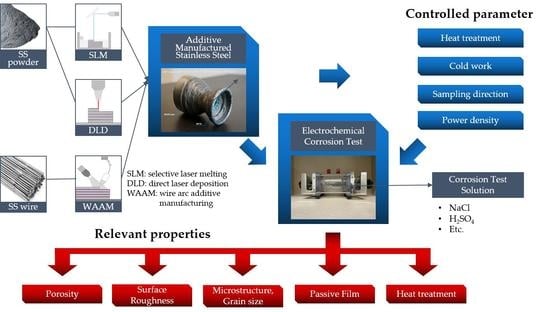
The Corrosion of Stainless Steel Made by Additive Manufacturing: A Review
Abstract
:1. Introduction
2. AM Process Review
2.1. Powder Bed Fusion
2.2. Direct Energy Deposition
3. Corrosion Behaviors Depending on the AM Process, the SS Type, and the Corrosion Environment
3.1. Corrosion Behaviors of SLM SS
3.1.1. Corrosion Behaviors of SLM 316L SS
Corrosion Behaviors of SLM 316 SS in NaCl Solutions
Corrosion Behaviors of SLM 316 SS in Miscellaneous Solutions
3.1.2. Corrosion Behaviors of the SLM Miscellaneous Type SS
Corrosion Behaviors of the SLM Miscellaneous Type SS in NaCl Solutions
Corrosion Behaviors of the SLM Miscellaneous Type SS in Miscellaneous Solutions
3.2. Corrosion Behaviors of the DLD SS
3.2.1. Corrosion Behaviors of the DLD 316L SS
3.2.2. Corrosion Behaviors of the DLD Miscellaneous Type SS
3.3. Corrosion Behaviors of the WAAM SS
3.4. Corrosion Behaviors of the SS Fabricated by Miscellaneous AM Processes
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel. JOM 2016, 68, 820–859. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Moyle, M.; Primig, S. Additive manufacturing of steels: A review of achievements and challenges. J. Mater. Sci. 2021, 56, 64–107. [Google Scholar] [CrossRef]
- Shahriari, A.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Amirkhiz, B.S.; Mohammadi, M. Microstructure and corrosion behavior of a novel additively manufactured maraging stainless steel. Electrochim. Acta 2020, 339, 135925. [Google Scholar] [CrossRef]
- Fayazfar, H.; Salarian, M.; Rogalsky, A.; Sarker, D.; Russo, P.; Paserin, V.; Toyserkani, E. A critical review of powder-based additive manufacturing of ferrous alloys: Process parameters, microstructure and mechanical properties. Mater. Des. 2018, 144, 98–128. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Sandeep, D.C.; Chhabra, D. Comparison and analysis of different 3d printing techniques. Int. J. Latest Trends Eng. Technol. 2017, 8, 264–272. [Google Scholar]
- Chen, Y.T.; Zhang, X.C.; Parvez, M.M.; Liou, F. A review on metallic alloys fabrication using elemental powder blends by laser powder directed energy deposition process. Materials 2020, 13, 3562. [Google Scholar] [CrossRef]
- Fang, J.X.; Dong, S.Y.; Wang, Y.J.; Xu, B.S.; Zhang, Z.H.; Xia, D.; Ren, W.B.; He, P. Microstructure and properties of an as-deposited and heat treated martensitic stainless steel fabricated by direct laser deposition. J. Manuf. Process. 2017, 25, 402–410. [Google Scholar] [CrossRef]
- Tabernero, I.; Paskual, T.; Álvarez, P.; Suárez, A. study on arc welding processes for high deposition rate additive manufacturing. Procedia CIRP 2018, 68, 358–362. [Google Scholar]
