Effect of Trace Elements on the Crystallization Temperature Interval and Properties of 5xxx Series Aluminum Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
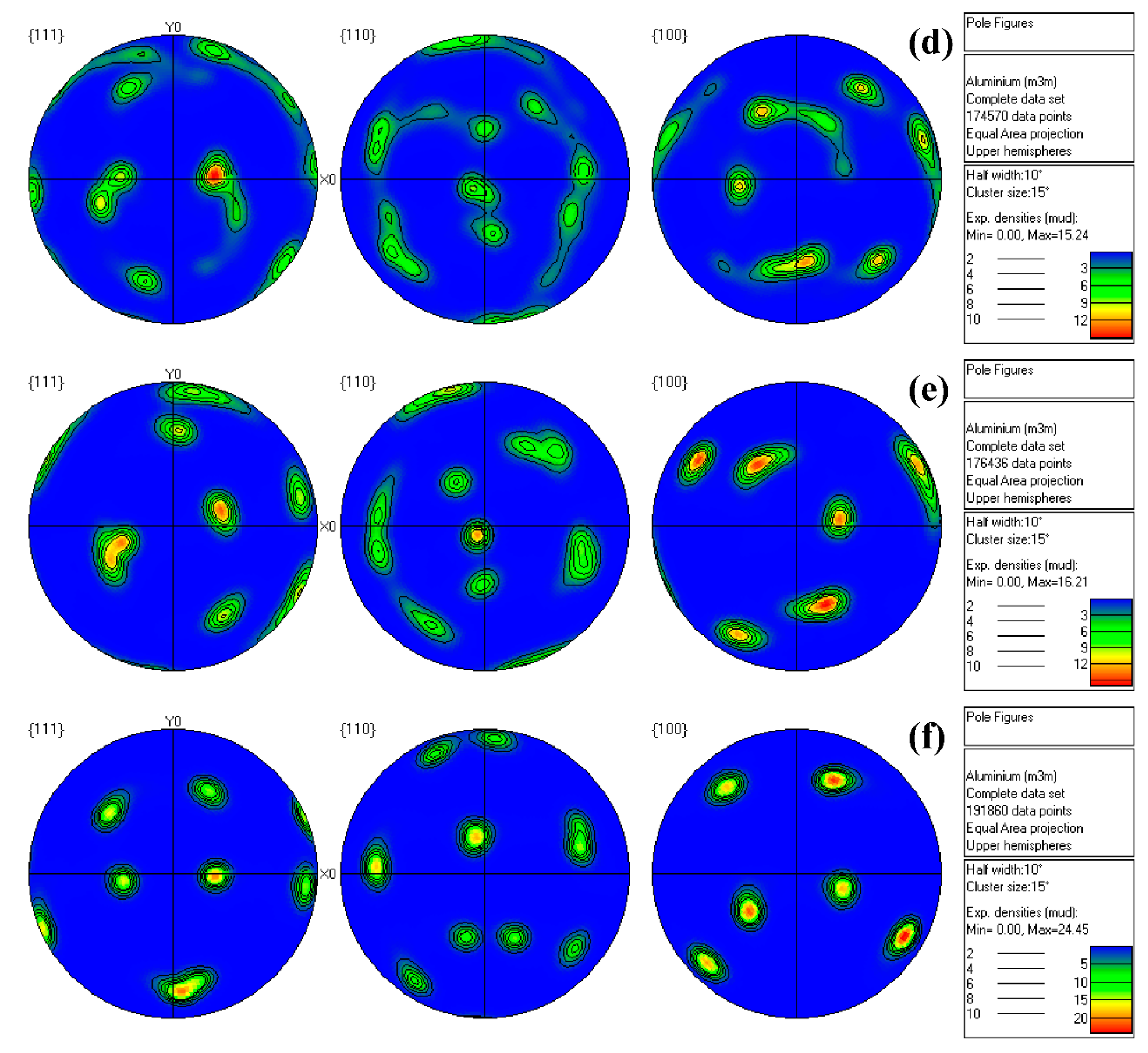
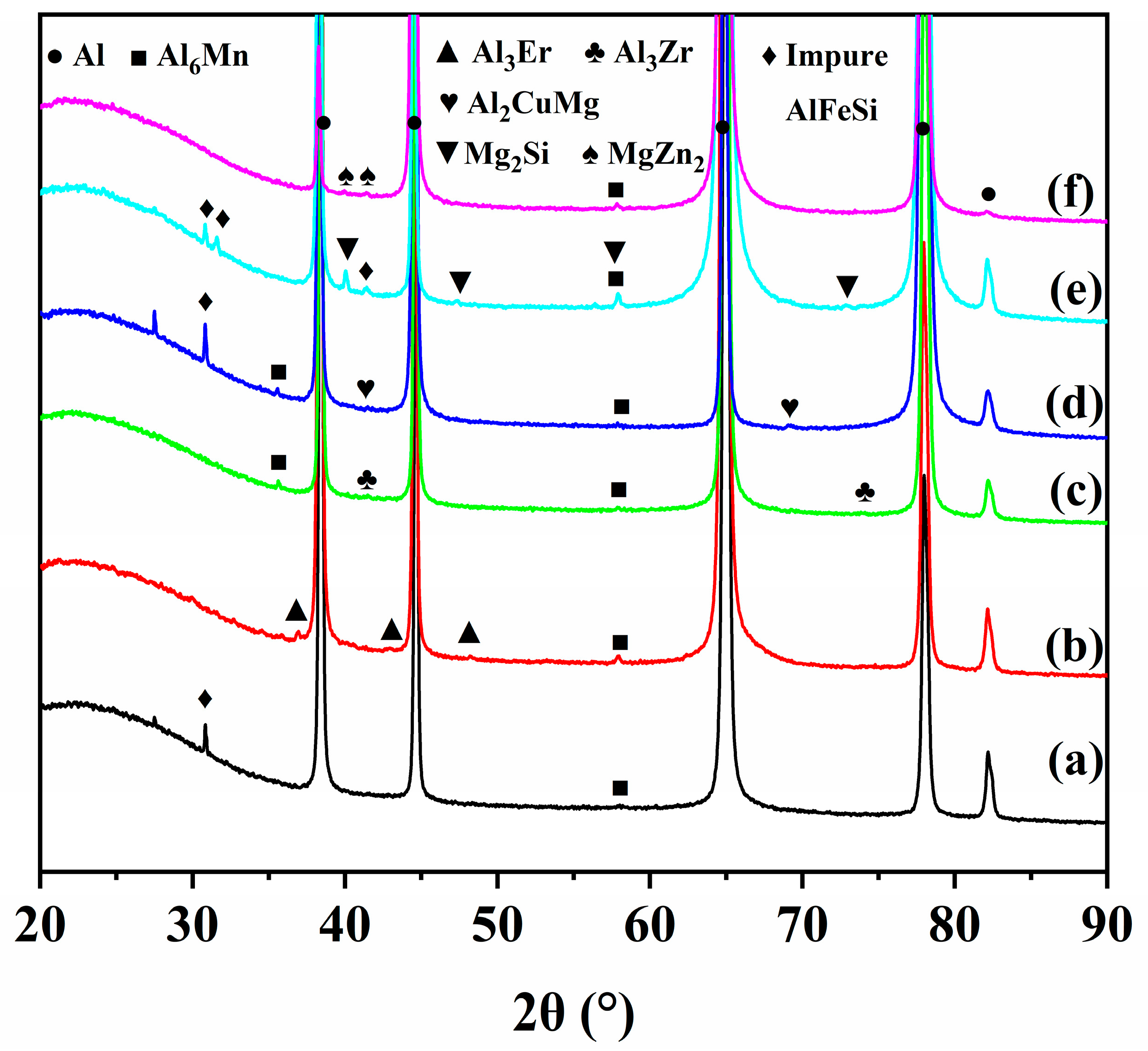
3.1. Microstructure Analysis
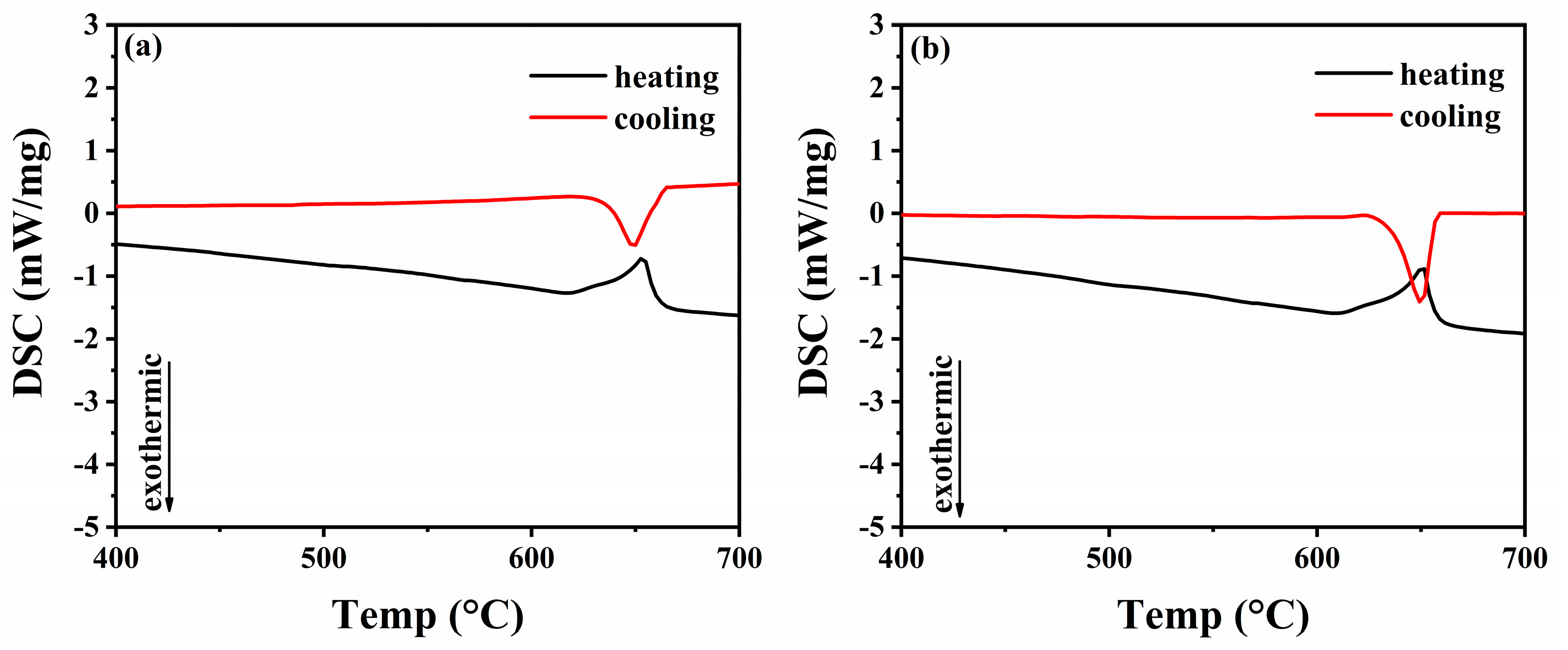
3.2. As-Cast DSC Analysis
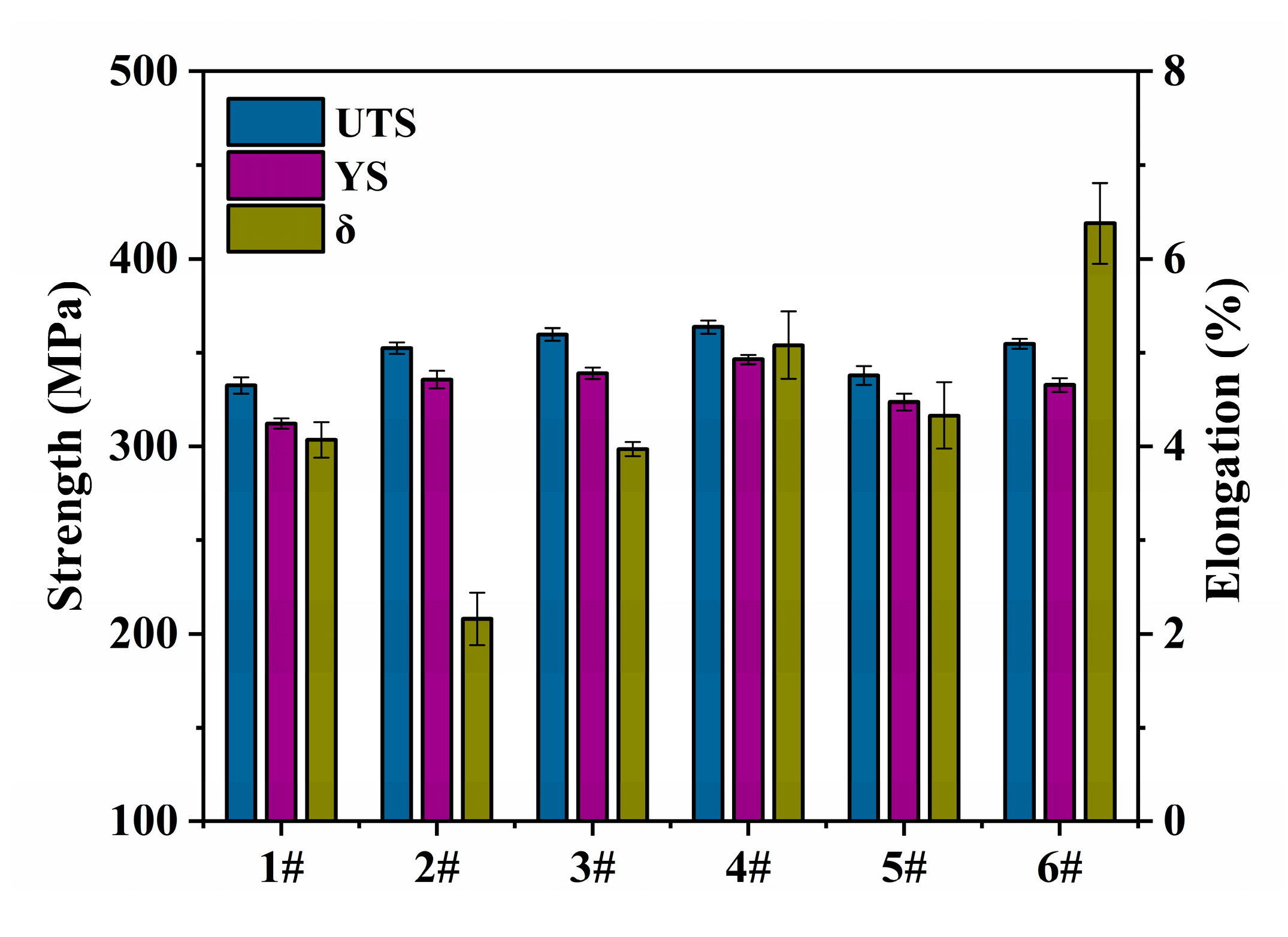
3.3. Analysis of Mechanical Properties
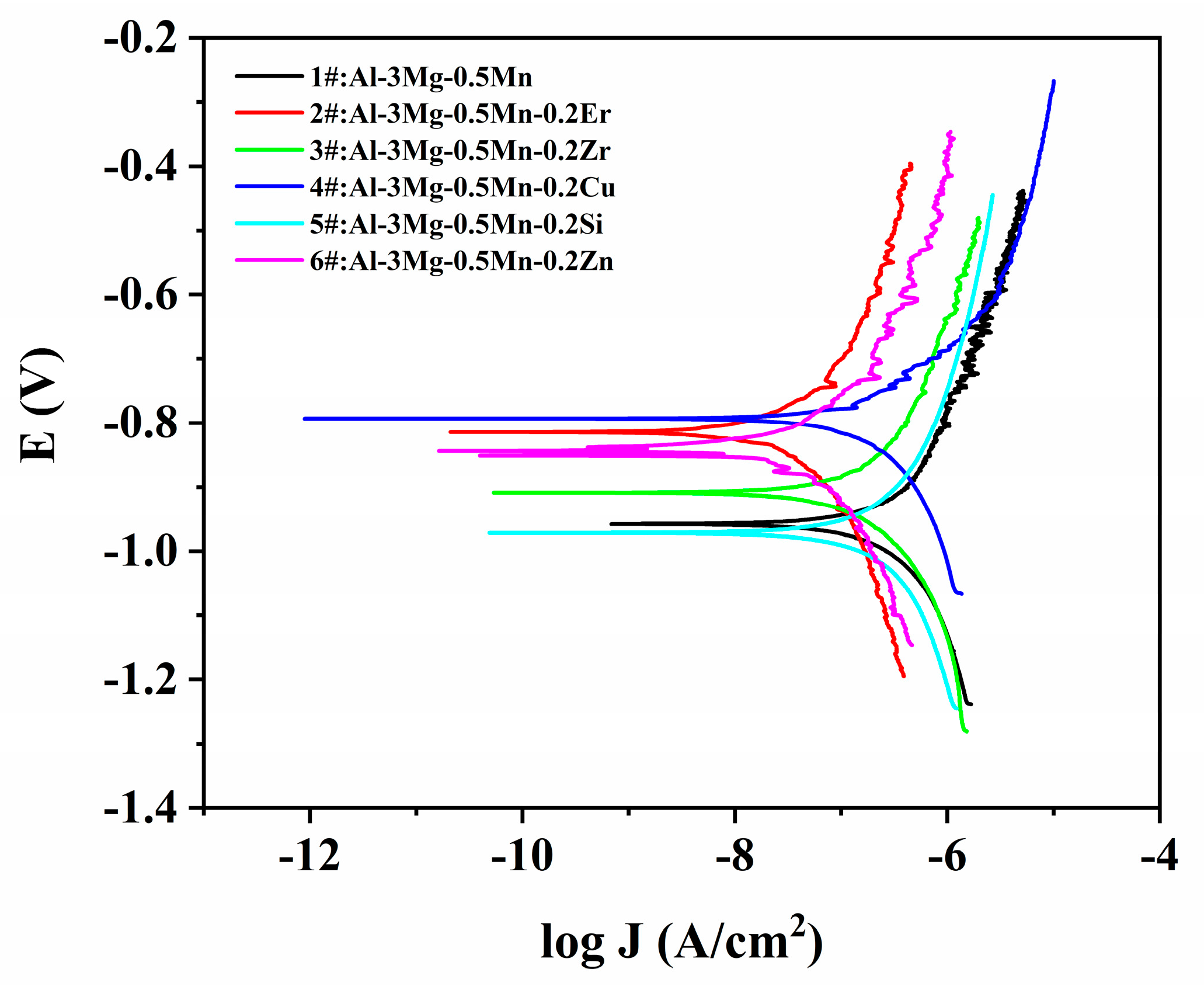
3.4. Electrochemical Analysis
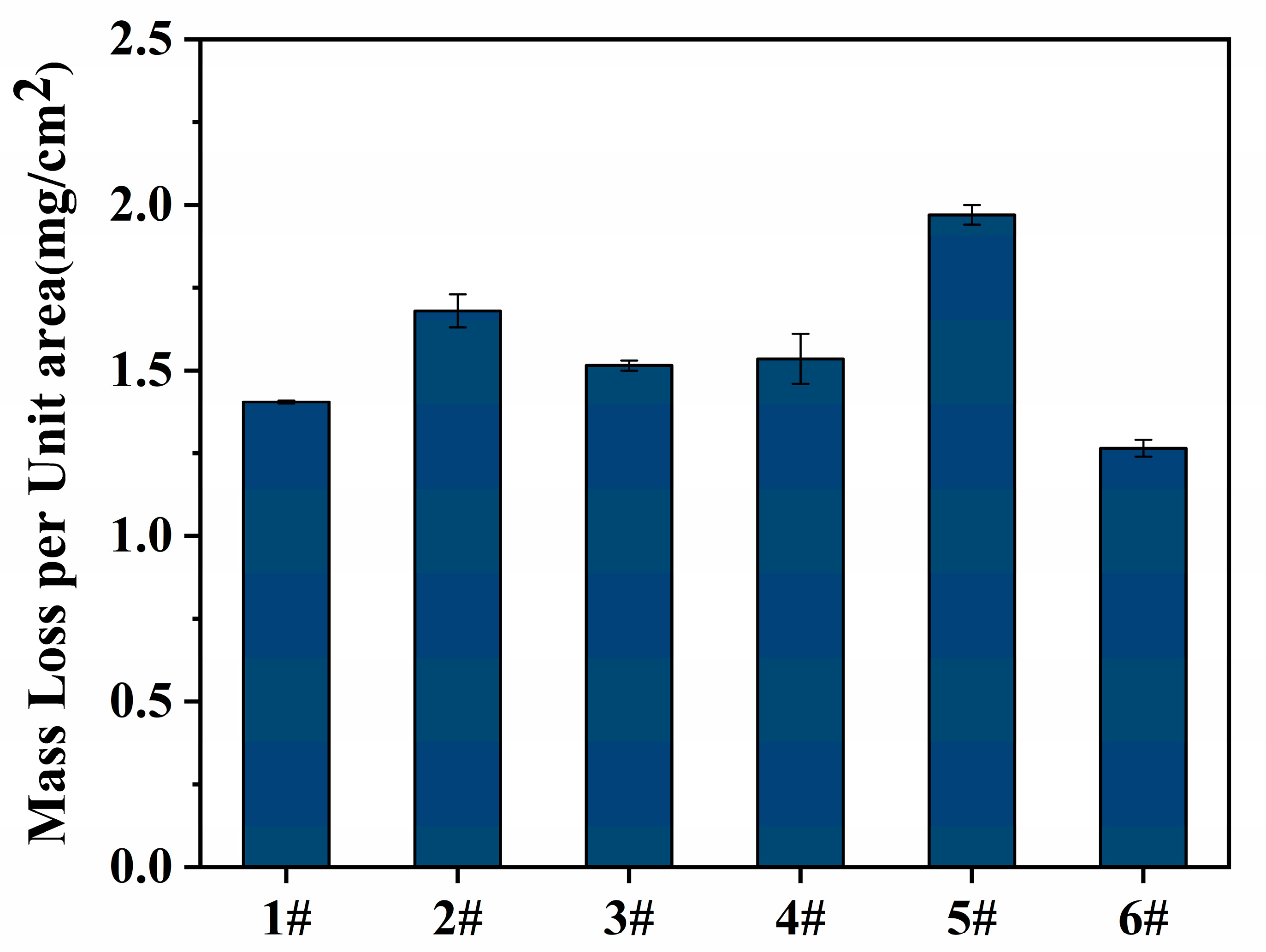
3.5. Analysis of Intergranular Corrosion Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kramer, L.; Phillippi, M.; Tack, W.T.; Wong, C. Locally Reversing Sensitization in 5xxx Aluminum Plate. J. Mater. Eng. Perform. 2011, 21, 1025–1029. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Lathabai, S.; Lloyd, P.G. The effect of scandium on the microstructure, mechanical properties and weldability of a cast Al–Mg alloy. Acta Mater. 2002, 50, 4275–4292. [Google Scholar] [CrossRef]
- Court, S.A.; Gatenby, K.M.; Lloyd, D.J. Factors affecting the strength and formability of alloys based on Al–3 wt.% Mg. Mater. Sci. Eng. A 2001, 319, 443–447. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Li, S.; Deng, Y.; Zhou, H.; Xu, G.F.; Yin, Z.M. Synergetic effects of Sc and Zr microalloying and heat treatment on mechanical properties and exfoliation corrosion behavior of Al-Mg-Mn alloys. Mater. Sci. Eng. A 2016, 666, 61–71. [Google Scholar] [CrossRef]
