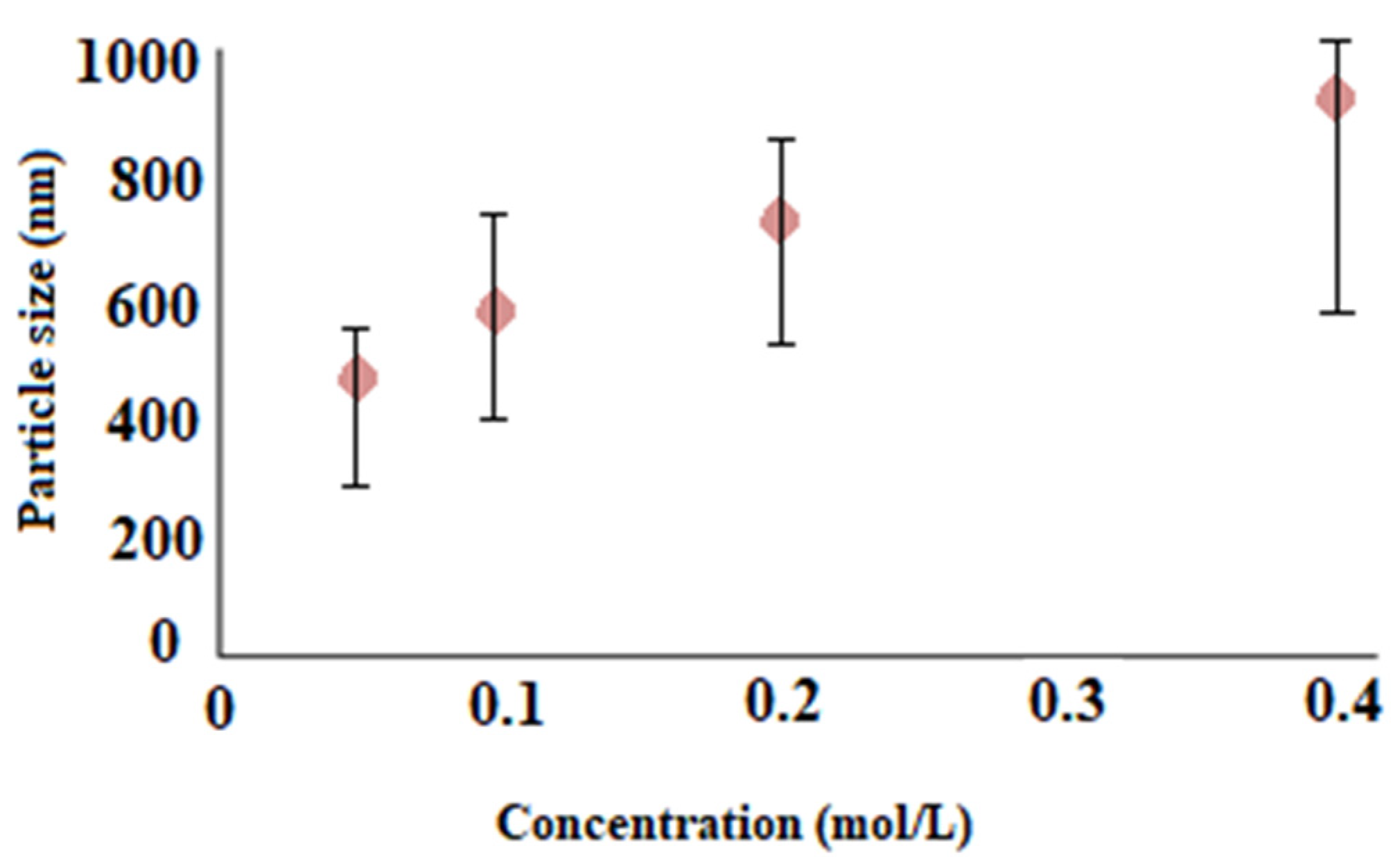
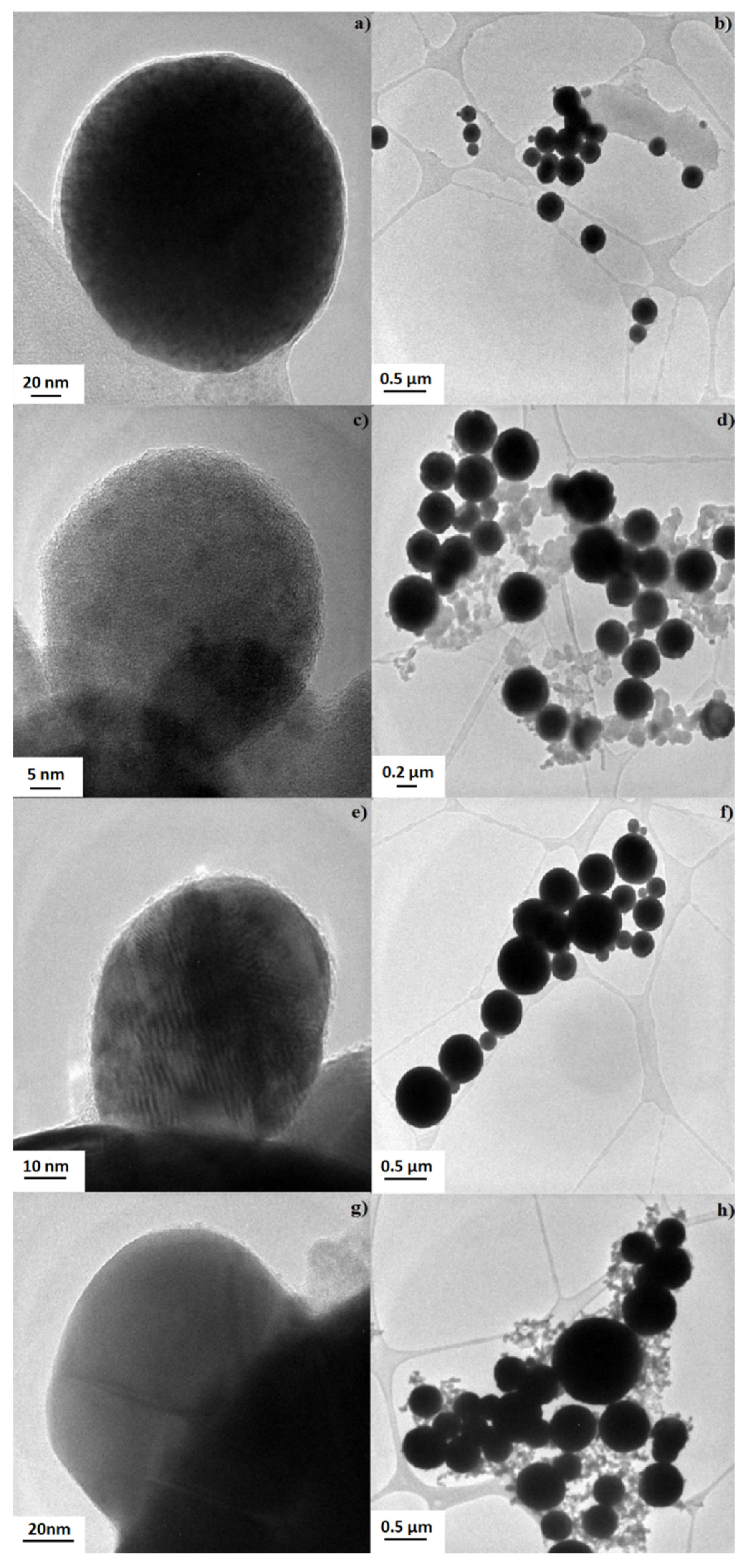
3.3. Morphological Characterization of AgCu Nanocomposite Particles
3.4. Antibacterial Properties of AgCu Nanosized Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laasonen, K.; Panizon, E.; Bochicchio, D.; Ferrando, R. Competition between Icosahedral Motifs in AgCu, AgNi, and AgCo Nanoalloys: A Combined Atomistic−DFT Study. J. Phys. Chem. C 2013, 117, 26405–26413. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Jabbareh, M.A.; Monji, F. Thermodynamic modeling of Ag-Cu nanoalloy phase diagram. Calphad 2018, 60, 208–213. [Google Scholar] [CrossRef]
- Khan, N.A.; Uhl, A.; Shaikhutdinov, S.; Freund, H.J. Alumina supported model Pd–Ag catalysts: A combined STM, XPS, TPD and IRAS study. Surf. Sci. 2006, 600, 1849–1853. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, N.; Bulusu, S.; Zeng, X.C. Effective CO Oxidation on Endohedral Gold-Cage Nanoclusters. J. Phys. Chem. C 2008, 112, 8234–8238. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.H.; Yeo, S.C.; Lee, H.M. Structural stability of AgCu bimetallic nanoparticles and their application as a catalyst: A DFT study. Catal. Today 2012, 185, 94–98. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, S.; Zhang, W.; Fang, J.; Wang, H. The Tunable and Highly Selective Reduction Products on Ag@Cu Bimetallic Catalysts toward CO2 Electrochemical Reduction Reaction. J. Phys. Chem. C 2017, 121, 11368–11379. [Google Scholar] [CrossRef]
- Ciacci, L.; Vassura, I.; Passarini, F. Urban Mines of Copper: Size and Potential for Recycling in the EU. Resources 2017, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Shahcheraghi, S.H.; Schaffie, M.; Ranjbar, M. Development of an electrochemical process for production of nano-copper oxides: Agglomeration kinetics modeling. Ultrason. Sonochem. 2018, 44, 162–170. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Chang, X.; Dong, H.; Li, A.; Hu, C.; Huang, Z.; Zhao, Z.J.; Gong, J. Morphological and Compositional Design of Pd-Cu Bimetallic Nanocatalysts with Controllable Product Selectivity toward CO2 Electroreduction. Nano-Micro Small 2018, 14, 1703314. [Google Scholar] [CrossRef]