- Qi, L.H.; Hao, Y.; Luo, J.; Zhang, D.C.; Shen, H. Embedded printing trace planning for aluminum droplets depositing on dissolvable supports with varying section. Robot. Comput. Integr. Manuf. 2020, 63, 101898. [Google Scholar] [CrossRef]
- Yi, H.; Qi, L.H.; Luo, J.; Zhang, D.C.; Li, N. Direct fabrication of metal tubes with high-quality inner surfaces via droplet deposition over soluble cores. J. Mater. Process. Technol. 2019, 264, 145–154. [Google Scholar] [CrossRef]
- Yi, H.; Qi, L.H.; Luo, J.; Zhang, D.C.; Li, H.J.; Hou, X.H. Effect of the surface morphology of solidified droplet on remelting between neighboring aluminum droplets. Int. J. Mach. Tools Manuf. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Visser, C.W.; Pohl, R.; Sun, C.; Roemer, G.W.; in ’t Veld, B.H.; Lohse, D. Toward 3D printing of pure metals by laser-induced forward transfer. Adv. Mater. 2015, 27, 4087–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Moroz, A.; Alrbaey, K. Sliding wear characteristics and corrosion behaviour of selective laser melted 316L stainless steel. J. Mater. Eng. Perform 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On the corrosion and metastable pitting characteristics of 316L stainless steel produced by selective laser melting. J. Electrochem. Soc. 2017, 164, C250–C257. [Google Scholar] [CrossRef]
- Murkute, P.; Pasebani, S.; Isgor, O.B. Production of corrosion-resistant 316L stainless steel clads on carbon steel using powder bed fusion-selective laser melting. J. Mater. Process. Technol. 2019, 273, 116243. [Google Scholar] [CrossRef]
- Lin, K.J.; Gu, D.D.; Xi, L.X.; Yuan, L.H.; Niu, S.Q.; Lv, P.; Ge, Q. Selective laser melting processing of 316L stainless steel: Effect of microstructural differences along building direction on corrosion behavior. Int. J. Adv. Manuf. Technol. 2019, 104, 2669–2679. [Google Scholar] [CrossRef]
- Zaharia, S.M.; Lancea, C.; Chicos, L.A.; Pop, M.A.; Caputo, G.; Serra, E. Mechanical properties and corrosion behaviour of 316l stainless steel honeycomb cellular cores manufactured by selective laser melting. Trans. Famena 2017, 41, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Guo, S.M. Electrochemical behavior of AISI316L stainless steel parts produced by laser-based powder bed fusion process and the effect of post annealing process. Addit. Manuf. 2018, 22, 153–156. [Google Scholar]
- Yusuf, S.M.; Nie, M.Y.; Chen, Y.; Yang, S.F.; Gao, N. Microstructure and corrosion performance of 316L stainless steel fabricated by Selective Laser Melting and processed through high-pressure torsion. J. Alloy. Compd. 2018, 763, 360–375. [Google Scholar] [CrossRef]