- Zhou, S.A.; Zhang, Z.; Li, M.; Pan, D.J.; Su, H.L.; Du, X.D.; Li, P.; Wu, Y.C. Effect of Sc on microstructure and mechanical properties of as-cast Al–Mg alloys. Mater. Des. 2016, 90, 1077–1084. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Liang, X.; Wang, Y.; Liu, H. Effect of Sc and Nd on the Microstructure and Mechanical Properties of Al-Mg-Mn Alloy. J. Mater. Eng. Perform. 2010, 21, 83–88. [Google Scholar] [CrossRef]
- Karnesky, R.A.; Dunand, D.C.; Seidman, D.N. Evolution of nanoscale precipitates in Al microalloyed with Sc and Er. Acta Mater. 2009, 57, 4022–4031. [Google Scholar] [CrossRef]
- Wu, X.L.; Nie, Z.R.; Wen, S.P.; Gao, K.Y.; Huang, H. New Progress on Er-Containing Micro-Alloying Aluminum Alloys. Mater. Sci. Forum 2016, 877, 211–217. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Yarasu, V.; Barkov, R.Y.; Yakovtseva, O.A.; Makhov, S.V.; Napalkov, V.I. Microstructure and mechanical properties of novel Al-Mg-Mn-Zr-Sc-Er alloy. Mater. Lett. 2017, 202, 116–119. [Google Scholar] [CrossRef]
- Che, H.; Jiang, X.; Qiao, N.; Liu, X. Effects of Er/Sr/Cu additions on the microstructure and mechanical properties of Al-Mg alloy during hot extrusion. J. Alloys Compd. 2017, 708, 662–670. [Google Scholar] [CrossRef]
- Fu, L.; Peng, Y.Y.; Huang, J.W.; Deng, Y.; Yin, Z.M. Microstructures and mechanical properties of Gas Tungsten Arc Welded joints of new Al–Mg–Sc and Al–Mg–Er alloy plates. Mater. Sci. Eng. A 2015, 620, 149–154. [Google Scholar] [CrossRef]
