Effect of Sigma Phase in Wire Arc Additive Manufacturing of Superduplex Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Deposition of Layers
2.3. Testing and Characterization
3. Results and Discussion
3.1. Macroscopic Inspection
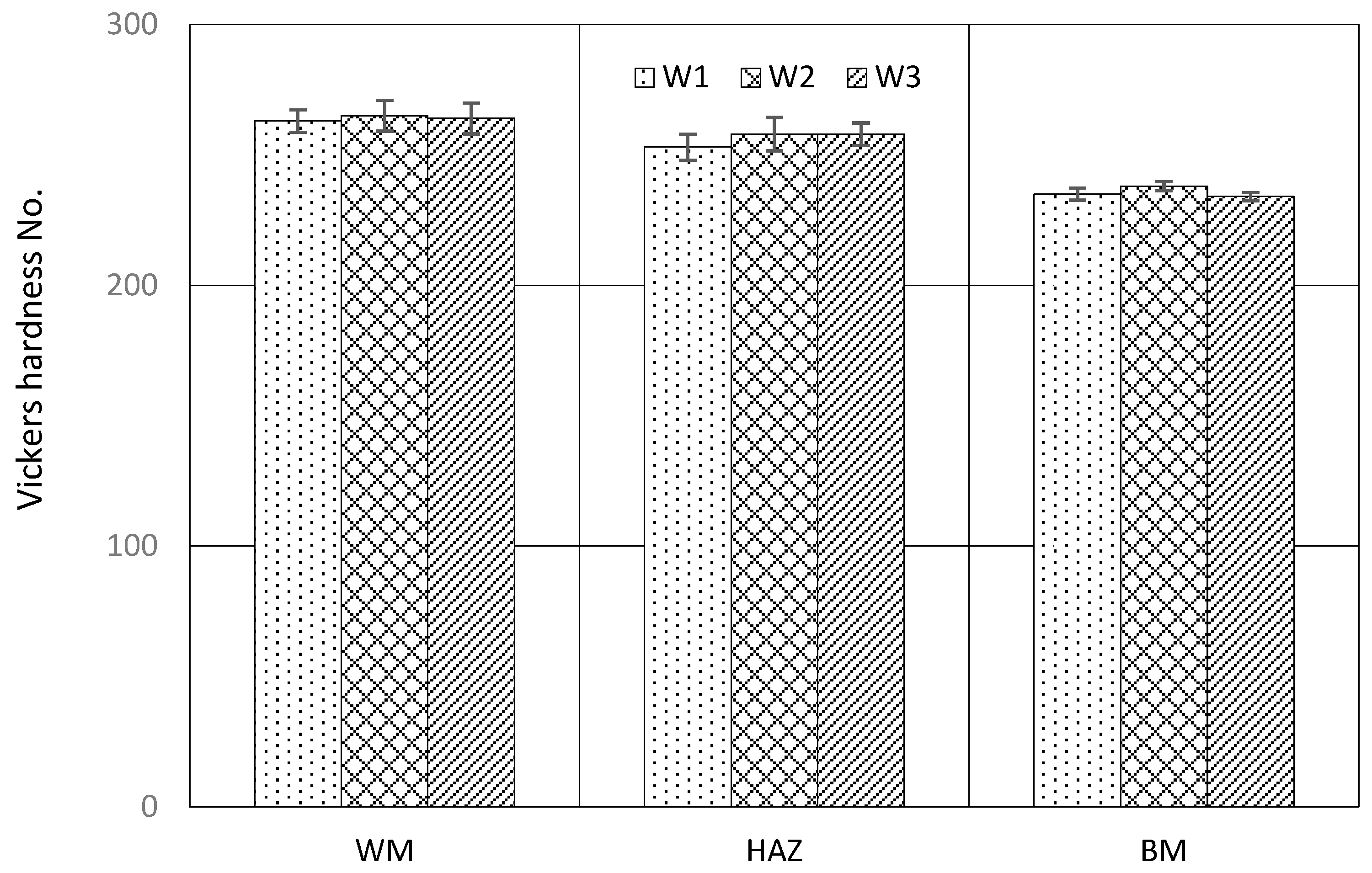
3.2. Hardness
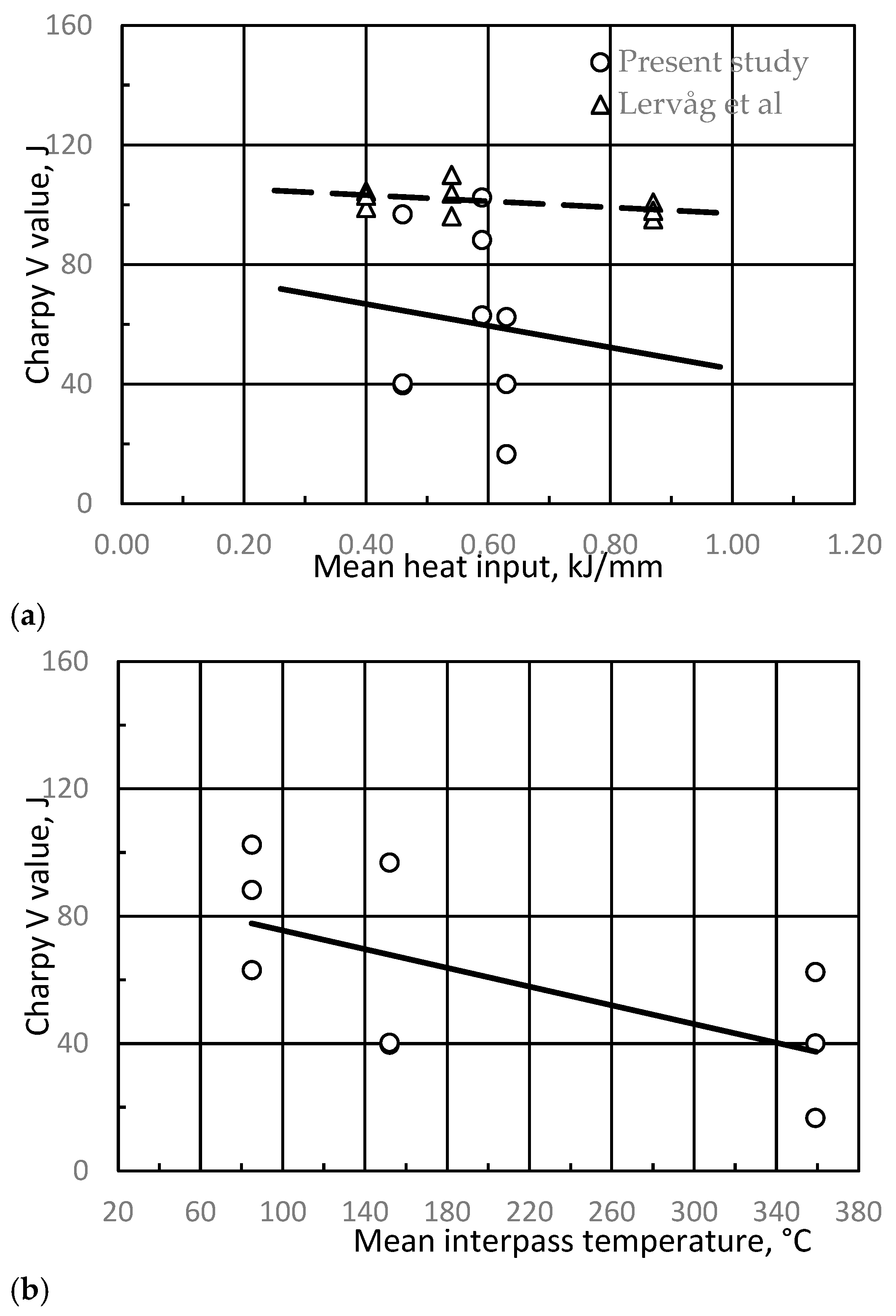
3.3. Charpy V Toughness