- Sun, S.H.; Ishimoto, T.; Hagihara, K.; Tsutsumi, Y.; Hanawa, T.; Nakano, T. Excellent mechanical and corrosion properties of austenitic stainless steel with a unique crystallographic lamellar microstructure via selective laser melting. Scr. Mater. 2019, 159, 89–93. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, J.; Bai, P.K.; Qu, H.Q.; Liang, M.J.; Liao, H.H.; Wu, L.Y.; Huo, P.C.; Liu, H.; Zhang, J.X. Microstructure and mechanical properties of TiC-Reinforced 316L stainless steel composites fabricated using selective laser melting. Materials 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Laleh, M.; Hughes, A.E.; Xu, W.; Gibson, I.; Tan, M.Y. Unexpected erosion-corrosion behaviour of 316L stainless steel produced by selective laser melting. Corrosion Sci. 2019, 155, 67–74. [Google Scholar] [CrossRef]
- Duan, Z.W.; Man, C.; Dong, C.F.; Cui, Z.Y.; Kong, D.C.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corrosion Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Geenen, K.; Rottger, A.; Theisen, W. Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater. Corros. 2017, 68, 764–775. [Google Scholar] [CrossRef]
- Lou, X.; Othon, M.A.; Rebak, R.B. Corrosion fatigue crack growth of laser additively-manufactured 316L stainless steel in high temperature water. Corrosion Sci. 2017, 127, 120–130. [Google Scholar] [CrossRef]
- Lou, X.; Song, M.; Emigh, P.W.; Othon, M.A.; Andresen, P.L. On the stress corrosion crack growth behaviour in high temperature water of 316L stainless steel made by laser powder bed fusion additive manufacturing. Corrosion Sci. 2017, 128, 140–153. [Google Scholar] [CrossRef]
- Lou, X.; Andresen, P.L.; Rebak, R.B. Oxide inclusions in laser additive manufactured stainless steel and their effects on impact toughness and stress corrosion cracking behavior. J. Nucl. Mater. 2018, 499, 182–190. [Google Scholar] [CrossRef]
- Prieto, C.; Singer, M.; Cyders, T.; Young, D. Investigation of pitting corrosion initiation and propagation of a type 316L stainless steel manufactured by the direct metal laser sintering process. Corrosion 2019, 75, 140–143. [Google Scholar] [CrossRef]
- Stašić, J.; Božić, D. Densification behavior of 316L-NiB stainless steel powder and surface morphology during selective laser melting process using pulsed Nd: YAG laser. Rapid Prototyping J. 2019, 25, 47–54. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Romlay, F.R.M.; Sharif, S.; Jan, N.H.M.; Tsumori, F. Surface characterisation and corrosion behaviour of oxide layer for SLMed-316L stainless steel. J. Alloy. Compd. 2018, 748, 1044–1052. [Google Scholar] [CrossRef]
