In Situ Time-Resolved Decomposition of β-Hydride Phase in Palladium Nanoparticles Coated with Metal-Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pd@HKUST-1
2.2. Ex Situ Characterization
2.3. In Situ X-Ray Powder Diffraction
3. Results
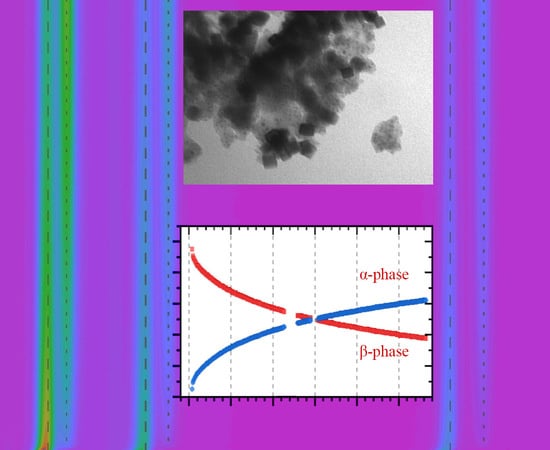
3.1. Characterization of the Initial Pd@HKUST-1 Material
3.2. Evolution of Palladium Structure During Adsorption/Desorption of Hydrogen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manchester, F.D.; San-Martin, A.; Pitre, J.M. The H-Pd (hydrogen-palladium) system. J. Phase Equilib. 1994, 15, 62–83. [Google Scholar] [CrossRef]
- Kishore, S.; Nelson, J.A.; Adair, J.H.; Eklund, P.C. Hydrogen storage in spherical and platelet palladium nanoparticles. J. Alloy. Compd. 2005, 389, 234–242. [Google Scholar] [CrossRef]
- Langhammer, C.; Zhdanov, V.P.; Zoric, I.; Kasemo, B. Size-dependent hysteresis in the formation and decomposition of hydride in metal nanoparticles. Chem. Phys. Lett. 2010, 488, 62–66. [Google Scholar] [CrossRef]
- Langhammer, C.; Zhdanov, V.P.; Zorić, I.; Kasemo, B. Size-dependent kinetics of hydriding and dehydriding of Pd nanoparticles. Phys. Rev. Lett. 2010, 104, 135502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugaev, A.L.; Guda, A.A.; Lomachenko, K.A.; Shapovalov, V.V.; Lazzarini, A.; Vitillo, J.G.; Bugaev, L.A.; Groppo, E.; Pellegrini, R.; Soldatov, A.V.; et al. Core–shell structure of palladium hydride nanoparticles revealed by combined X-ray absorption spectroscopy and X-ray diffraction. J. Phys. Chem. C 2017, 121, 18202–18213. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Srabionyan, V.V.; Soldatov, A.V.; Bugaev, L.A.; Van Bokhoven, J.A. The role of hydrogen in formation of Pd XANES in Pd-nanoparticles. J. Physics Conf. Ser. 2013, 430, 012028. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Guda, A.A.; Lomachenko, K.A.; Lazzarini, A.; Srabionyan, V.V.; Vitillo, J.G.; Piovano, A.; Groppo, E.; Bugaev, L.A.; Soldatov, A.V.; et al. Hydride phase formation in carbon supported palladium hydride nanoparticles by in situ EXAFS and XRD. J. Physics Conf. Ser. 2016, 712, 012032. [Google Scholar] [CrossRef]
- Tew, M.W.; Miller, J.T.; Van Bokhoven, J.A. Particle size effect of hydride formation and surface hydrogen adsorption of nanosized palladium catalysts: L3 edge vs K edge X-ray absorption spectroscopy. J. Phys. Chem. C 2009, 113, 15140–15147. [Google Scholar] [CrossRef]
- Tew, M.W.; Janousch, M.; Huthwelker, T.; Van Bokhoven, J.A. The roles of carbide and hydride in oxide-supported palladium nanoparticles for alkyne hydrogenation. J. Catal. 2011, 283, 45–54. [Google Scholar] [CrossRef]
- Bugaev, A.; Polyakov, V.; Tereshchenko, A.; Isaeva, A.; Skorynina, A.; Kamyshova, E.; Budnyk, A.; Lastovina, T.; Soldatov, A. Chemical synthesis and characterization of Pd/SiO2: The effect of chemical reagent. Metals 2018, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Bugaev, A.L.; Guda, A.A.; Pankin, I.A.; Groppo, E.; Pellegrini, R.; Longo, A.; Soldatov, A.V.; Lamberti, C. The role of palladium carbides in the catalytic hydrogenation of ethylene over supported palladium nanoparticles. Catal. Today 2019, 336, 40–44. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Guda, A.A.; Pankin, I.A.; Groppo, E.; Pellegrini, R.; Longo, A.; Soldatov, A.V.; Lamberti, C. Operando X-ray absorption spectra and mass spectrometry data during hydrogenation of ethylene over palladium nanoparticles. Data Brief 2019, 24, 103954. [Google Scholar] [CrossRef]