- Wen, S.P.; Xing, Z.B.; Huang, H.; Li, B.L.; Wang, W.; Nie, Z.R. The effect of erbium on the microstructure and mechanical properties of Al–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2009, 516, 42–49. [Google Scholar] [CrossRef]
- Xu, G.F.; Mou, S.Z.; Yang, J.J.; Jin, T.N.; Nie, Z.R.; Yin, Z.M. Effect of trace rare earth element Er on Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2006, 16, 598–603. [Google Scholar] [CrossRef]
- Forbord, B.; Lefebvre, W.; Danoix, F.; Hallem, H.; Marthinsen, K. Three dimensional atom probe investigation on the formation of Al3(Sc,Zr)-dispersoids in aluminium alloys. Scr. Mater. 2004, 51, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Kendig, K.L.; Miracle, D.B. Strengthening mechanisms of an Al-Mg-Sc-Zr alloy. Acta Mater. 2002, 50, 4165–4175. [Google Scholar] [CrossRef]
- Knipling, K.E.; Karnesky, R.A.; Lee, C.P.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–0.1Sc, Al–0.1Zr and Al–0.1Sc–0.1Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184–5195. [Google Scholar] [CrossRef]
- Ikeshita, S.; Strodahs, A.; Saghi, Z.; Yamada, K.; Burdet, P.; Hata, S.; Ikeda, K.I.; Midgley, P.A.; Kaneko, K. Hardness and microstructural variation of Al-Mg-Mn-Sc-Zr alloy. Micron 2016, 82, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, G.F.; Yin, Z.M.; Lei, X.F.; Huang, J.W. Effects of Sc and Zr microalloying additions on the recrystallization texture and mechanism of Al–Zn–Mg alloys. J. Alloys Compd. 2013, 580, 412–426. [Google Scholar] [CrossRef]