- Kong, D.C.; Ni, X.Q.; Dong, C.F.; Lei, X.W.; Zhang, L.; Man, C.; Yao, J.Z.; Cheng, X.Q.; Li, X.G. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Kong, D.C.; Ni, X.Q.; Dong, C.F.; Lei, X.W.; Zhang, L.; Man, C.; Yao, J.Z.; Xiao, K.; Li, X.G. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Yang, G.Q.; Yu, S.L.; Mo, J.K.; Kang, Z.Y.; Dohrmann, Y.; List, F.A.; Green, J.B.; Babu, S.S.; Zhang, F.Y. Bipolar plate development with additive manufacturing and protective coating for durable and high-efficiency hydrogen production. J. Power Sources 2018, 396, 590–598. [Google Scholar] [CrossRef]
- Lyczkowska-Widlak, E.; Lochynski, P.; Nawrat, G.; Chlebus, E. Comparison of electropolished 316L steel samples manufactured by SLM and traditional technology. Rapid Prototyping J. 2019, 25, 566–580. [Google Scholar] [CrossRef]
- Chen, W.; Yin, G.F.; Huang, Z.B.; Feng, Z. Effect of the particle size of 316L stainless steel on the corrosion characteristics of the steel fabricated by selective laser melting. Int. J. Electrochem. Sci. 2018, 13, 10217–10232. [Google Scholar] [CrossRef]
- Song, M.; Wang, M.; Lou, X.Y.; Rebak, R.B.; Was, G.S. Radiation damage and irradiation-assisted stress corrosion cracking of additively manufactured 316L stainless steels. J. Nucl. Mater. 2019, 513, 33–44. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Haghdadi, N.; Wang, K.; Cizek, P.; Gibson, I.; Tan, M.Y. On the unusual intergranular corrosion resistance of 316L stainless steel additively manufactured by selective laser melting. Corrosion Sci. 2019, 161, 108189. [Google Scholar] [CrossRef]
- Quan, J.F.; Lin, K.J.; Gu, D.D. Selective laser melting of silver submicron powder modified 316L stainless steel: Influence of silver addition on microstructures and performances. Powder Technol. 2020, 364, 478–483. [Google Scholar] [CrossRef]
- Wozniak, A.; Adamiak, M.; Chladek, G.; Kasperski, J. The influence of the process parameters on the microstructure and properties slm processed 316 l stainless steel. Arch. Metall. Mater. 2020, 65, 73–80. [Google Scholar]
- Schaller, R.F.; Taylor, J.M.; Rodelas, J.; Schindelholz, E.J. Corrosion properties of powder bed fusion additively manufactured 17-4 ph stainless steel. Corrosion 2017, 73, 796–807. [Google Scholar] [CrossRef]
- Stoudt, M.R.; Ricker, R.E.; Lass, E.A.; Levine, L.E. Influence of postbuild microstructure on the electrochemical behavior of additively manufactured 17-4 PH stainless steel. JOM 2017, 69, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnajjar, M.; Christien, F.; Barnier, V.; Bosch, C.; Wolski, K.; Fortes, A.D.; Telling, M. Influence of microstructure and manganese sulfides on corrosion resistance of selective laser melted 17-4 PH stainless steel in acidic chloride medium. Corrosion Sci. 2020, 168, 108585. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.F.; Man, C.; Kong, D.C.; Xiao, K.; Li, X.G. Enhancing the corrosion resistance of selective laser melted 15-5PH martensite stainless steel via heat treatment. Corrosion Sci. 2020, 166, 108427. [Google Scholar] [CrossRef]
