Corrosion Behavior and Microstructure of Stir Zone in Fe-30Mn-3Al-3Si Twinning-Induced Plasticity Steel after Friction Stir Welding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation and FSW Condition
2.2. Microstructural Characterization
2.3. Corrosion Tests
3. Results
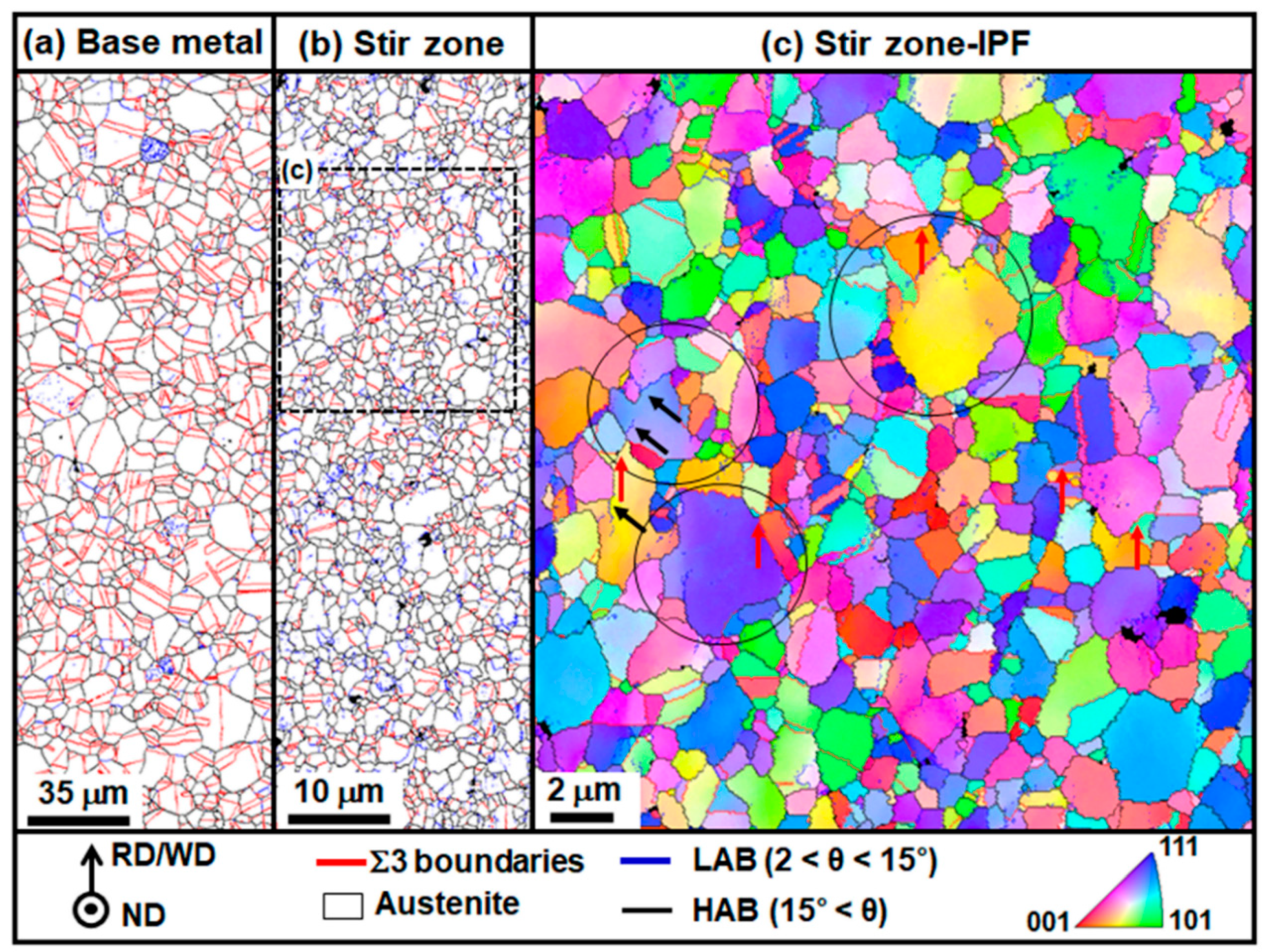
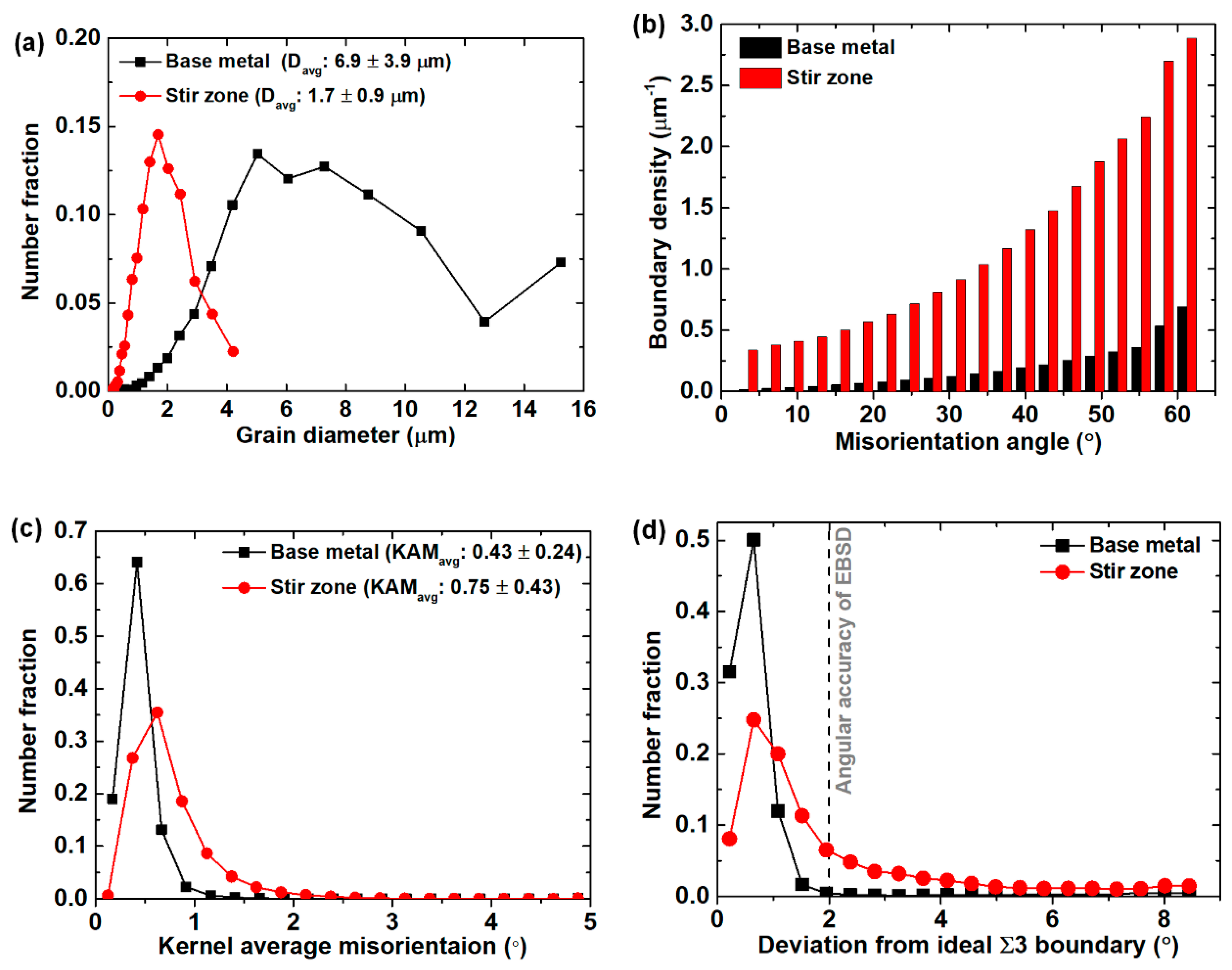
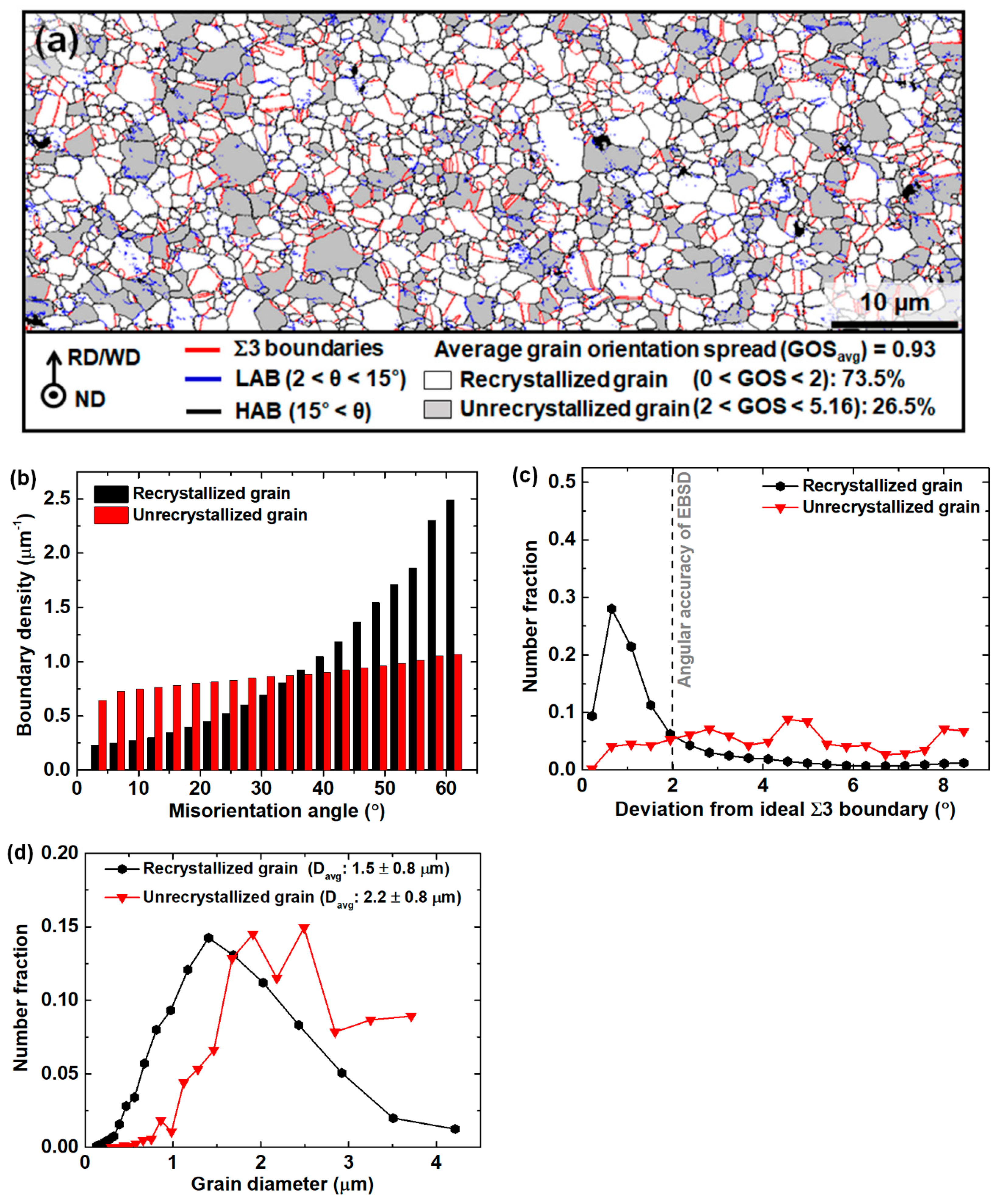
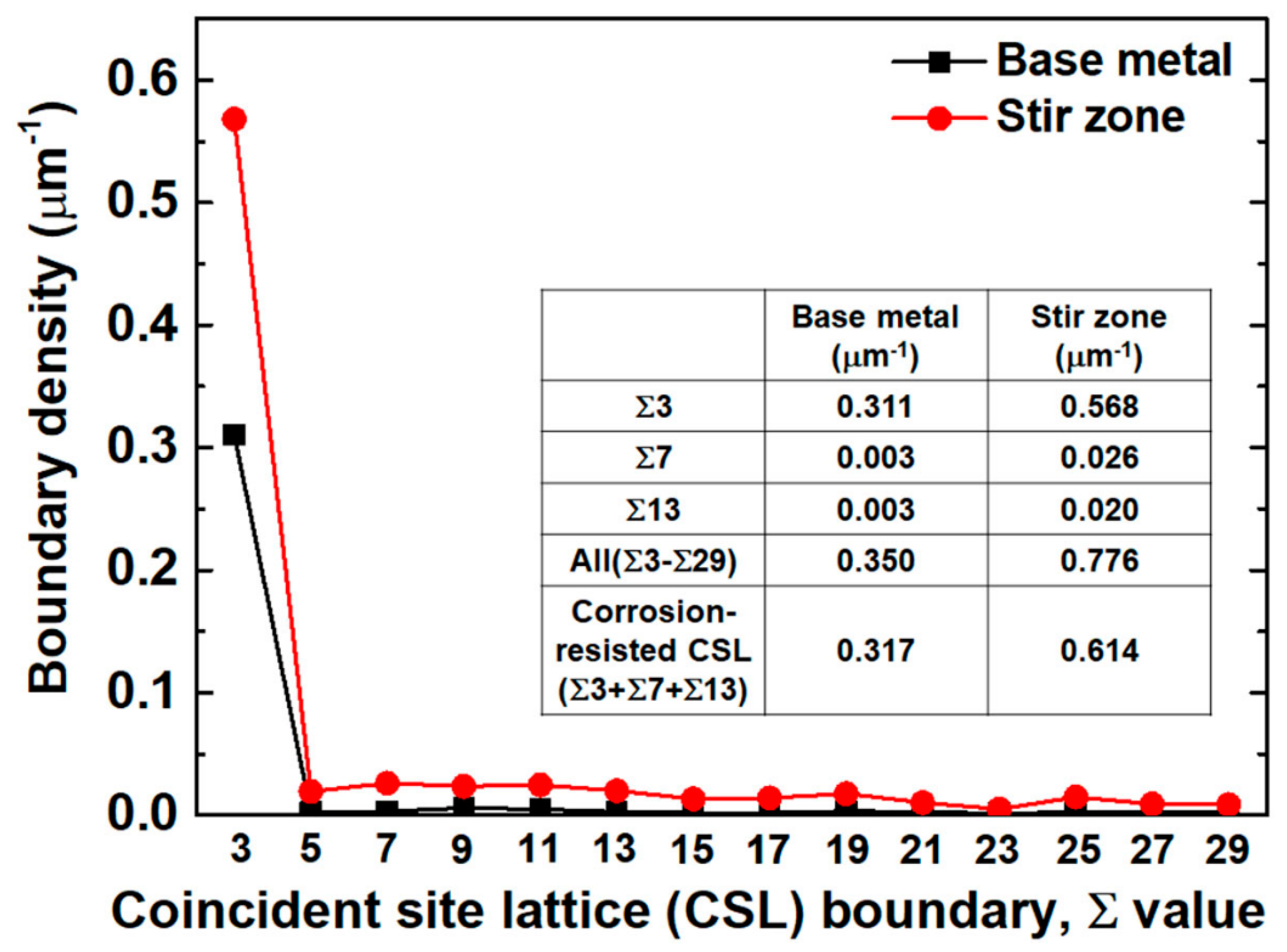