- Pajor-Swierzy, A.; Farraj, Y.; Kamyshny, A.; Magdassi, S. Effect of carboxylic acids on conductivity of metallic films formed by inks based on copper@silver core-shell particles. Colloids Surf. A 2017, 522, 320–327. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Saveant, J.M. Catalysis of the Electrochemical Reduction of Carbon Dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Kawashita, M.; Tsuneyama, S.; Miyaji, F.; Kokubo, T.; Kozuka, H.; Yamamoto, K. Antibacterial silver-containing silica glass prepared by sol-gel method. Biomaterialia 2000, 21, 393–398. [Google Scholar] [CrossRef]
- Qin, D.; Yang, G.; Wang, Y.; Zhou, Y.; Zhang, L. Green synthesis of biocompatible trypsin-conjugated Ag nanocomposite with antibacterial activity. Appl. Surf. Sci. 2019, 469, 528–536. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.; Ann, L.C.; Bakhori, S.K.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Cao, G.; Qu, J.; Deng, Y.; Tang, J. Antibacterial activity of Cu2O and Ag co-modified rice grains-like ZnO nanocomposites. J. Mater. Sci. Technol. 2018, 34, 2359–2367. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Raee, M.; Manafi, Z.; Sotoodeh Jahromi, A.; Ghasemi, Y. Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med. Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Mena, X.; Chen, D.; Tang, F.; Jiao, J. Using silver nanoparticle to enhance current response of biosensor. Biosens. Bioelectron. 2005, 21, 433–437. [Google Scholar] [CrossRef]
- Zuo, F.; Zhang, C.; Zhang, H.; Tan, X.; Chen, S.; Yuan, R.A. solid-state electrochemiluminescence biosensor for Con A detection based on CeO2@Ag nanoparticles modified graphene quantum dots as signal probe. Electrochim. Acta 2019, 294, 76–83. [Google Scholar] [CrossRef]
- Amiri, A.; Tang, S.; Steinberger-Wilckens, R.; Tade, M.O. Evaluation of fuel diversity in Solid Oxide Fuel Cell system. Int. J. Hydrogen Energy 2018, 43, 27. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Tae Lee, J.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-Lopez, R.; Carvalho, M.; Pasaoglu, P. The lithium-ion battery: State of the art and future perspective. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Simakin, A.V.; Voronov, V.V.; Shafeev, G.A.; Brayner, R.; Bozon-Verduraz, F. Nanodisks of Au and Ag produced by laser ablation in liquid environment. Chem. Phys. Lett. 2001, 348, 182–186. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yeh, C.S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Sharma, P.; Lotey, G.S.; Singh, S. Solution-combustion: The versatile route to synthesize silver nanoparticles. J. Nanopart. Res. 2011, 13, 2553–2561. [Google Scholar] [CrossRef]