- Fuller, C.B.; Krause, A.R.; Dunand, D.C.; Seidman, D.N. Microstructure and mechanical properties of a 5754 aluminum alloy modified by Sc and Zr additions. Mater. Sci. Eng. A 2002, 338, 8–16. [Google Scholar] [CrossRef]
- Yang, D.X.; Li, X.Y.; He, D.Y.; Huang, H. Effect of minor Er and Zr on microstructure and mechanical properties of Al–Mg–Mn alloy (5083) welded joints. Mater. Sci. Eng. A 2013, 561, 226–231. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Q.L.; Song, Y.F.; Li, C.; Li, Z.F.; Chen, Q.; Yin, Z.M. Recrystallization of Al-5.8Mg-Mn-Sc-Zr alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 3235–3241. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhang, D.; Cui, H.; Zhuang, L.Z.; Zhang, J.S. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al–Mg alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al–Mg–Zn alloys. J. Alloys Compd. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhang, D.; Liu, P.P.; Zhuang, L.Z.; Zhang, J.S. Microstructure characterization in a sensitized Al-Mg-Mn-Zn alloy. Rare Met. 2018, 37, 129–135. [Google Scholar] [CrossRef]
- Sukiman, N.L.; Gupta, R.K.; Buchheit, R.G.; Birbilis, N. Influence of microalloying additions on Al–Mg alloy. Part 1: Corrosion and electrochemical response. Corros. Eng. Sci. Technol. 2013, 49, 254–262. [Google Scholar] [CrossRef]