- Nath, S.D.; Irrinki, H.; Gupta, G.; Kearns, M.; Gulsoy, O.; Atre, S. Microstructure-property relationships of 420 stainless steel fabricated for by laser-powder bed fusion. Powder Technol. 2019, 343, 738–746. [Google Scholar] [CrossRef]
- Pateras, A.; Brandt, M.; Song, M.; Chen, X.-B.; Easton, M.; Yagodzinskyy, Y.; Virkkunen, I.; Hänninen, H.; Papula, S. Selective laser melting of duplex stainless steel 2205: Effect of post-processing heat treatment on microstructure, mechanical properties, and corrosion resistance. Materials 2019, 12, 2468. [Google Scholar]
- Shang, F.; Chen, X.Q.; Wang, Z.Y.; Ji, Z.C.; Ming, F.; Ren, S.B.; Qu, X.H. The microstructure, mechanical properties, and corrosion resistance of UNS S32707 hyper-duplex stainless steel processed by selective laser melting. Metal 2019, 9, 1012. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wen, S.F.; Duan, L.C.; Wang, C.; Chen, K.Y.; Wei, Q.S.; Zhou, Y.; Shi, Y.S. Enhanced corrosion behavior of selective laser melting S136 mould steel reinforced with nano-TiB2. Opt. Laser Technol. 2019, 119, 105588. [Google Scholar] [CrossRef]
- Zeng, G.H.; Song, T.; Dai, Y.H.; Tang, H.P.; Yan, M. 3D printed breathable mould steel: Small micrometer-sized, interconnected pores by creatively introducing foaming agent to additive manufacturing. Mater. Des. 2019, 169, 107693. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, Y.; Li, F.Z.; Huang, W.P.; Jing, G.Y.; Wang, Z.M.; Zeng, X.Y. Weldability, microstructure and mechanical properties of laser-welded selective laser melted 304 stainless steel joints. J. Mater. Sci. Technol. 2019, 35, 1817–1824. [Google Scholar] [CrossRef]
- Schaller, R.F.; Mishra, A.; Rodelas, J.M.; Taylor, J.M.; Schindelholz, E.J. The role of microstructure and surface finish on the corrosion of selective laser melted 304L. J. Electrochem. Soc. 2018, 165, C234–C242. [Google Scholar] [CrossRef]
- Miller, J.T.; Cudjoe, E.; Martin, H.J. Comparison of the effects of a sulfuric acid environment on traditionally manufactured and additive manufactured stainless steel 316L alloy. Addit. Manuf. 2018, 23, 272–286. [Google Scholar] [CrossRef]
- Schmidt, D.P.; Jelis, E.; Clemente, M.P.; Ravindra, N.M. Corrosion of 3D printed steel. In Proceedings of the Materials Science and Technology Conference and Exhibition, Columbus, OH, USA, 4–8 October 2015. [Google Scholar]
- Carluccio, D.; Bermingham, M.; Kent, D.; Demir, A.G.; Previtali, B.; Dargusch, M.S. Comparative study of pure iron manufactured by selective laser melting, laser metal deposition, and casting processes. Adv. Eng. Mater. 2019, 21, 7. [Google Scholar] [CrossRef]
