Selective and Mutual Separation of Palladium (II), Platinum (IV), and Rhodium (III) Using Aliphatic Primary Amines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metal Precipitation Experiments from Metal-Containing Solutions
2.3. Mutual Separation of Pd(II), Pt(IV), and Rh(III)
2.4. Isolation of Metal-Containing Precipitate
2.5. Measurements
3. Results and Discussion
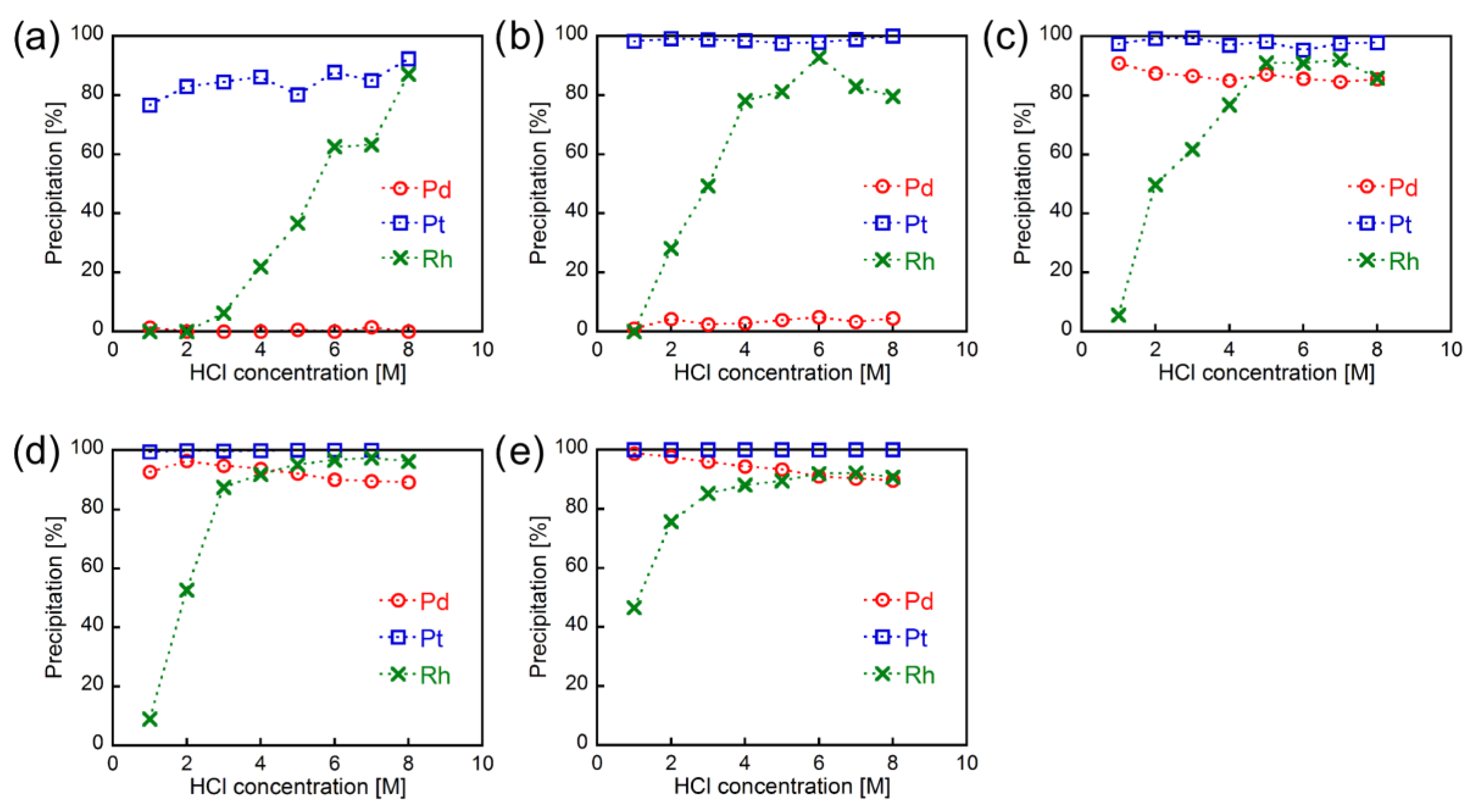
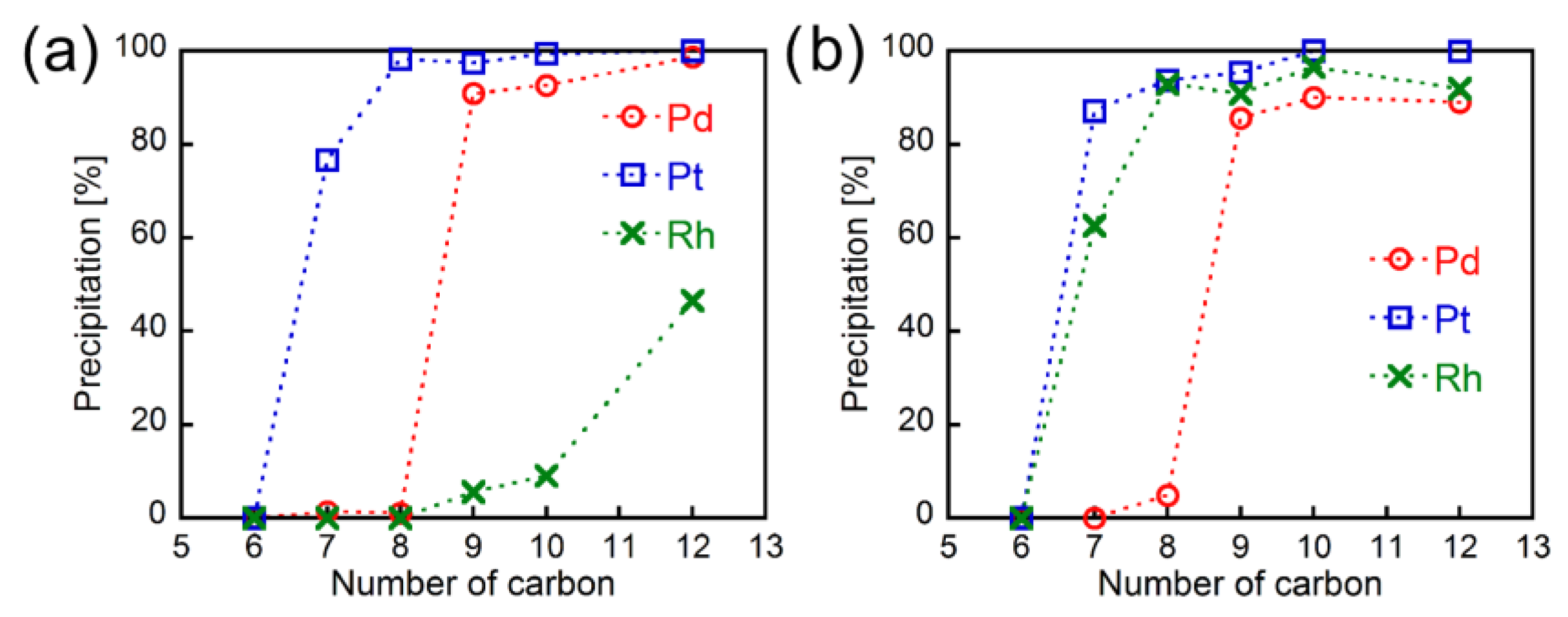
3.1. Metal Precipitation Experiments
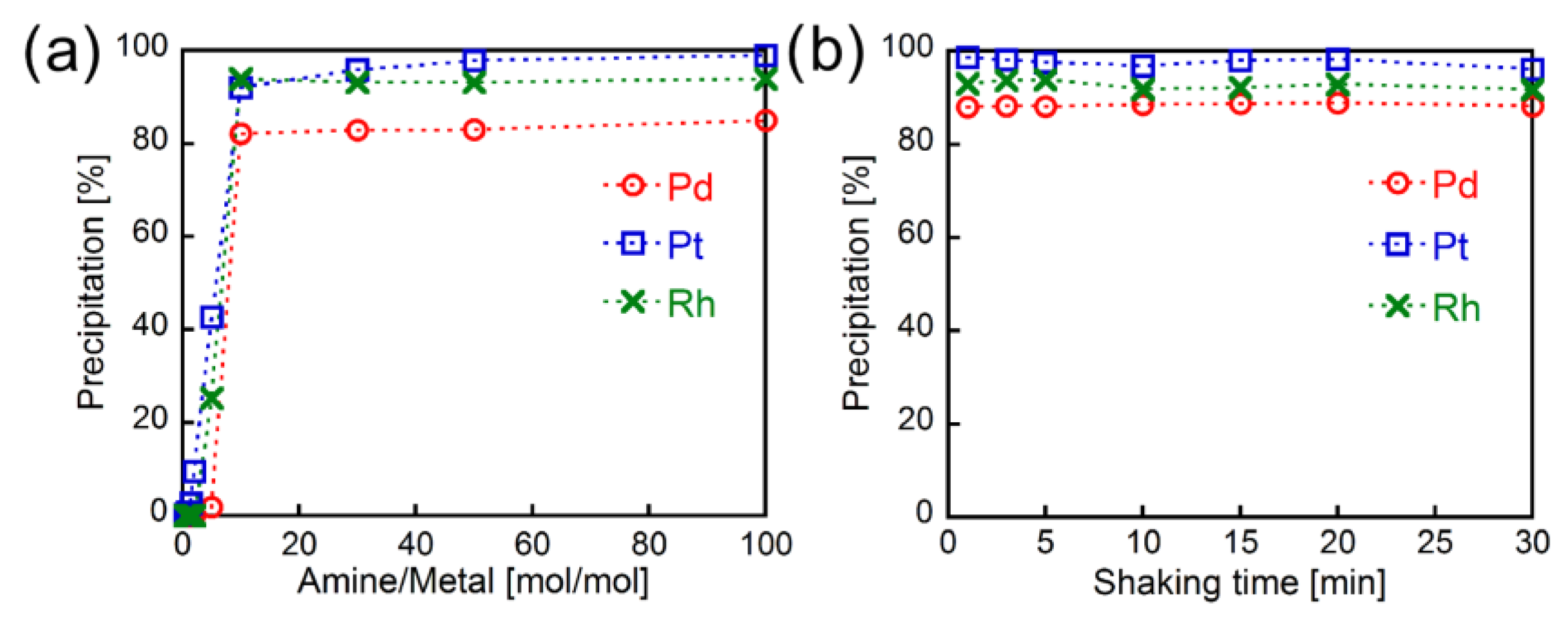
3.2. Loading Amount and Shaking Time
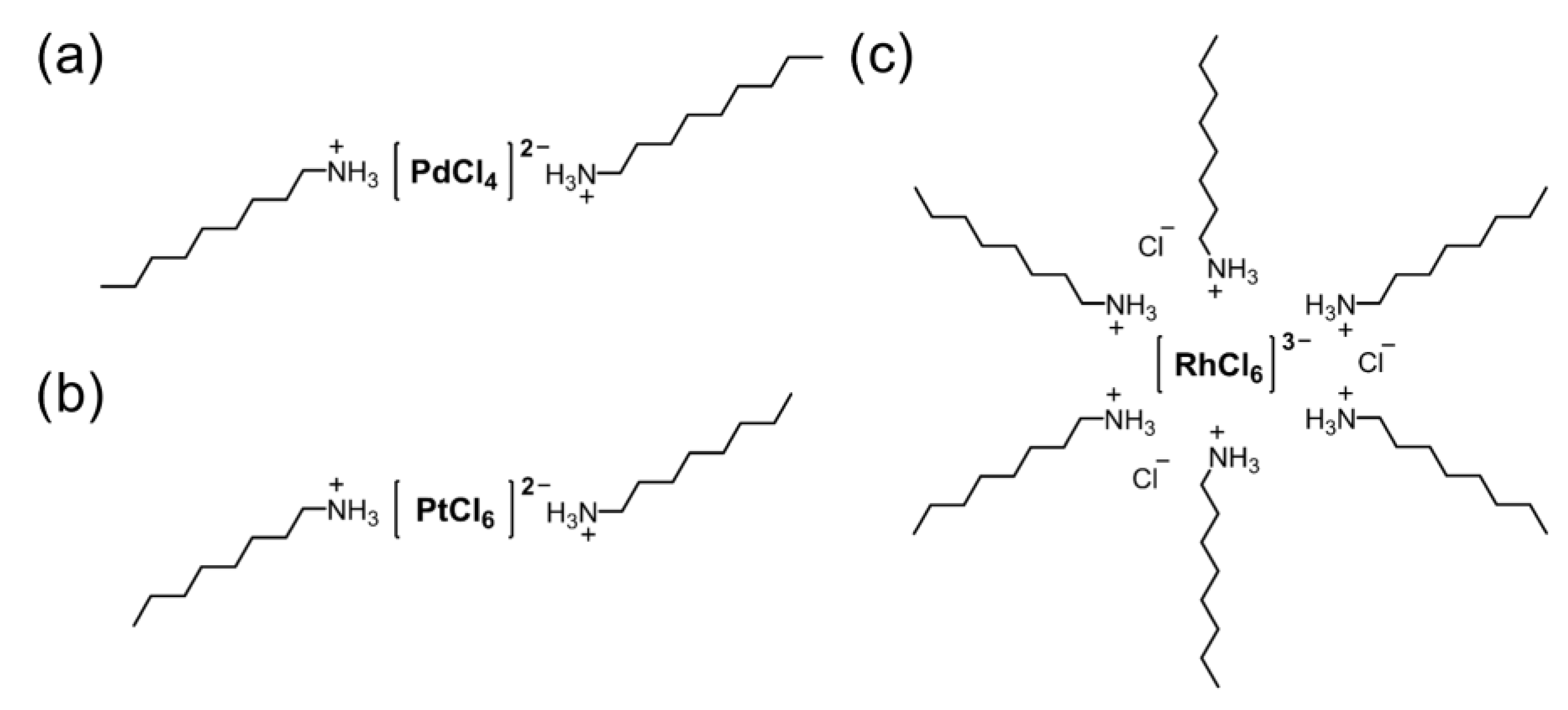
3.3. Mutual Separation of Pd(II), Pt(IV), and Rh(III)
3.4. XPS Analysis of Metal-Containing Precipitates
3.5. Thermogravimetric Analysis of Metal-Containing Precipitates
3.6. Metal Precipitation Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alonso, E.; Field, F.R.; Kirchain, R.E. Platinum availability for future automotive technologies. Environ. Sci. Technol. 2012, 46, 12986–12993. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Froehlich, P.; Lorenz, T.; Martin, G.; Brett, B.; Bertau, M. Valuable metals—recovery processes, current trends, and recycling strategies. Angew. Chem. Int. Ed. 2017, 56, 2544–2580. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the platinum group metals: A European perspective. Platinum Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, J.Y.; Lee, M.S. Separation of Pt (IV) and Rh (III) from chloride solution by solvent extraction with amine and neutral extractants. Mater. Trans. 2011, 52, 2071–2076. [Google Scholar] [CrossRef] [Green Version]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium (II), platinum (IV), gold (III) and rhodium (III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery form real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Senanayake, G. Separation of Pt (IV), Rh (III) and Fe (III) in acid chloride leach solutions of glass scraps by solvent extraction with various extractants. Hydrometallurgy 2018, 175, 232–239. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Kubota, F.; Goto, M. Solvent extraction of Pt (IV), Pd (II), and Rh (III) with the ionic liquid trioctyl(dodecyl) phosphonium chloride. J. Chem. Technol. Biotechnol. 2018, 93, 1714–1721. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt (IV), Pd (II), Ru (III) and Rh (III) from model chloride solutions by liquid-liquid extraction with phosphonium ionic liquids. Sep. Purif. Technol. 2019, 212, 791–801. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K. Palladium extraction with N,N,N′,N′-tetra-n-octyl-thiodiglycolamide. Miner. Eng. 2008, 21, 483–488. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover palladium (II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G.R. Solvent Extraction Principles and Practice, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Nikoloski, A.N.; Ang, K.L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extract. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamakawa, S.; Jikei, M. Selective recovery of platinum (IV) from palladium (II)-containing solution using 4-(hexyloxy)aniline. Chem. Lett. 2017, 46, 22–24. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamakawa, S.; Sezaki, Y.; Katagiri, H.; Jikei, M. Preferential precipitation and selective separation of Rh(III) from Pd(II) and Pt(IV) using 4-alkylanilines as precipitants. ACS Omega 2019, 4, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Hata, Y.; Sezaki, Y.; Katagiri, H.; Jikei, M. Highly selective Rh(III) recovery from HCl solutions using aromatic primary diamines via formation of three-dimensional ionic crystals. ACS Omega 2019, 4, 14613–14620. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Yamakawa, S.; Haga, K.; Ishibashi, K.; Jikei, M.; Shibayama, A. Selective and preferential separation of rhodium (III) from palladium (II) and platinum (IV) using a m-phenylene diamine-containing precipitant. Sci. Rep. 2019, 9, 12414. [Google Scholar] [CrossRef] [Green Version]