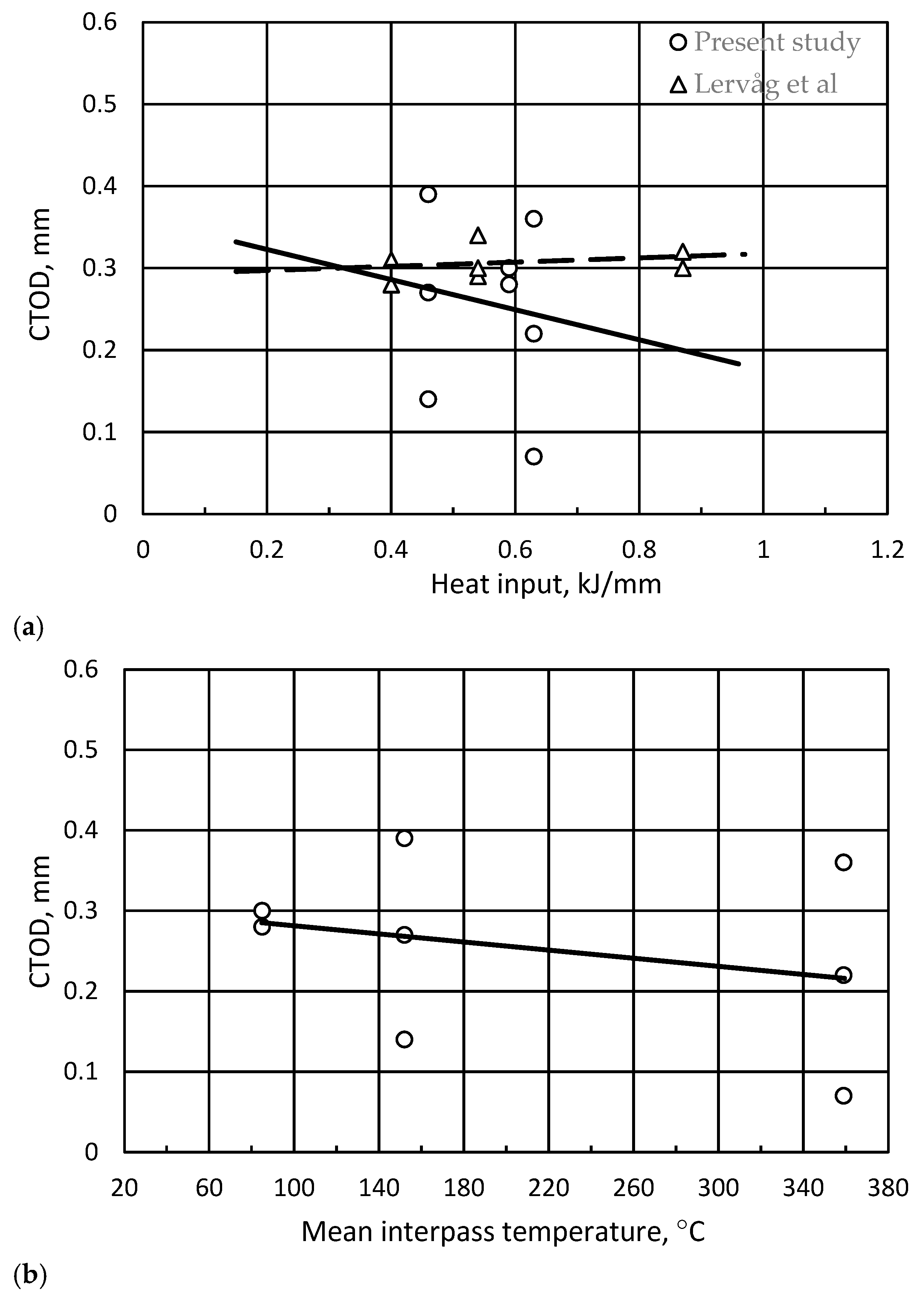
3.4. Fracture Toughness
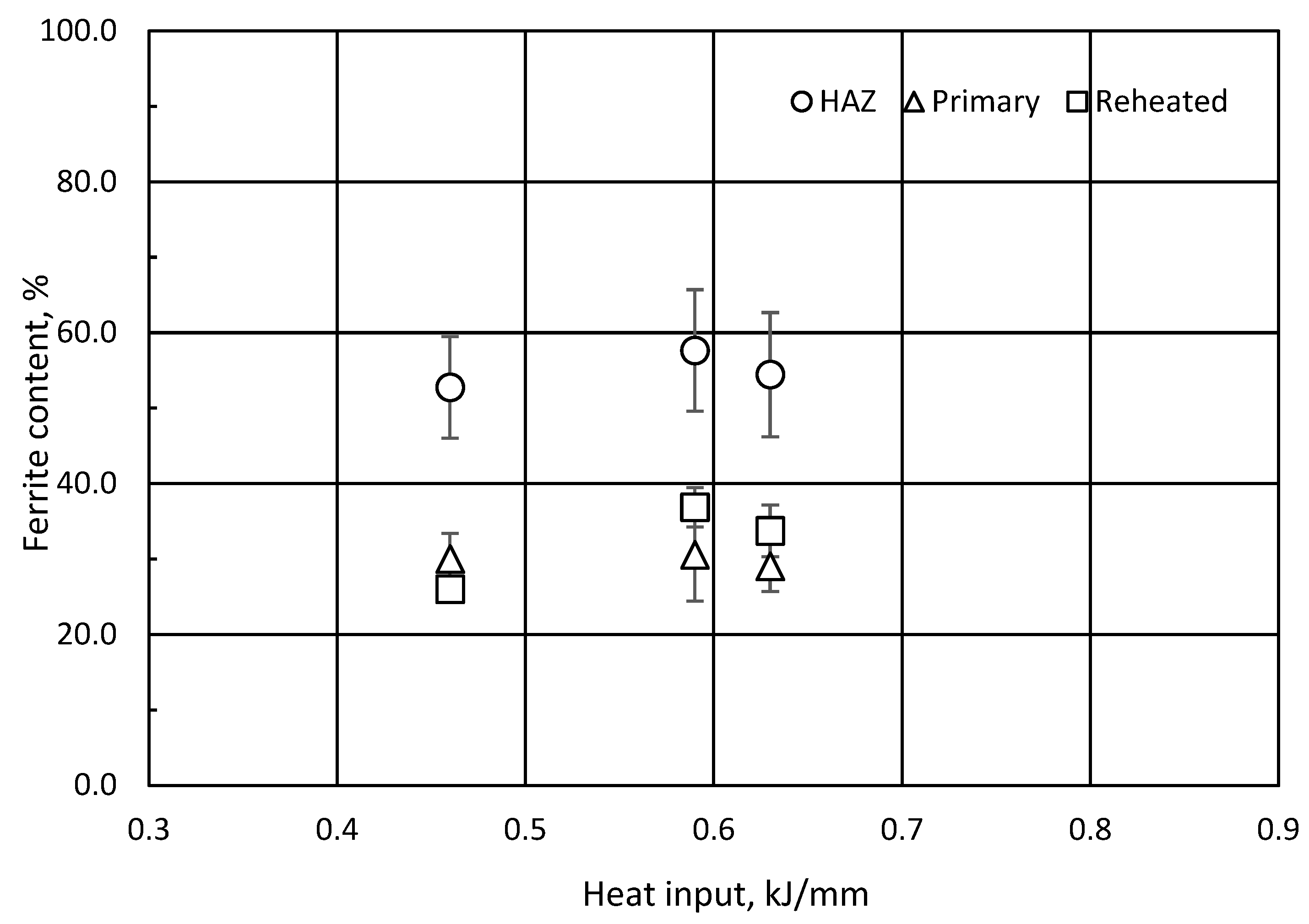
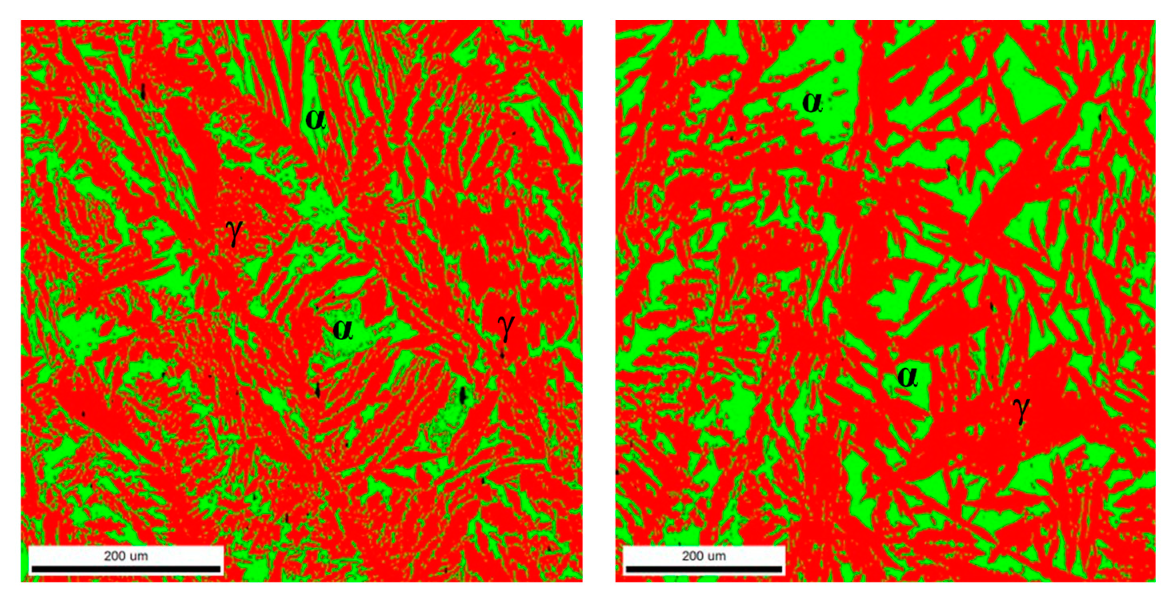
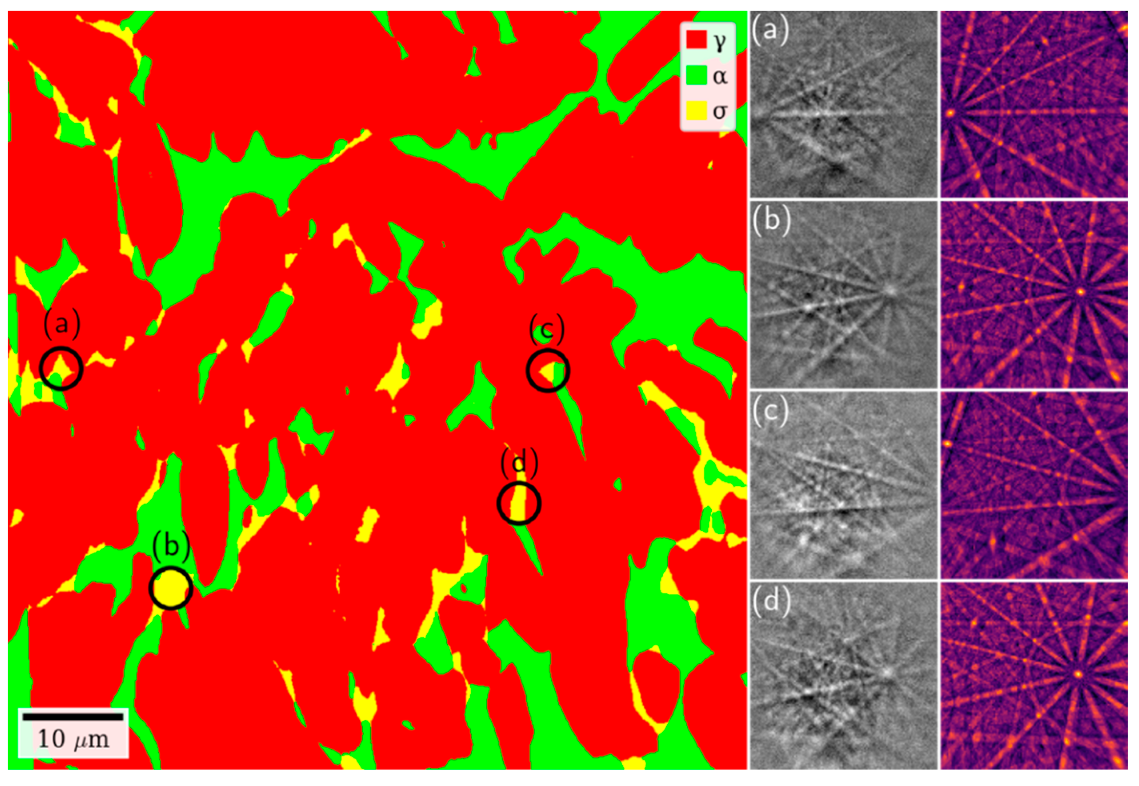