- Engler, O.; Brinkman, H.J.; Hentschel, T. Simulation-Based Design of 5xxx Series Alloys with Improved Resistivity against Intergranular Corrosion for Automotive Applications. Mater. Sci. Forum 2014, 794–796, 622–627. [Google Scholar] [CrossRef]
- Medrano, S.; Zhao, H.; De Geuser, F.; Gault, B.; Stephenson, L.T.; Deschamps, A.; Ponge, D.; Raabe, D.; Sinclair, C.W. Cluster hardening in Al-3Mg triggered by small Cu additions. Acta Mater. 2018, 161, 12–20. [Google Scholar] [CrossRef]
- Engler, O.; Marioara, C.D.; Hentschel, T.; Brinkman, H.J. Influence of copper additions on materials properties and corrosion behaviour of Al–Mg alloy sheet. J. Alloys Compd. 2017, 710, 650–662. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, H. Corrosion Behavior of Extruded near Eutectic Al–Si–Mg and 6063 Alloys. J. Mater. Sci. Technol. 2013, 29, 380–386. [Google Scholar] [CrossRef]
- Zeng, F.L.; Wei, Z.L.; Li, J.F.; Li, C.X.; Tan, X.; Zhang, Z.; Zheng, Z.Q. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Eckermann, F.; Suter, T.; Uggowitzer, P.J.; Afseth, A.; Schmutz, P. The influence of MgSi particle reactivity and dissolution processes on corrosion in Al–Mg–Si alloys. Electrochim. Acta 2008, 54, 844–855. [Google Scholar] [CrossRef]
- AQSIQ, SAC. GB/T 16865-2013. Test Pieces and Method for Tensile Test for Wrought Aluminum and Magnesium Alloys Products; AQSIQ, SAC: Beijing, China, 2013.