- Yin, B.; Ma, H.; Wang, S.; Chen, S. Electrochemical synthesis of silver nanoparticles under protection of poly(N-vinylpyrrolidone). J. Phys. Chem. B 2003, 107, 8898–8904. [Google Scholar] [CrossRef]
- Ishizaki, T.; Watanabe, R. A New One-Pot Method for the Synthesis of Cu Nanoparticles for Low Temperature Bonding. J. Mater. Chem. 2012, 22, 25198–25206. [Google Scholar] [CrossRef]
- Ouhaibi, A.; Ghamnia, M.; Dahamni, A.; Heresanu, V.; Fauquet, C.; Tonneau, D. The effect of strontium doping on structural and morphological properties of ZnO nanofilms synthesized by ultrasonic spray pyrolysis method. J. Sci. Adv. Mater. Devices 2018, 3, 29–36. [Google Scholar] [CrossRef]
- Qiu, S.; Dong, J.; Chen, G. Preparation of Cu nanoparticle from water-in-oil microemulsions. J. Colloid Interface Sci. 1999, 216, 230. [Google Scholar] [CrossRef]
- Wu, S.H.; Chen, D.H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165. [Google Scholar] [CrossRef]
- Zhu, H.T.; Zhang, C.Y.; Yin, Y.S. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradation. J. Cryst. Growth 2004, 270, 722. [Google Scholar] [CrossRef]
- Stopic, S.; Schroeder, M.; Weirich, T.; Friedrich, B. Synthesis of TiO2 Core/RuO2 Shell Particles using Multistep Ultrasonic Spray Pyrolysis. Mater. Res. Bull. 2013, 48, 3633–3635. [Google Scholar] [CrossRef]
- Jokanovic, V.; Spasic, A.M.; Uskokovic, D. Designing of nanostructured hollow TiO2 spheres obtained by ultrasonic spray pyrolysis. J. Colloid Interface Sci. 2004, 278, 342–352. [Google Scholar] [CrossRef]
- Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Çakmak, T.; Kaya, E.E.; Küçük, D.; Ebin, B.; Balci, O.; Gürmen, S. Novel Strategy for One-Step Production of Attenuated Ag-Containing AgCu/ZnO Antibacterial-Antifungal Nanocomposite Particles. Powder Metall. Met. Ceram. 2020, 59, 261–270. [Google Scholar] [CrossRef]
- Stopic, S.; Dvorak, P.; Friedrich, B. Synthesis of nanopowder of copper by ultrasonic spray pyrolysis method. World Metall. Erzmetall 2005, 58, 191–197. [Google Scholar]