3.5. Quantitative Microstructure Analyses
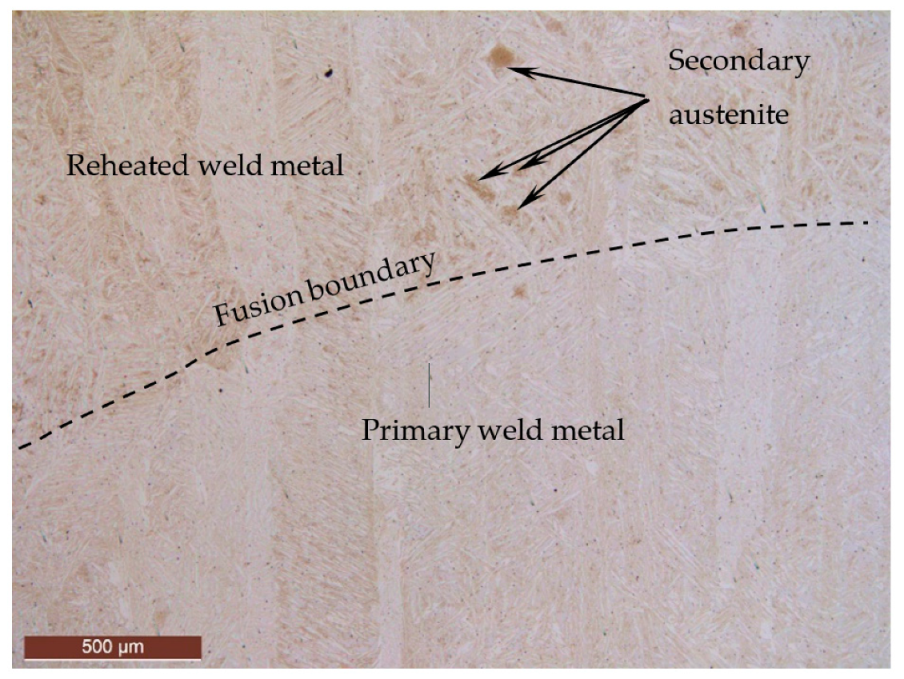
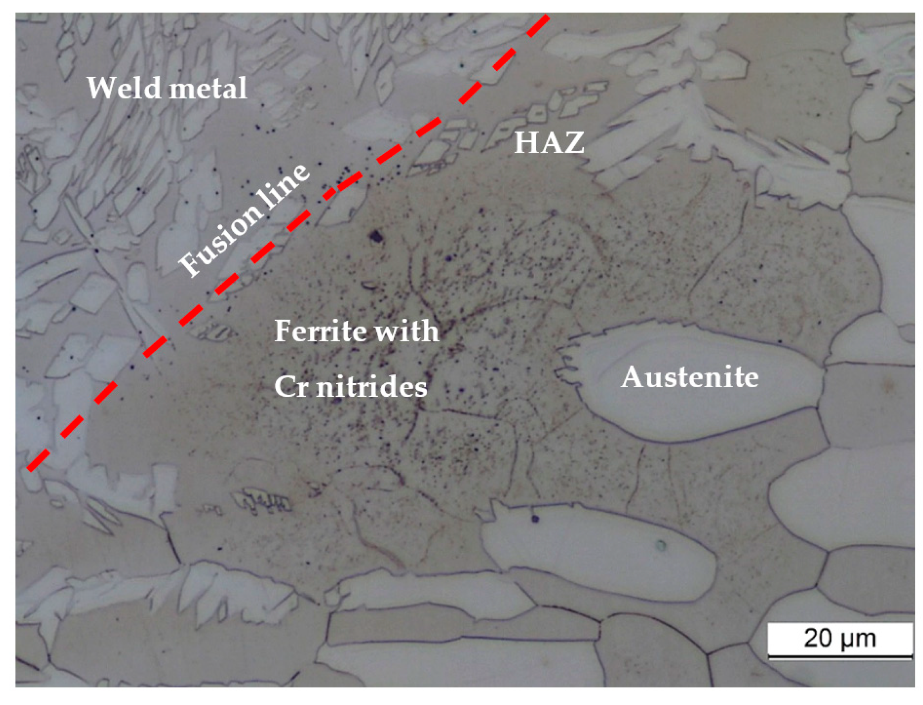
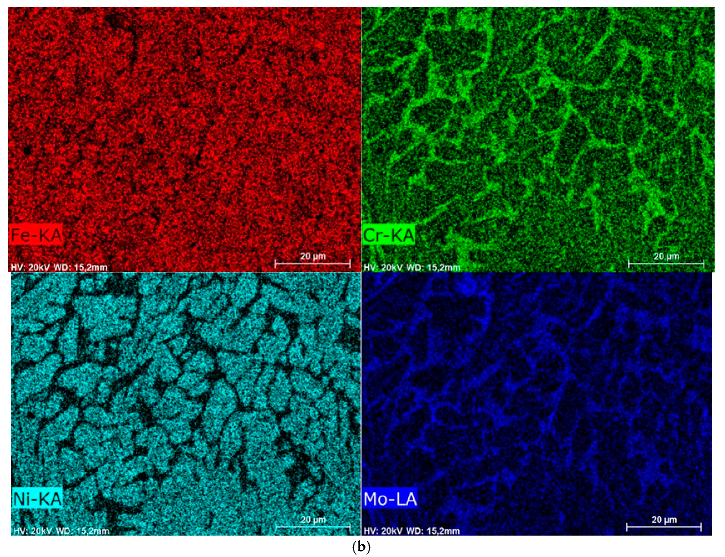