- Sander, J.; Kochta, F.; Pilz, S.; Voss, A.; Kuhn, U.; Gebert, A.; Hufenbach, J. Effect of selective laser melting on microstructure, mechanical, and corrosion properties of biodegradable FeMnCS for implant applications. Adv. Eng. Mater. 2020, 22, 10. [Google Scholar]
- Peters, K.; Taube, A.; Keller, A.; Hoyer, K.P.; Niendorf, T.; Grundmeier, G.; Wiesener, M. Corrosion properties of bioresorbable FeMn-Ag alloys prepared by selective laser melting. Mater. Corros. 2017, 68, 1028–1036. [Google Scholar]
- Zietala, M.; Durejko, T.; Polanski, M.; Kunce, I.; Plocinski, T.; Zielinski, W.; Lazinska, M.; Stepniowski, W.; Czujko, T.; Kurzydlowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316 L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- Stull, J.A.; Hill, M.A.; Lienert, T.J.; Tokash, J.; Bohn, K.R.; Hooks, D.E. Corrosion characteristics of laser-engineered net shaping additively-manufactured 316L stainless steel. JOM 2018, 70, 2677–2683. [Google Scholar] [CrossRef]
- Jung, G.S.; Park, Y.H.; Kim, D.J.; Lim, C.S. Study on corrosion properties of additive manufactured 316L stainless steel and alloy 625 in seawater. Corros. Sci. Tech. 2019, 18, 258–266. [Google Scholar]
- Lefky, C.S.; Zucker, B.; Nassar, A.R.; Simpson, T.W.; Hildreth, O.J. Impact of compositional gradients on selectivity of dissolvable support structures for directed energy deposited metals. Acta Mater. 2018, 153, 1–7. [Google Scholar] [CrossRef]
- Melia, M.A.; Nguyen, H.-D.A.; Rodelas, J.M.; Schindelholz, E.J. Corrosion properties of 304L stainless steel made by directed energy deposition additive manufacturing. Corros. Sci. 2019, 152, 20–30. [Google Scholar] [CrossRef]
- Fang, J.X.; Dong, S.Y.; Li, S.B.; Wang, Y.J.; Xu, B.S.; Li, J.; Li, B.; Jiang, Y.L. Direct laser deposition as repair technology for a low transformation temperature alloy: Microstructure, residual stress, and properties. Mater. Sci. Eng. A 2019, 748, 119–127. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.H.; Wang, Q.; Wu, C.L.; Chen, J.; Abdullah, A.O.; Zhang, S. Effect of Ni content on stainless steel fabricated by laser melting deposition. Opt. Laser Technol. 2018, 101, 363–371. [Google Scholar] [CrossRef]