3.1. Microstructure before and after the Friction Stir Welding
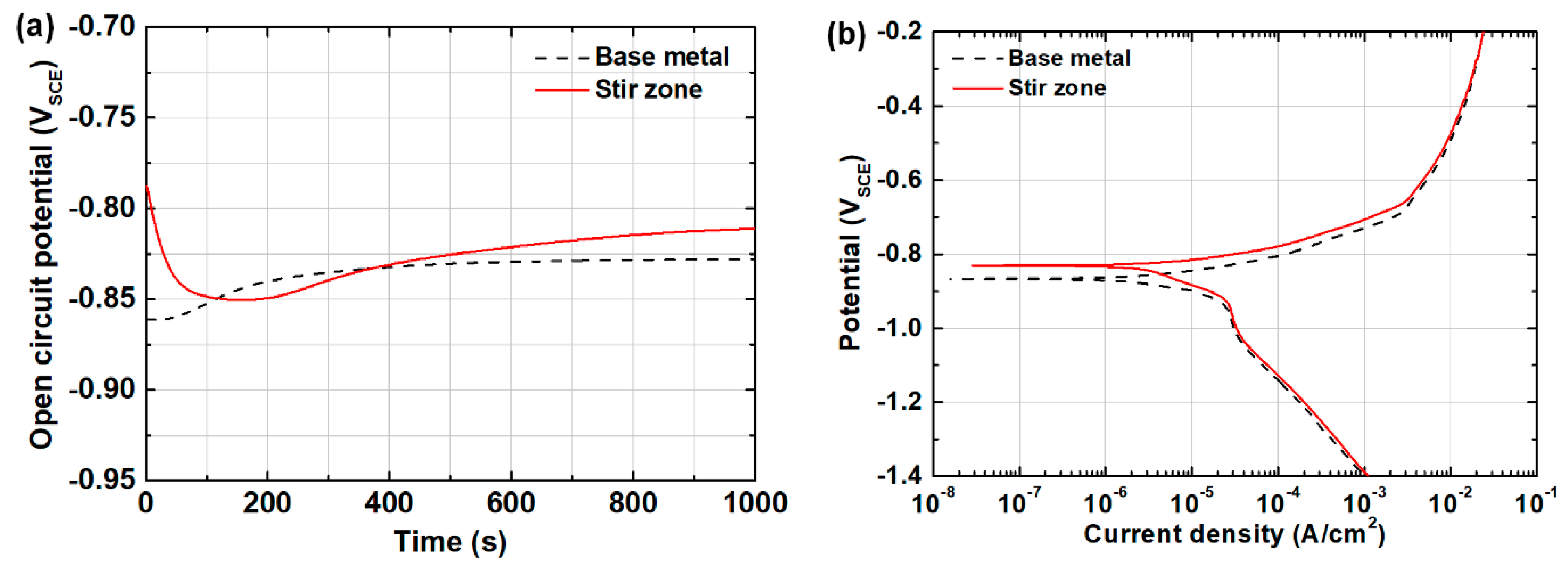
3.2. Corrosion Behavior before and after the Friction Stir Welding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-induced plasticity (TWIP) steels. Acta Mater. 2018, 142, 283–362. [Google Scholar]
- Steinmetz, D.R.; Jäpel, T.; Wietbrock, B.; Eisenlohr, P.; Gutierrez-Urrutia, I.; Saeed–Akbari, A.; Hickel, T.; Roters, F.; Raabe, D. Revealing