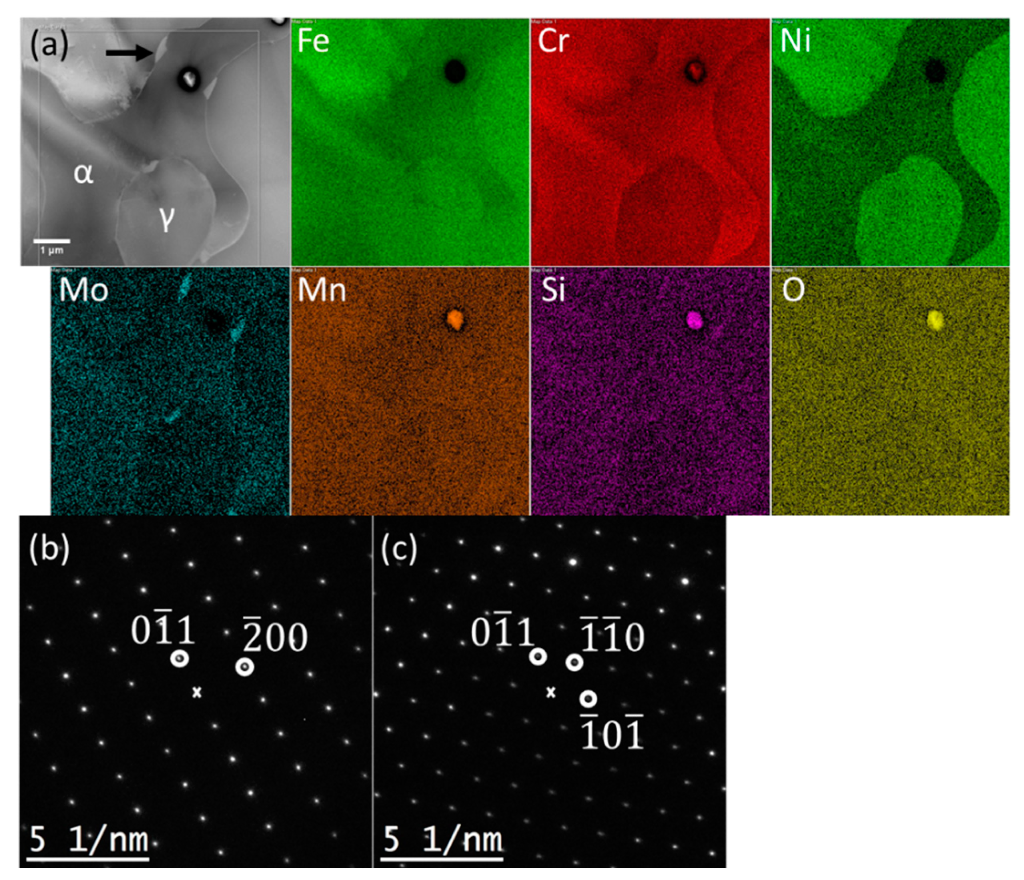
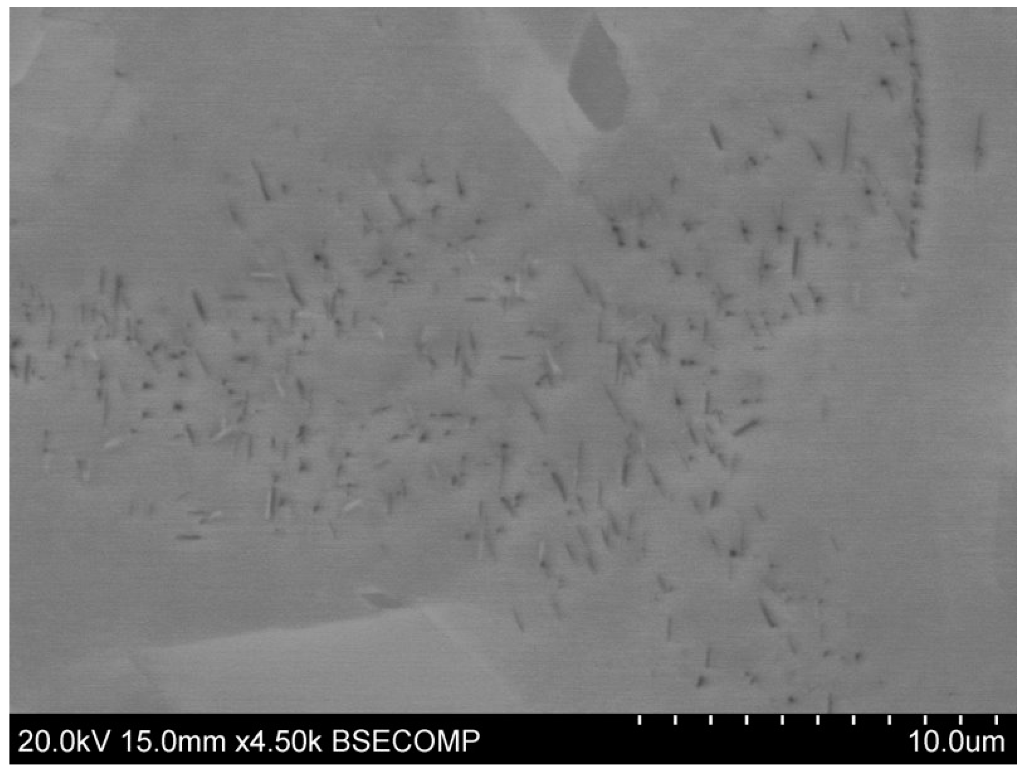
3.6. SEM Examination
4. Conclusions
- WAAM of superduplex stainless steels are feasible for industrial building or repair of components, but attention is needed to control the cooling rate to be sufficiently fast to prevent brittle intermetallic sigma phase from forming.