- ASTM. G67-13: Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss After Exposure to Nitric Acid (NAMLT Test); ASTM: West Conshohocken, PA, USA, 2013. [CrossRef]
- Engler, O.; Kuhnke, K.; Westphal, K.; Hasenclever, J. Impact of chromium on the microchemistry evolution during solidification and homogenization of the Al-Mg alloy AA 5052. J. Alloys Compd. 2018, 744, 561–573. [Google Scholar] [CrossRef]
- Sukiman, N.L.; Zhou, X. Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments; IntechOpen Limited: London, UK, 2008; pp. 154–196. [Google Scholar]
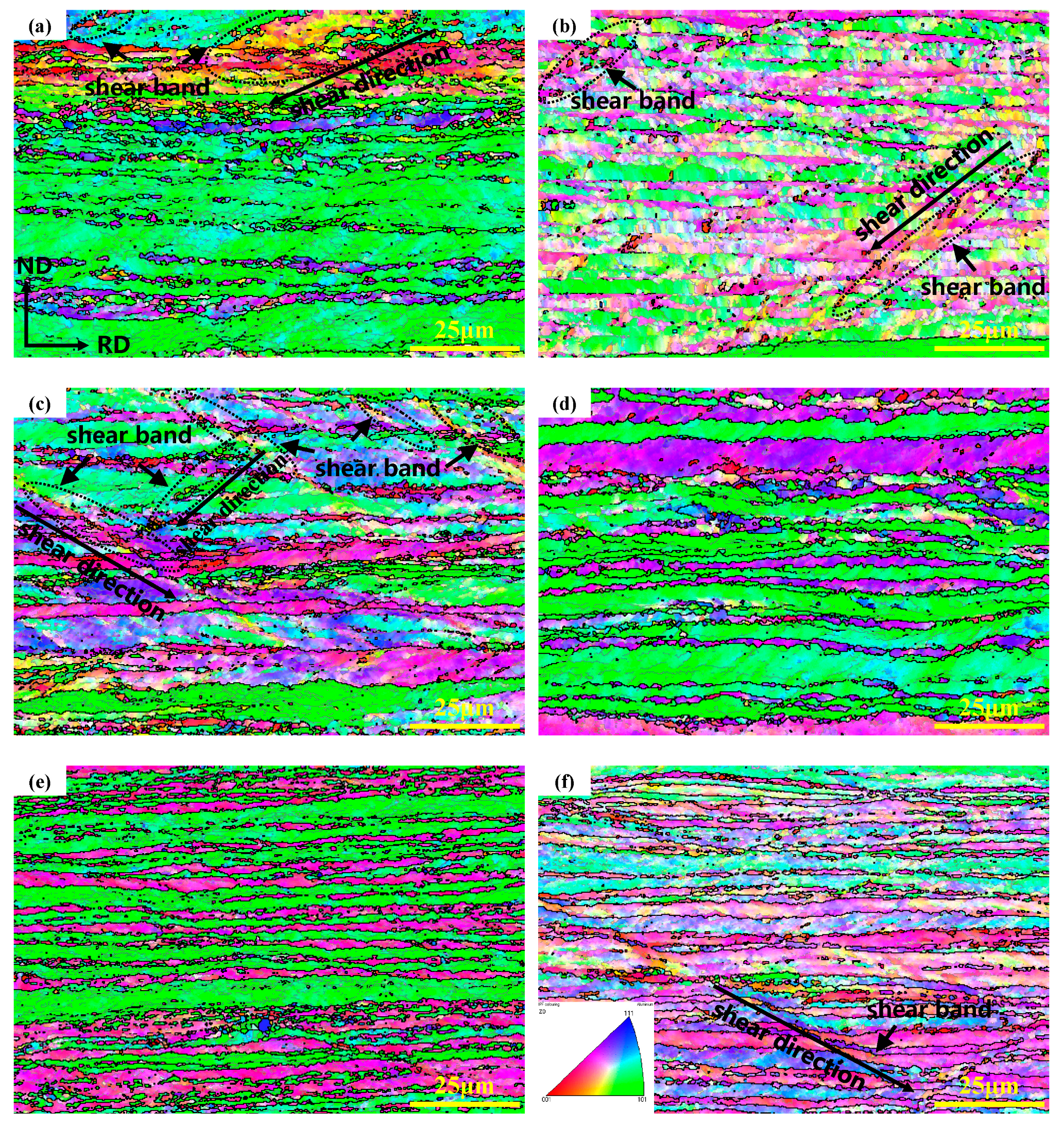
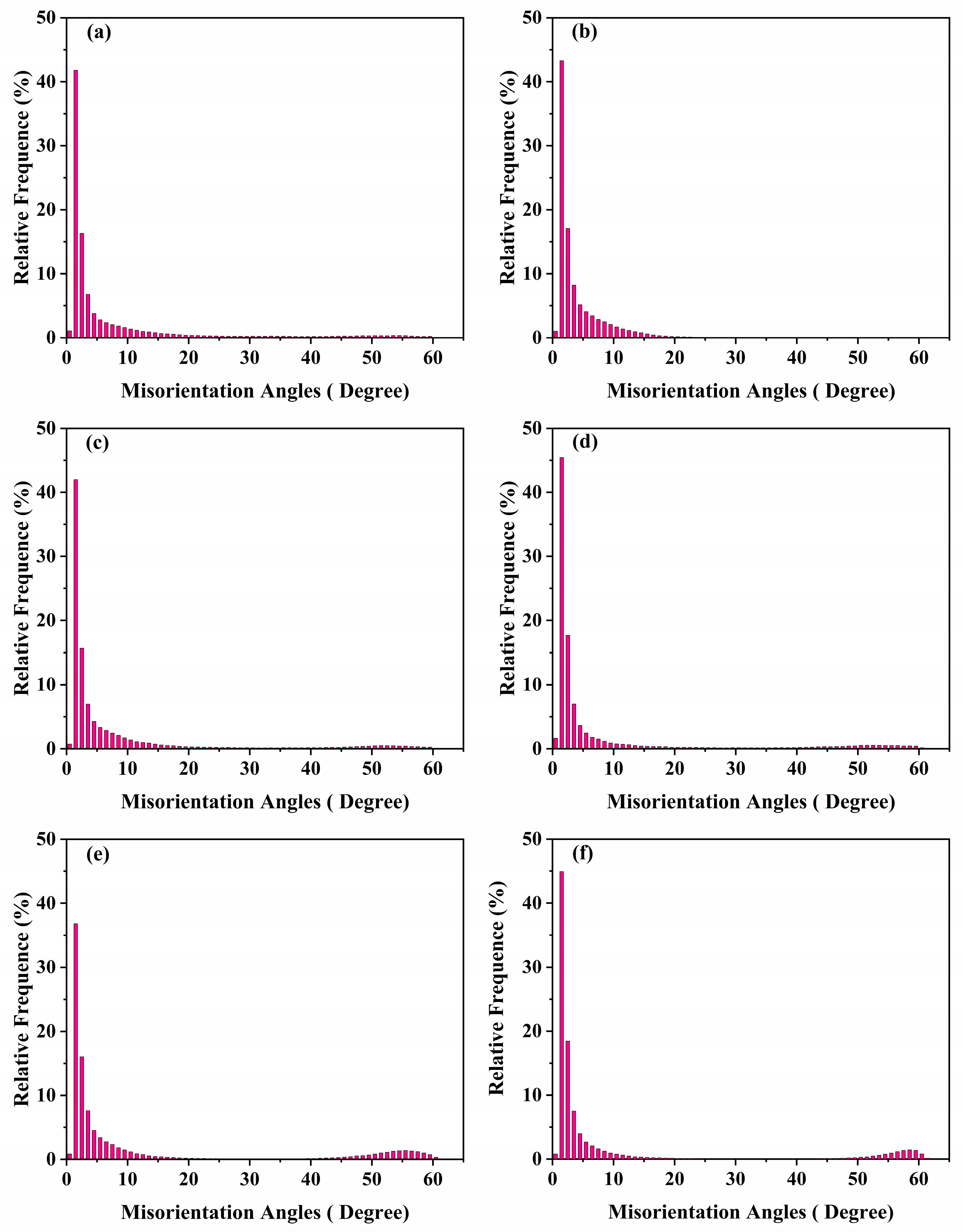
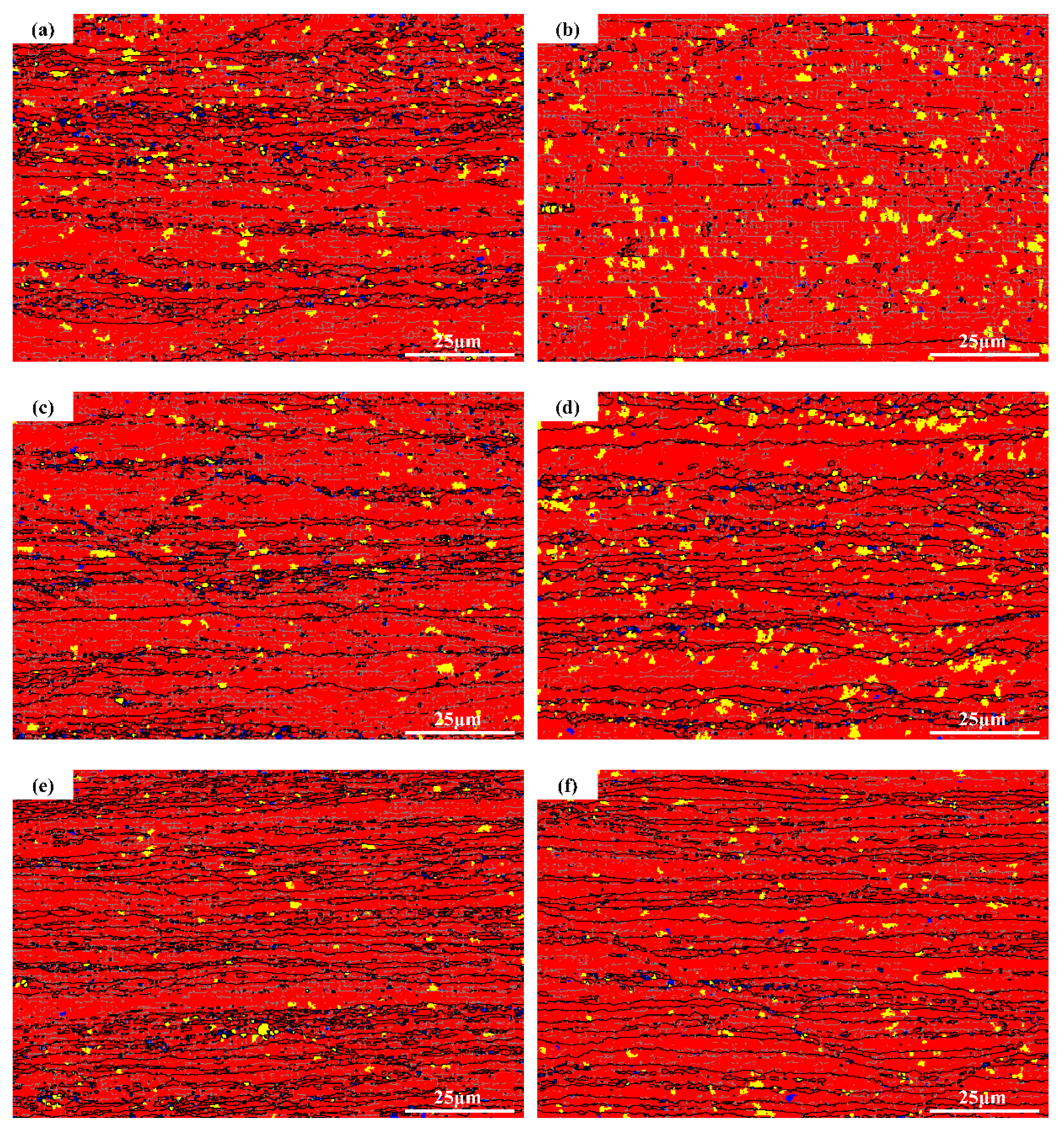

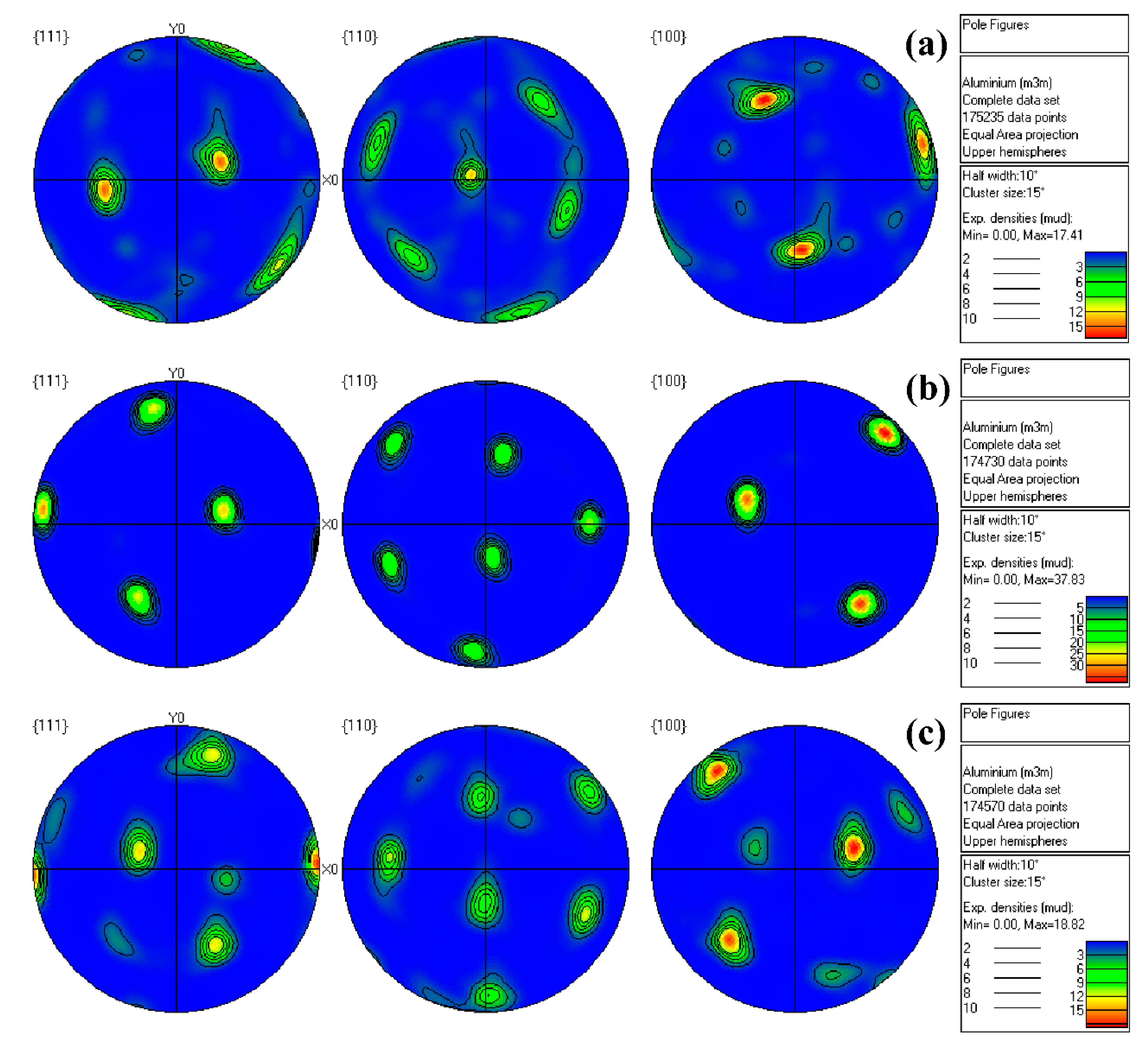




| Alloys | Mg | Mn | Er | Zr | Cu | Si | Zn | Al |
|---|---|---|---|---|---|---|---|---|
| 1#: Al-3Mg-0.5Mn | 3 | 0.5 | ― | ― | ― | ― | ― | Bal |
| 2#: Al-3Mg-0.5Mn-0.2Er | 3 | 0.5 | 0.2 | ― | ― | ― | ― | Bal |
| 3#: Al-3Mg-0.5Mn-0.2Zr | 3 | 0.5 | ― | 0.2 | ― | ― | ― | Bal |
| 4#: Al-3Mg-0.5Mn-0.2Cu | 3 | 0.5 | ― | ― | 0.2 | ― | ― | Bal |