- Usoltsev, O.A.; Bugaev, A.L.; Guda, A.A.; Guda, S.A.; Soldatov, A.V. Absorption of hydrocarbons on palladium catalysts: From simple models towards machine learning analysis of X-ray absorption spectroscopy data. Top. Catal. 2020. [Google Scholar] [CrossRef]
- Skorynina, A.A.; Tereshchenko, A.A.; Usoltsev, O.A.; Bugaev, A.L.; Lomachenko, K.A.; Guda, A.A.; Groppo, E.; Pellegrini, R.; Lamberti, C.; Soldatov, A. Time-dependent carbide phase formation in palladium nanoparticles. Radiat. Phys. Chem. 2018. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Usoltsev, O.A.; Lazzarini, A.; Lomachenko, K.A.; Guda, A.A.; Pellegrini, R.; Carosso, M.; Vitillo, J.G.; Groppo, E.; Van Bokhoven, J.A.; et al. Time-resolved operando studies of carbon supported Pd nanoparticles under hydrogenation reactions by X-ray diffraction and absorption. Faraday Discuss. 2018, 208, 187–205. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Guda, A.A.; Lomachenko, K.A.; Soldatov, A.V. Kinetics of the atomic structure of palladium nanoparticles during the desorption of hydrogen according to X-ray diffraction. JETP Lett. 2019, 109, 594–599. [Google Scholar] [CrossRef]
- Wadell, C.; Pingel, T.; Olsson, E.; Zoric, I.; Zhdanov, V.P.; Langhammer, C. Thermodynamics of hydride formation and decomposition in supported sub-10 nm Pd nanoparticles of different sizes. Chem. Phys. Lett. 2014, 603, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Zhdanov, V.P.; Kasemo, B. Kinetics of the formation of a new phase in nanoparticles. Chem. Phys. Lett. 2008, 460, 158–161. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Krozer, A.; Kasemo, B. Kinetics of first-order phase transitions initiated by diffusion of particles from the surface into the bulk. Phys. Rev. B 1993, 47, 11044–11048. [Google Scholar] [CrossRef]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Esken, D.; Zhang, X.; Lebedev, O.I.; Schröder, F.; Fischer, R.A. Pd@MOF-5: Limitations of gas-phase infiltration and solution impregnation of [Zn4O(bdc)3] (MOF-5) with metal–organic palladium precursors for loading with Pd nanoparticles. J. Mater. Chem. 2009, 19. [Google Scholar] [CrossRef]
- Wang, T.; Gao, L.; Hou, J.; Herou, S.J.A.; Griffiths, J.T.; Li, W.; Dong, J.; Gao, S.; Titirici, M.M.; Kumar, R.V.; et al. Rational approach to guest confinement inside MOF cavities for low-temperature catalysis. Nat. Commun. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Proch, S.; Herrmannsdorfer, J.; Kempe, R.; Kern, C.; Jess, A.; Seyfarth, L.; Senker, J. Pt@MOF-177: Synthesis, room-temperature hydrogen storage and oxidation catalysis. Chem. Eur. J. 2008, 14, 8204–8212. [Google Scholar] [CrossRef]
- Braglia, L.; Borfecchia, E.; Lomachenko, K.A.; Bugaev, A.L.; Guda, A.A.; Soldatov, A.V.; Bleken, B.T.L.; Oien-Odegaard, S.; Olsbye, U.; Lillerud, K.P.; et al. Tuning Pt and Cu sites population inside functionalized UiO-67 MOF by controlling activation conditions. Faraday Discuss. 2017, 201, 277–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braglia, L.; Borfecchia, E.; Maddalena, L.; Øien, S.; Lomachenko, K.A.; Bugaev, A.L.; Bordiga, S.; Soldatov, A.V.; Lillerud, K.P.; Lamberti, C.; et al. Exploring structure and reactivity of Cu sites in functionalized UiO-67 MOFs. Catal. Today 2017, 283, 89–103. [Google Scholar] [CrossRef]