- Benguerel, E.; Demopoulos, G.P.; Harris, G.B. Speciation and separation of rhodium (III) from chloride solutions: A critical review. Hydrometallurgy 1996, 40, 135–152. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Maniecki, T.P. Influence of atmosphere kind on temperature programmed decomposition of noble metal chlorides. Thermochim. Acta 2005, 435, 151–161. [Google Scholar] [CrossRef]



| Metal | Ash at 750 °C a | Expected Weight Fraction b |
|---|---|---|
| Pd(II) | 22.8% | 22.5% |
| Pt(IV) | 28.7% | 29.2% |
| Rh(III) | 10.3% | 10.5% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.; Sezaki, Y.; Yamakawa, S.; Hata, Y.; Jikei, M. Selective and Mutual Separation of Palladium (II), Platinum (IV), and Rhodium (III) Using Aliphatic Primary Amines. Metals 2020, 10, 324. https://doi.org/10.3390/met10030324
Matsumoto K, Sezaki Y, Yamakawa S, Hata Y, Jikei M. Selective and Mutual Separation of Palladium (II), Platinum (IV), and Rhodium (III) Using Aliphatic Primary Amines. Metals. 2020; 10(3):324. https://doi.org/10.3390/met10030324
Chicago/Turabian StyleMatsumoto, Kazuya, Yuto Sezaki, Sumito Yamakawa, Yuki Hata, and Mitsutoshi Jikei. 2020. "Selective and Mutual Separation of Palladium (II), Platinum (IV), and Rhodium (III) Using Aliphatic Primary Amines" Metals 10, no. 3: 324. https://doi.org/10.3390/met10030324