| 5#: Al-3Mg-0.5Mn-0.2Si | 3 | 0.5 | ― | ― | ― | 0.2 | ― | Bal |
| 6#: Al-3Mg-0.5Mn-0.2Zn | 3 | 0.5 | ― | ― | ― | ― | 0.2 | Bal |
| Alloys | Cube Texture | Goss Texture | Brass Texture | Copper Texture | S Texture | Random Texture |
|---|---|---|---|---|---|---|
| 1# | 0.06 | 19.10 | 6.19 | 0.40 | 21.60 | 52.65 |
| 2# | 0 | 2.88 | 0.16 | 21.30 | 72.30 | 3.36 |
| 3# | 0.11 | 0.50 | 0.34 | 19.40 | 68.20 | 11.45 |
| 4# | 0.08 | 16.20 | 4.64 | 22.30 | 20.90 | 35.88 |
| 5# | 0.01 | 10.60 | 16.60 | 0.41 | 54.3 | 18.08 |
| 6# | 0 | 0 | 1.11 | 8.81 | 75.2 | 14.88 |
| Alloys | Liquidus Temperature | Solidus Temperature | Crystallization Temperature Interval |
|---|---|---|---|
| 1#:Al-3Mg-0.5Mn | 661.5 | 639.0 | 22.5 |
| 2#:Al-3Mg-0.5Mn-0.2Er | 656.5 | 634.3 | 22.2 |
| 3#:Al-3Mg-0.5Mn-0.2Zr | 669.7 | 636.1 | 33.6 |
| 4#:Al-3Mg-0.5Mn-0.2Cu | 667.4 | 641.4 | 26.0 |
| 5#:Al-3Mg-0.5Mn-0.2Si | 664.6 | 639.7 | 24.9 |
| 6#:Al-3Mg-0.5Mn-0.2Zn | 659.5 | 641.7 | 17.8 |
| Samples | Icorr (A/cm2) | Ecorr (V) |
|---|---|---|