- Welding with low heat input and low interpass temperature can be performed without any intermetallics present.
- The ferrite content in HAZ in the support plate, primary and non-reheated region, was slightly reduced with increasing heat input and interpass temperature.
- The ferrite content approached the lower acceptance level in the phase balance of ferrite–austenite of 25–60%.
- The present results conflicts with the push towards more production efficiency and lower costs. High interpass temperature benefits productivity, but promotes sigma, and hence, severe deterioration of the toughness. A post weld solution heat treatment can be used to remove the intermetallic phases, but is costly and therefore not desirable.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martina, F.; Mehnen, J.; Williams, S.W.; Colegrove, P.; Wang, F. Investigation of the benefits of plasma deposition for the additive layer manufacture of Ti–6Al–4V. J. Mater. Process. Technol. 2012, 212, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Szost, B.A.; Terzi, S.; Martina, F.; Boisselier, D.; Prytuliak, A.; Pirling, T.; Hofmann, M.; Jarvis, D.J. A comparative study of additive manufacturing techniques: Residual stress and microstructural analysis of CLAD and WAAM printed Ti–6Al–4V components. Mater. Des. 2016, 89, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire plus Arc Additive Manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Asala, G.; Khan, A.K.; Andersson, J.; Ojo, O.A. Microstructural Analyses of ATI 718Plus® Produced by Wire-ARC Additive Manufacturing Process. Metall. Mater. Trans. 2017, 48, 4211–4228. [Google Scholar] [CrossRef] [Green Version]
- Haden, C.V.; Zeng, G.; Carter, F.M.; Ruhl, C.; Krick, B.A.; Harlow, D.G. Wire and arc additive manufactured steel: Tensile and wear properties. Addit. Manuf. 2017, 16, 115–123. [Google Scholar] [CrossRef]
- Ji, L.; Lu, J.; Liu, C.; Jing, C.; Fan, H.; Ma, S. Microstructure and mechanical properties of 304L steel fabricated by arc additive manufacturing. In Proceedings of the 2017 International Conference on Electronic Information Technology and Computer Engineering (EITCE 2017), MATEC Web of Conferences 128, Singapore, 27–29 December 2017. [Google Scholar]
- Rodriguez, N.; Vázquez, L.; Huarte, I.; Arruti, E.; Tabernero, I.; Alvarez, P. Wire and arc additive manufacturing: A comparison between CMT and TopTIG processes applied to stainless steel. Weld. World 2018, 62, 1083–1096. [Google Scholar] [CrossRef]
- Martina, F.; Ding, J.; Williams, S.; Caballero, A.; Pardal, G.; Quintino, L. Tandem metal inert gas process for high productivity wire arc additive manufacturing in stainless steel. Addit. Manuf. 2019, 25, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire + Arc Additive Manufacture of 17-4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2018, 268, 54–62. [Google Scholar] [CrossRef]
- Eriksson, M.; Lervik, M.; Sørensen, C.; Robertstad, A.; Brønstad, B.M.; Nyhus, B.; Aune, R.; Ren, X.; Akselsen, O.M. Additive manufacture of superduplex stainless steel using WAAM. In Proceedings of the 5th International Conference of Engineering Against Failure, ICEAF-V 2018, MATEC Web of Conferences, Chios Island, Greece, 20–22 June 2018; Volume 188, p. 03014. [Google Scholar] [CrossRef]
- Ahn, Y.S.; Kang, J.P. Effect of aging treatments on microstructure and impact properties of tungsten substituted 2205 duplex stainless steel. Mater. Sci. Technol. 2000, 16, 382–388. [Google Scholar] [CrossRef]
- Chen, T.H.; Weng, K.L.; Yang, J.R. The effect of high-temperature exposure on the microstructural stability and toughness property in a 2205 duplex stainless steel. Mater. Sci. Eng. 2002, 338, 259–270. [Google Scholar] [CrossRef]
- El Koussy, M.R.; El Mahallawi, I.S.; Khalifa, W.; Al Dawood, M.M.; Bueckins, M. Effects of thermal aging on microstructure and mechanical properties of duplex stainless steel weldments. Mater. Sci. Technol. 2004, 20, 375–381. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Kasper, J.S. The ordering of atoms in the chi-phase of the iron-chromium-molybdenum system. Acta Metall. 1954, 2, 456–461. [Google Scholar] [CrossRef]
- Ånes, H.W.; Natlandsmyr, O.; Bergh, T.; Lervik, L. pyxem/kikuchipy: Kikuchipy 0.4.0. 2021. Available online: https://zenodo.org/record/5082225#.Ybv5bmhBxPY (accessed on 12 December 2021).