- Hao, Z.Z.; Ao, S.S.; Cai, Y.C.; Zhang, W.; Luo, Z. Formation of SUS304/aluminum alloys using wire and arc additive manufacturing. Metals 2018, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Ron, T.; Levy, G.K.; Dolev, O.; Leon, A.; Shirizly, A.; Aghion, E. Environmental behavior of low carbon steel produced by a wire arc additive manufacturing process. Metals 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, P.; Giri, R.; Kaul, R.; Sankar, P.R.; Tiwari, P.; Atulkar, A.; Porwal, R.K.; Dayal, R.K.; Kukrej, L.M. Studies on pitting corrosion and sensitization in laser rapid manufactured specimens of type 316L stainless steel. Mater. Des. 2012, 39, 509–521. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, J.; Cheng, X.; Wang, H.M.; Huang, Z. Effect of heat treatment on microstructure, mechanical and corrosion properties of austenitic stainless steel 316L using arc additive manufacturing. Mater. Sci. Eng. A 2018, 715, 307–314. [Google Scholar] [CrossRef]
- Zhang, P.R.; Liu, Z.Q. Physical-mechanical and electrochemical corrosion behaviors of additively manufactured Cr-Ni-based stainless steel formed by laser cladding. Mater. Des. 2016, 100, 254–262. [Google Scholar] [CrossRef]
- Tarasov, S.Y.; Filippov, A.V.; Shamarin, N.N.; Fortuna, S.V.; Maier, G.G.; Kolubaev, E.A. Microstructural evolution and chemical corrosion of electron beam wire-feed additively manufactured AISI 304 stainless steel. J. Alloy Compd. 2019, 803, 364–370. [Google Scholar] [CrossRef]
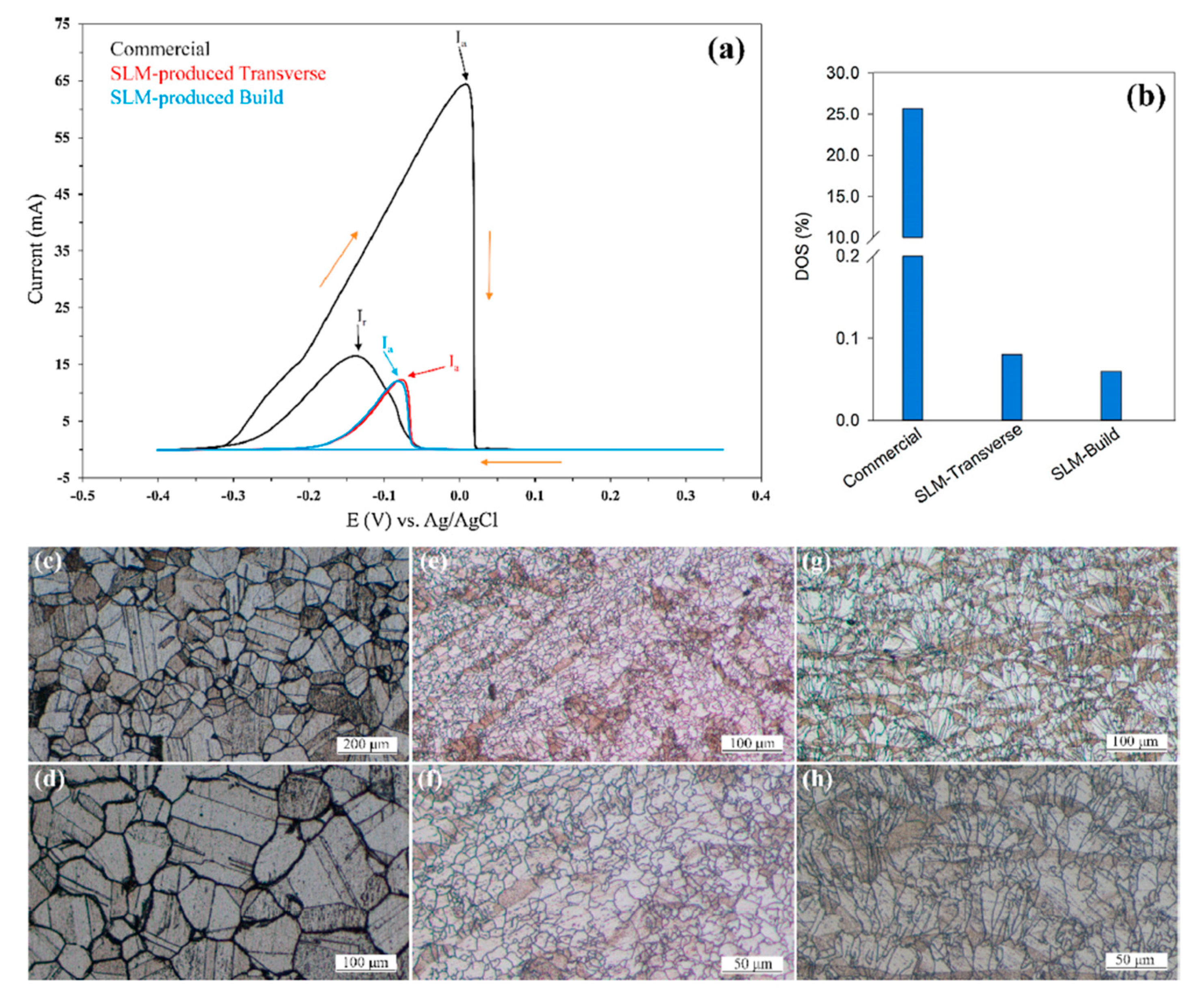
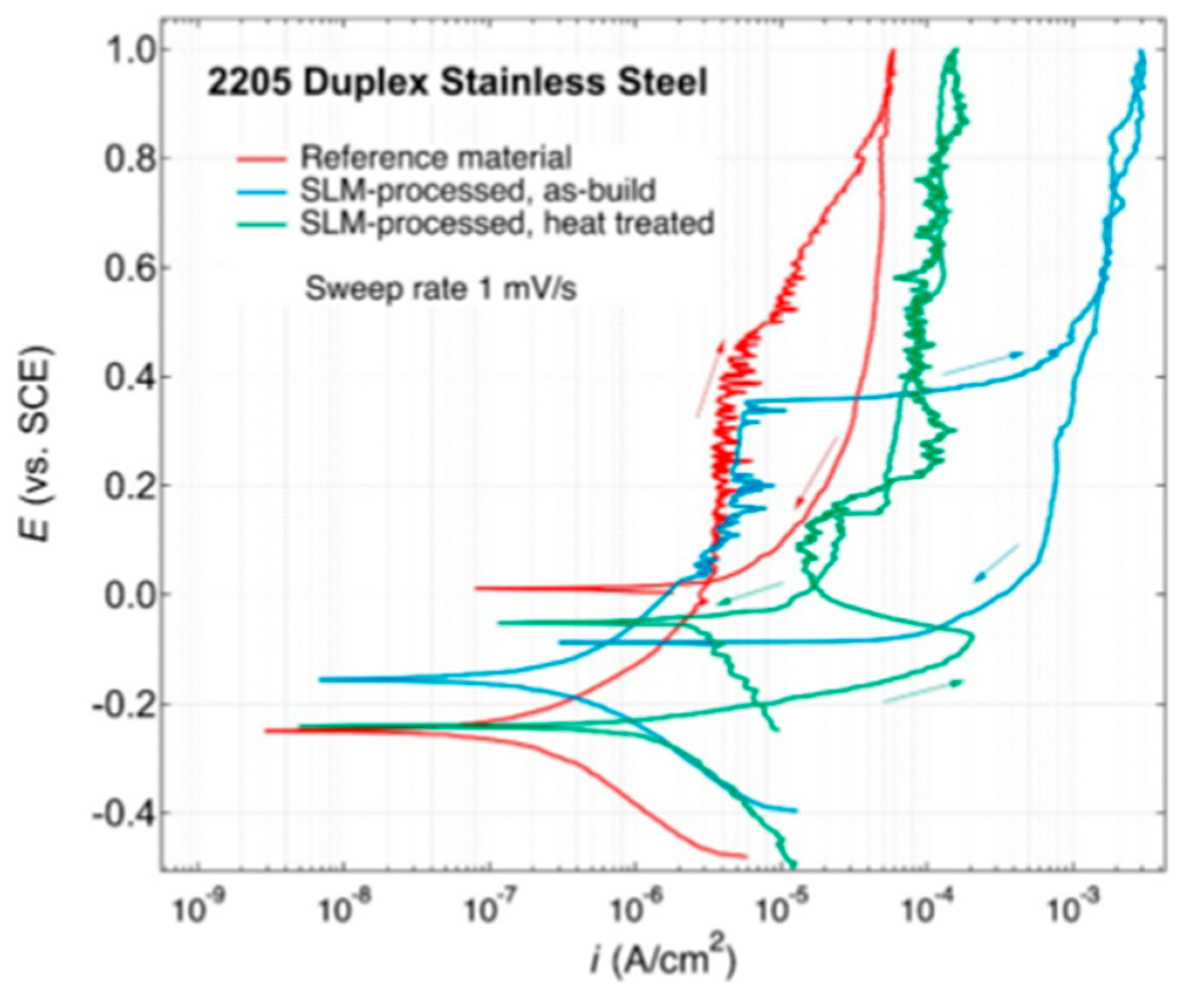
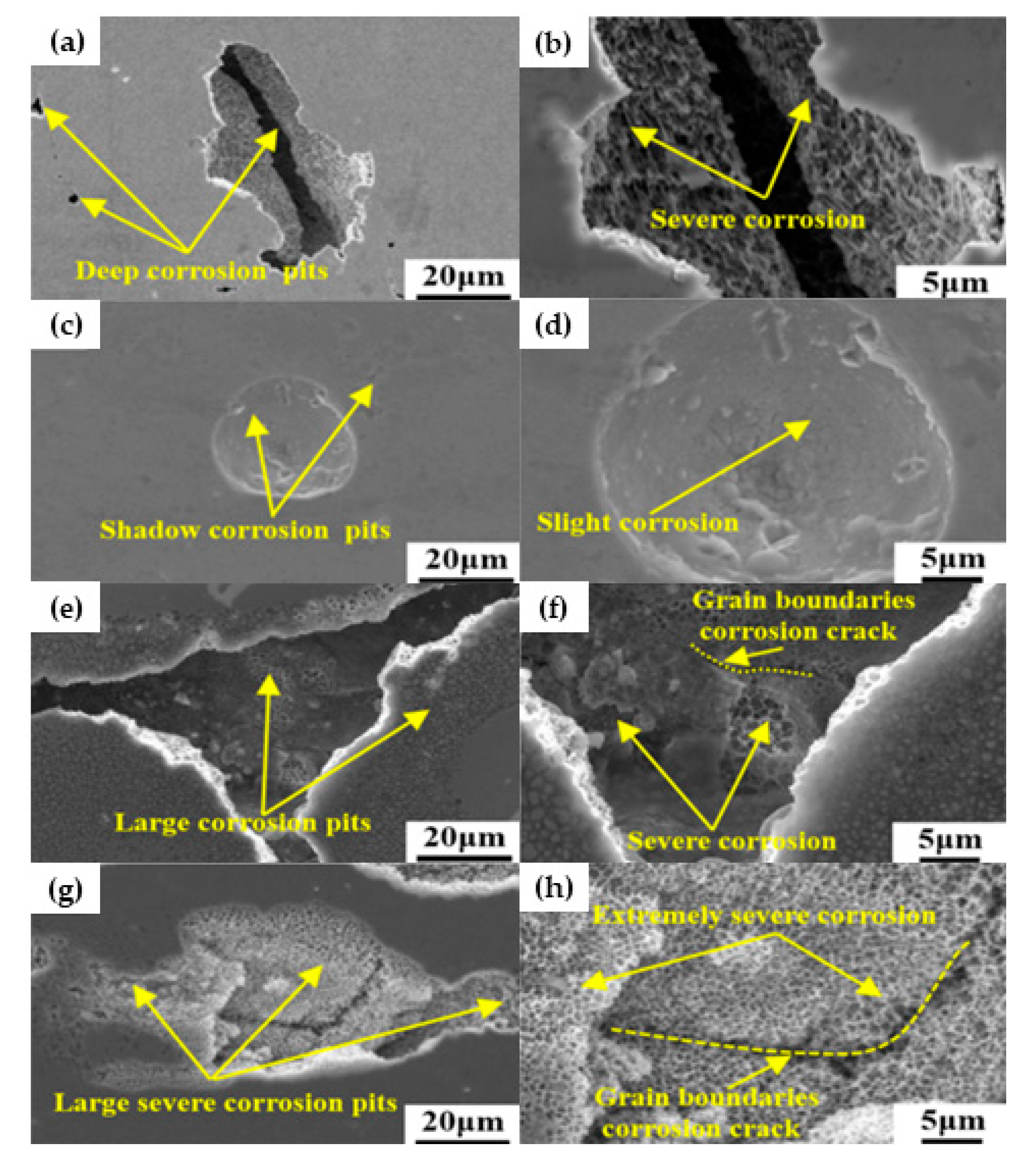

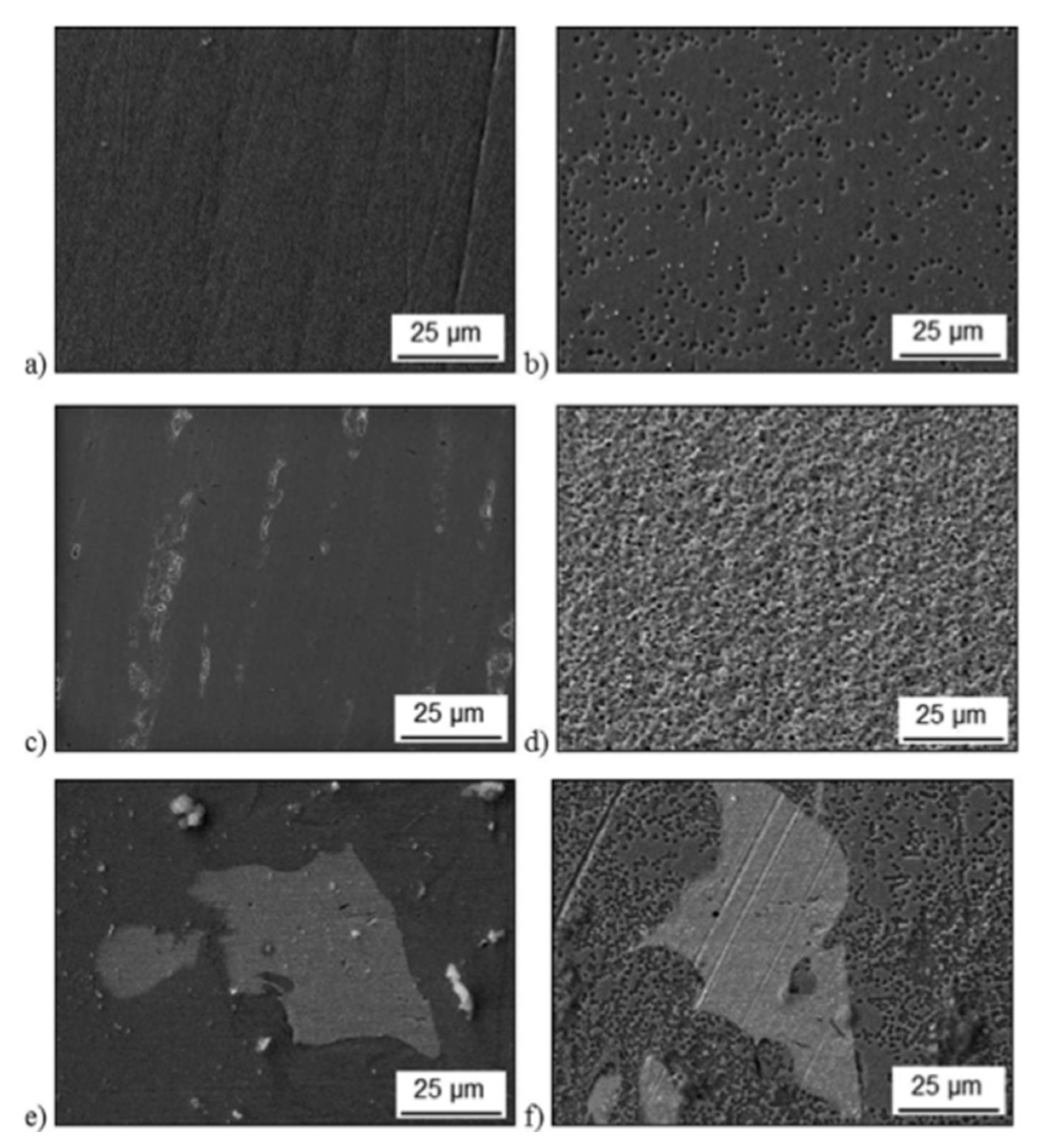



| AM Process | SS Type | Controlled Parameter | Relevant Properties | Corrosion Characterization | Ref. Num. |
|---|---|---|---|---|---|
| SLM | 316L | scan speed, laser powers | - | PDP, potentiostatic test | [15] |
| - | - | scan speed | - | PDP, OCP, EIS, linear polarization resistance | [16] |
| - | - | scan speed | closely-packed crystal planes (111), grain size | PDP | [17] |
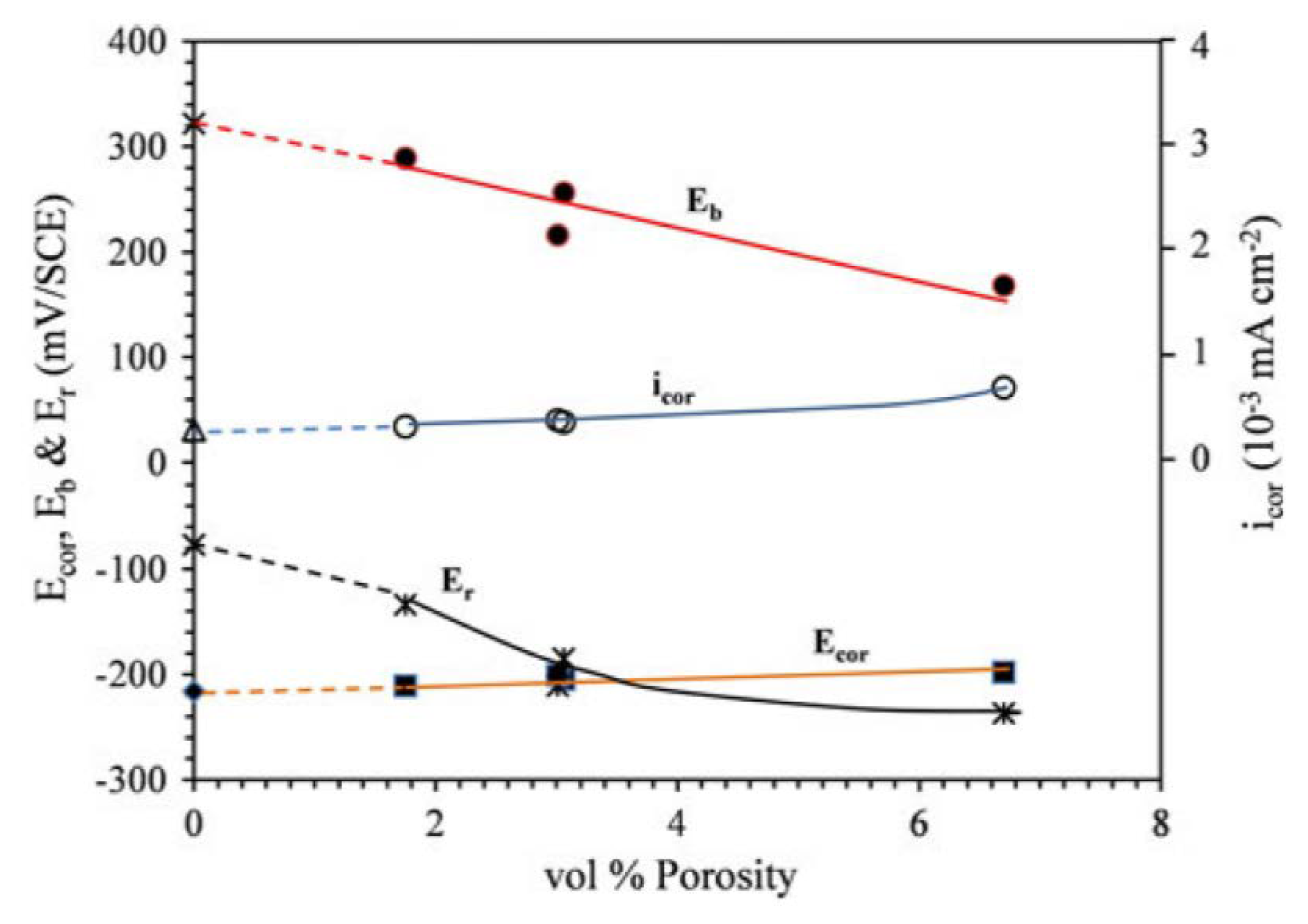
| - | - | scan speed | porosity | PDP | [14] |
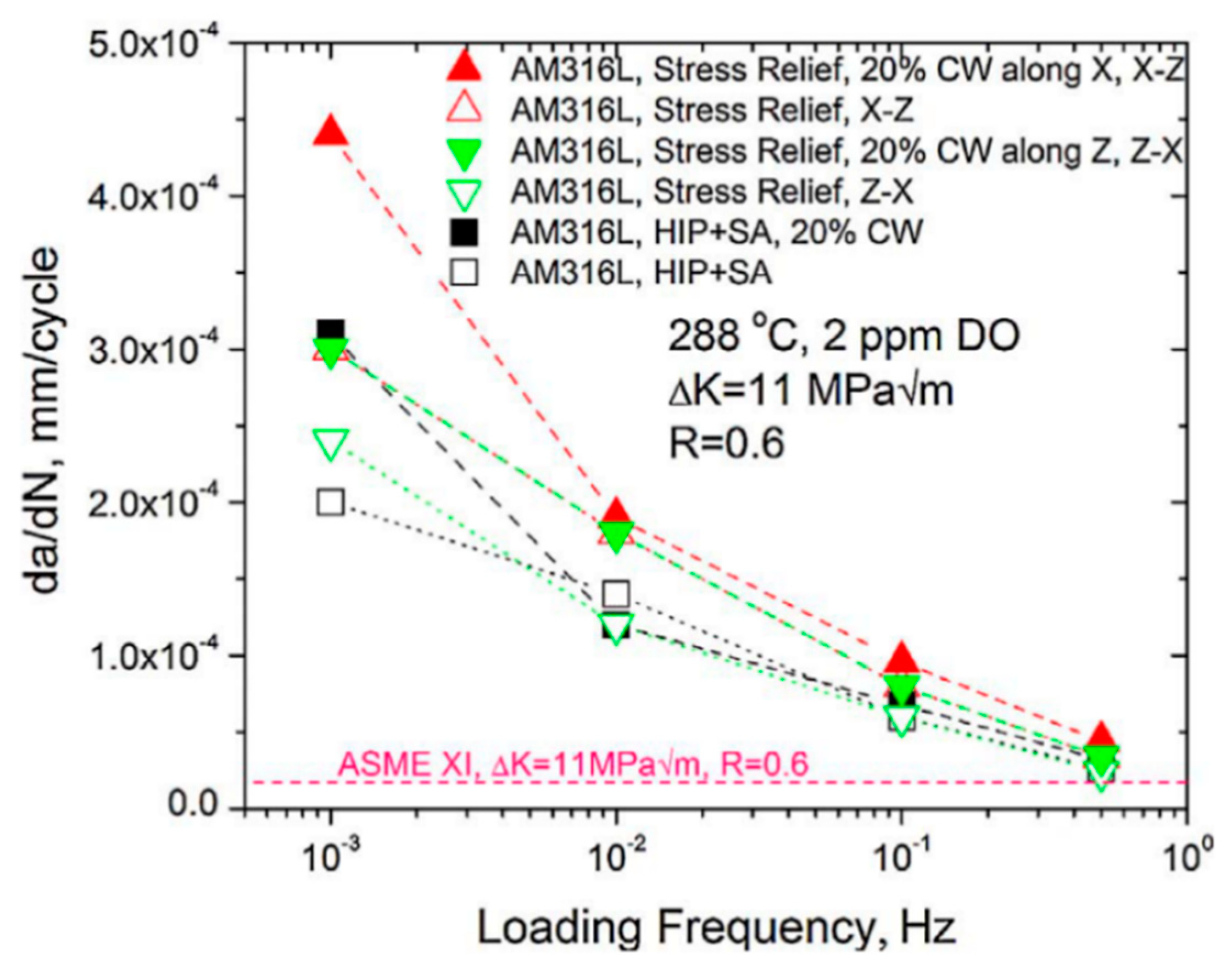
| - | - | heat treatments, cold work | - | corrosion fatigue crack growth tests | [27] |
| - | - | heat treatments | residual stress | PDP, EIS | [20] |
| - | - | heat treatments(thermal oxidized) | oxide composition | PDP | [32] |
| - | - | heat treatments | oxide composition | PDP | [34] |
| - | - | heat treatments | pit shape, corrosion damage pattern | PDP | [30] |
| - | - | cold work | porosity | PDP | [28] |
| - | - | HIP | - | SCC | [38] |
| - | - | HIP | porosity, si-rich oxide, grain size | PDP, OCP | [26] |
| - | - | laser powers | - | PDP, EIS | [33] |
| - | - | - | PDP, weight loss | [37] | |
| - | - | laser energy density | - | PDP | [41] |
| - | - | HPT plastic defarmation | cellular structure and voids | PDP, OCP, EIS | [21] |
| - | - | add TiC | - | PDP | [23] |
| - | - | Au coating | - | PDP, EIS | [35] |
| - | - | electropolising | surface roughness | PDP | [36] |
| - | - | NiB | - | weight loss | [31] |