| 1#:Al-3Mg-0.5Mn | 8.20 × 10−8 | −0.93 |
| 2#:Al-3Mg-0.5Mn-0.2Er | 8.89 × 10−9 | −0.81 |
| 3#:Al-3Mg-0.5Mn-0.2Zr | 3.98 × 10−8 | −0.91 |
| 4#:Al-3Mg-0.5Mn-0.2Cu | 6.97 × 10−8 | −0.79 |
| 5#:Al-3Mg-0.5Mn-0.2Si | 6.25 × 10−8 | −0.97 |
| 6#:Al-3Mg-0.5Mn-0.2Zn | 9.34 × 10−9 | −0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, M.; Lu, G.; Guan, S.; Zhang, E.; Xu, C. Effect of Trace Elements on the Crystallization Temperature Interval and Properties of 5xxx Series Aluminum Alloys. Metals 2020, 10, 483. https://doi.org/10.3390/met10040483
Li Q, Li M, Lu G, Guan S, Zhang E, Xu C. Effect of Trace Elements on the Crystallization Temperature Interval and Properties of 5xxx Series Aluminum Alloys. Metals. 2020; 10(4):483. https://doi.org/10.3390/met10040483
Chicago/Turabian StyleLi, Qianqian, Mengjia Li, Guangxi Lu, Shaokang Guan, Engui Zhang, and Cong Xu. 2020. "Effect of Trace Elements on the Crystallization Temperature Interval and Properties of 5xxx Series Aluminum Alloys" Metals 10, no. 4: 483. https://doi.org/10.3390/met10040483