- Callahan, P.G.; De Graef, M. Dynamical Electron Backscatter Diffraction Patterns. Part I: Pattern Simulations. Microsc. Microanal. 2013, 19, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; De Graef, M. Orientation sampling for dictionary-based diffraction pattern indexing methods. Model. Simul. Mater. Sci. Eng. 2016, 24, 085013. [Google Scholar] [CrossRef]
- Lervåg, M.; Sørensen, C.; Robertstad, A.; Brønstad, B.M.; Nyhus, B.; Eriksson, M.; Aune, R.; Ren, X.; Akselsen, O.M.; Bunaziv, I. Additive manufacturing with superduplex stainless steel wire by CMT process. Metals 2020, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.M.; Dulls, E.J.; Carroll, K.G. Identification of the precipitate accompanying 885 °F embrittlement in chromium steels. Trans. AIME 1953, 197, 690–695. [Google Scholar]
- API (American Petroleum Institute). Use of Duplex Stainless Steels in the Oil Refining Industry, 2nd ed.; Technical Report 938-C; American Petroleum Institute (API): Washington, DC, USA, 2011. [Google Scholar]
- NACE—National Association for Corrosion. Engineers-ANSI/NACE MR0175/ISO 15156: Petroleum, Petrochemical, and Natural Gas Industries—Materials for Use in H2S-Containing Environments in Oil and Gas Production—General Principles for Selection of Cracking-Resistant Materials; National Association for Corrosion: Houston, TX, USA, 2015. [Google Scholar]
- Magnabosco, R. Kinetics of Sigma Phase Formation in a Duplex Staineless Steel. Mater. Res. 2009, 12, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.-O.; Karlsson, L.; Andersson, J.O. Secondary austenite formation and its relation to pitting corrosion in duplex stainless steel weld metal. Mater. Sci. Technol. 1995, 11, 276–283. [Google Scholar] [CrossRef]
- Ottonello, G.; Borketa, M.; Sciuto, P.F. Parameterization of energy and interactions in garnets: End-member Properties. Am. Mineral. 1996, 81, 429–447. [Google Scholar] [CrossRef]
- Atamert, S.; King, J.E. Sigma-phase formation and its prevention in duplex stainless steels. J. Mater. Sci. Lett. 1993, 12, 1144–1147. [Google Scholar] [CrossRef]
- Nilsson, J.O. Superduplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Sato, Y.S.; Kokawa, H. Preferential precipitation site of sigma phase in duplex stainless steel weld metal. Scr. Mater. 1999, 40, 659–663. [Google Scholar] [CrossRef]







| Material | C | Si | Mn | P | S | Cr | Ni | Mo | Cu | N | W |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wire | 0.018 | 0.3 | 0.7 | 0.02 | 0.001 | 25.0 | 9.5 | 3.7 | 0.6 | 0.23 | 0.6 |
| Plate | 0.020 | 0.32 | 0.85 | 0.023 | 0.0003 | 24.8 | 6.6 | 3.7 | 0.16 | 0.26 | - |
| Parameter | Weld No | ||
|---|---|---|---|
| W1 | W2 | W3 | |
| Average current (A) | 197 | 161 | 202 |
| Average voltage (V) | 21 | 20 | 22 |
| Travel speed (mm/s) | 7.0 | 7.0 | 7.0 |
| Wire feed rate (mm/s) | 7.7 | 5.8 | 8.2 |
| Heat input (kJ/mm) | 0.59 | 0.46 | 0.63 |
| Average interpass temperature (°C) | 85 | 152 | 359 |
| Average interpass time (s) | 360 | 120 | 40 |
| Polarity | DC+ | DC+ | DC+ |
| Wall Production Data | Weld No | ||
|---|---|---|---|
| W1 | W2 | W3 | |
| Number of layers | 17 | 16 | 16 |
| Average layer height (mm) | 2.6 | 2.0 | 2.1 |
| Wall width (mm) | 11.6 | 9.2 | 8.9 |
| Wall length (mm) | 230 | 230 | 230 |
| Deposition time (min) | 90.5 | 15.9 | 36.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akselsen, O.M.; Bjørge, R.; Ånes, H.W.; Ren, X.; Nyhus, B. Effect of Sigma Phase in Wire Arc Additive Manufacturing of Superduplex Stainless Steel. Metals 2021, 11, 2045. https://doi.org/10.3390/met11122045
Akselsen OM, Bjørge R, Ånes HW, Ren X, Nyhus B. Effect of Sigma Phase in Wire Arc Additive Manufacturing of Superduplex Stainless Steel. Metals. 2021; 11(12):2045. https://doi.org/10.3390/met11122045
Chicago/Turabian StyleAkselsen, Odd M., Ruben Bjørge, Håkon Wiik Ånes, Xiaobo Ren, and Bård Nyhus. 2021. "Effect of Sigma Phase in Wire Arc Additive Manufacturing of Superduplex Stainless Steel" Metals 11, no. 12: 2045. https://doi.org/10.3390/met11122045