| - | - | add Ag powder | pores | PDP | [40] |
| - | - | - | Non-equilibrium microstructure | PDP | [1] |
| - | - | - | Si-rich oxide | SCC | [29] |
| - | - | - | MnS content and presence of Cr deletion zones | PDP | [19] |
| - | - | - | lamellar microstructure | PDP | [22] |
| - | - | - | pores | PDP | [24] |
| SLM | 17-4 PH | heat treatment | microstructure, precipitates, passive film | PDP | [43] |
| - | - | heat treatment | MnS inclusion | PDP, OCP | [44] |
| - | - | - | pore | PDP, OCP | [42] |
| - | 420 | heat treatment | - | PDP | [46] |
| - | 2205 duplex | heat treatment | - | PDP | [47] |
| - | 15-5PH SS | solution treatment, aging treatment | passive film | PDP, OCP, EIS, potentiostatic pulse test | [45] |
| - | UNS S32707 hyper-duplex | solution treatment | passive film | PDP | [48] |
| - | S136 | add TiB2 | passive film, grain size, porosity | PDP, OCP, weight loss | [49] |
| - | AISI 420 SS | add CrNx | distribution of Cr | PDP | [50] |
| - | SS CX | sampling direction | microstructural, passive film | PDP, EIS | [3] |
| - | 304 | welded directions | - | PDP, OCP | [51] |
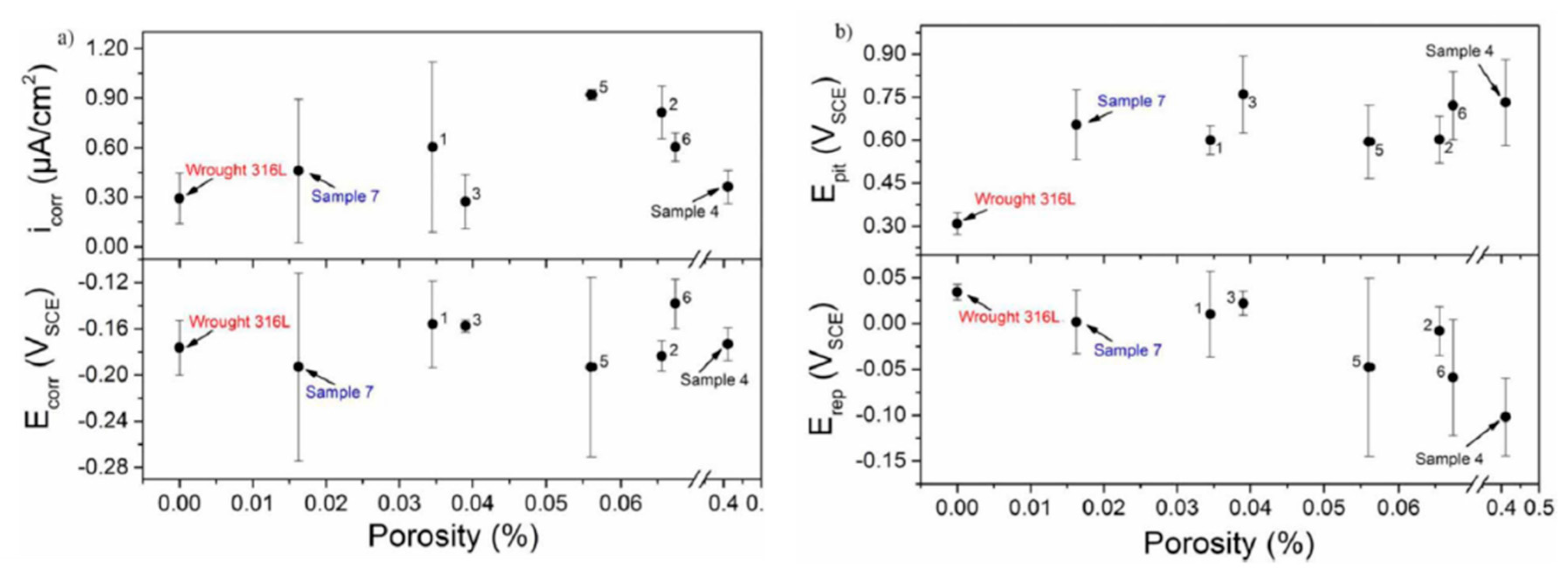
| - | 304L | surface finish | surface roughness, embedded particles | immersion test, PDP | [52] |
| - | AISI 4340 | printing parameters | porosity | PDP, EIS | [54] |
| DLD | 316L | heat treatment | - | PDP, single loop-EPR | [59] |
| - | heat treatment | passive film | CPT, CCT, PDP | [60] | |
| - | 304L | heat input | lack of fusion (LOF) pores | PDP, DL-EPR | [62] |
| - | Fe-Cr-Ni-Mn-Mo-B steel | heat treatment | Cr content | PDP | [8] |
| - | Fe-Cr-Ni-Mn-Mo-Nb-Si steel | - | Cr content | PDP | [63] |
| - | functionally gradient materials (FGM) | - | Cr content | OCP, PDP | [61] |
| - | 35CrMo | add Ni | - | PDP | [64] |
| WAAM | SUS304 | applying current type | content of Cr and Ni | PDP | [65] |
| LRM | 316L | solution annealing | sensitization | PDP, DL-EPR | [67] |
| GMA-AM | 316L | heat treatment | σ phase | OCP, PDP | [68] |
| electron beam wire-feed | AISI 304 | heat input | δ-ferrite | immersion tests, weight loss, flexural test | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, G.; Kim, W.; Kwon, K.; Lee, T.-K. The Corrosion of Stainless Steel Made by Additive Manufacturing: A Review. Metals 2021, 11, 516. https://doi.org/10.3390/met11030516
Ko G, Kim W, Kwon K, Lee T-K. The Corrosion of Stainless Steel Made by Additive Manufacturing: A Review. Metals. 2021; 11(3):516. https://doi.org/10.3390/met11030516
Chicago/Turabian StyleKo, Gyeongbin, Wooseok Kim, Kyungjung Kwon, and Tae-Kyu Lee. 2021. "The Corrosion of Stainless Steel Made by Additive Manufacturing: A Review" Metals 11, no. 3: 516. https://doi.org/10.3390/met11030516