- Braglia, L.; Borfecchia, E.; Martini, A.; Bugaev, A.L.; Soldatov, A.V.; Oien-Odegaard, S.; Lonstad-Bleken, B.T.; Olsbye, U.; Lillerud, K.P.; Lomachenko, K.A. The duality of UiO-67-Pt MOFs: Connecting treatment conditions and encapsulated Pt species by operando XAS. Phys. Chem. Chem. Phys. 2017, 19, 27489–27507. [Google Scholar] [CrossRef] [Green Version]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Guda, A.A.; Lomachenko, K.A.; Kamyshova, E.G.; Soldatov, M.A.; Kaur, G.; Øien-Ødegaard, S.; Braglia, L.; Lazzarini, A.; Manzoli, M.; et al. Operando study of palladium nanoparticles inside UiO-67 MOF for catalytic hydrogenation of hydrocarbons. Faraday Discuss. 2018, 208, 287–306. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Skorynina, A.A.; Braglia, L.; Lomachenko, K.A.; Guda, A.; Lazzarini, A.; Bordiga, S.; Olsbye, U.; Lillerud, K.P.; Soldatov, A.V.; et al. Evolution of Pt and Pd species in functionalized UiO-67 metal-organic frameworks. Catal. Today 2019, 336, 33–39. [Google Scholar] [CrossRef]
- Bugaev, A.L.; Skorynina, A.A.; Kamyshova, E.G.; Lomachenko, K.A.; Guda, A.A.; Soldatov, A.V.; Lamberti, C. In situ X-ray absorption spectroscopy data during formation of active Pt- and Pd-sites in functionalized UiO-67 metal-organic frameworks. Data Brief 2019, 25, 104280. [Google Scholar] [CrossRef] [PubMed]
- Kamyshova, E.G.; Skorynina, A.A.; Bugaev, A.L.; Lamberti, C.; Soldatov, A.V. Formation and growth of Pd nanoparticles in UiO-67 MOF by in situ EXAFS. Radiat. Phys. Chem. 2019. [Google Scholar] [CrossRef]
- Li, G.; Kobayashi, H.; Taylor, J.M.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Toh, S.; Matsumura, S.; et al. Hydrogen storage in Pd nanocrystals covered with a metal-organic framework. Nat. Mater. 2014, 13, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Collins, T. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Van Beek, W.; Safonova, O.V.; Wiker, G.; Emerich, H. SNBL, a dedicated beamline for combinedin situ X-ray diffraction, X-ray absorption and Raman scattering experiments. Phase Transit. 2011, 84, 726–732. [Google Scholar] [CrossRef]
- Kieffer, J.; Wright, J.P. PyFAI: A Python library for high performance azimuthal integration on GPU. Powder Diffr. 2013, 28, S339–S350. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Berube, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Karen, P.; Woodward, P.M. Liquid-mix disorder in crystalline solids: ScMnO3. J. Solid State Chem. 1998, 141, 78–88. [Google Scholar] [CrossRef]
- Vogel, W.; He, W.; Huang, Q.-H.; Zou, Z.; Zhang, X.-G.; Yang, H. Palladium nanoparticles “breathe” hydrogen; a surgical view with X-ray diffraction. Int. J. Hydrogen Energy 2010, 35, 8609–8620. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichkov, M.V.; Bugaev, A.L.; Skorynina, A.A.; Butova, V.V.; Budnyk, A.P.; Guda, A.A.; Trigub, A.L.; Soldatov, A.V. In Situ Time-Resolved Decomposition of β-Hydride Phase in Palladium Nanoparticles Coated with Metal-Organic Framework. Metals 2020, 10, 810. https://doi.org/10.3390/met10060810
Kirichkov MV, Bugaev AL, Skorynina AA, Butova VV, Budnyk AP, Guda AA, Trigub AL, Soldatov AV. In Situ Time-Resolved Decomposition of β-Hydride Phase in Palladium Nanoparticles Coated with Metal-Organic Framework. Metals. 2020; 10(6):810. https://doi.org/10.3390/met10060810
Chicago/Turabian StyleKirichkov, Mikhail V., Aram L. Bugaev, Alina A. Skorynina, Vera V. Butova, Andriy P. Budnyk, Alexander A. Guda, Alexander L. Trigub, and Alexander V. Soldatov. 2020. "In Situ Time-Resolved Decomposition of β-Hydride Phase in Palladium Nanoparticles Coated with Metal-Organic Framework" Metals 10, no. 6: 810. https://doi.org/10.3390/met10060810