- Stopic, S.; Friedrich, B.; Dvorak, P. Synthesis of nanosized spherical silver powder by ultrasonic spray pyrolysis. Metall 2006, 60, 377–382. [Google Scholar]
- Jankovic, B.; Stopic, S.; Bogovic, J.; Friedrich, B. Kinetic and thermodynamic investigations of non-isothermal decomposition process of a commercial silver nitrate in argon atmosphere used as the precursors for ultrasonic spray pyrolysis USP. Chem. Eng. Process. 2014, 82, 71–87. [Google Scholar] [CrossRef]
- Bogovic, J.; Schwinger, A.; Stopic, S.; Schroeder, J.; Gaukel, V.; Schuhmann, P.; Friedrich, B. Controlled droplet size distribution in ultrasonic spray pyrolysis. Metallurgica 2011, 10, 455–459. [Google Scholar]
- Emil Kaya, E.; Kaya, O.; Alkan, G.; Gürmen, S.; Stopic, S.; Friedrich, B. New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique. Materials 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| AgNO3 (mol/L) | Cu(NO3)2 (mol/L) | Temperature (°C) | H2 Flow Rate (L/min) | N2 Flow Rate (L/min) | Ultrasonic Frequency (MHz) |
|---|---|---|---|---|---|
| 0.05 | 0.05 | 800 | 1.0 | 0.5 | 1.3 |
| 0.1 | 0.1 | 800 | 1.0 | 0.5 | 1.3 |
| 0.2 | 0.2 | 800 | 1.0 | 0.5 | 1.3 |
| 0.4 | 0.4 | 800 | 1.0 | 0.5 | 1.3 |
| Concentration (mol/L) | Element (%) | ||
|---|---|---|---|
| Ag | Cu | O | |
| 0.05 | 32.2 | 46.4 | 21.4 |
| 0.1 | 48.1 | 51.9 | - |
| 0.2 | 52.1 | 47.9 | - |
| 0.4 | 49.1 | 50.9 | - |
| The Sample of Specimen | Bacterial Reduction (%) |
|---|---|
| Untreated reference sample | +140.00 |
| 0.05 mol/L | −100.00 |
| 0.1 mol/L | −100.00 |
| 0.2 mol/L | −100.00 |
| 0.4 mol/L | −100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köroğlu, M.; Ebin, B.; Stopic, S.; Gürmen, S.; Friedrich, B. One Step Production of Silver-Copper (AgCu) Nanoparticles. Metals 2021, 11, 1466. https://doi.org/10.3390/met11091466
Köroğlu M, Ebin B, Stopic S, Gürmen S, Friedrich B. One Step Production of Silver-Copper (AgCu) Nanoparticles. Metals. 2021; 11(9):1466. https://doi.org/10.3390/met11091466
Chicago/Turabian StyleKöroğlu, Münevver, Burçak Ebin, Srecko Stopic, Sebahattin Gürmen, and Bernd Friedrich. 2021. "One Step Production of Silver-Copper (AgCu) Nanoparticles" Metals 11, no. 9: 1466. https://doi.org/10.3390/met11091466