the strain-hardening behavior of twinning-induced plasticity steels: Theory, simulations, experiments. Acta Mater. 2013, 61, 494–510. [Google Scholar]
- Galindo-Nava, E.I.; Rivera-Díaz-del-Castillo, P.E.J. Understanding martensite and twin formation in austenitic steels: A model describing TRIP and TWIP effects. Acta Mater. 2017, 128, 120–134. [Google Scholar]
- Lee, S.-J.; Han, J.; Lee, S.; Kang, S.H.; Lee, S.M.; Lee, Y.K. Design for Fe-high Mn alloy with an improved combination of strength and ductility. Sci. Rep. 2017, 7, 3573. [Google Scholar]
- Chung, K.; Ahn, K.; Yoo, D.H.; Chung, K.H.; Seo, M.H.; Park, S.H. Formability of TWIP (twinning induced plasticity) automotive sheets. Int. J. Plast. 2011, 27, 52–81. [Google Scholar]
- Lee, S.-J.; Sun, Y.; Fujii, H. Stacking-fault energy, mechanical twinning and strain hardening of Fe-18Mn-0.6C-(0, 1.5)Al twinning-induced plasticity steels during friction stir welding. Acta Mater. 2018, 148, 235–248. [Google Scholar]
- Lee, S.-J.; Ushioda, K.; Fujii, H. Evaluation of stacking-fault energy in Fe-Mn based twinning-induced plasticity steels after friction stir welding. Mater. Charact. 2019, 147, 379–383. [Google Scholar]
- Park, G.; Kim, B.; Kang, Y.; Kang, H.; Lee, C. Characterization of bond line discontinuities in a high-Mn TWIP steel pipe welded by HF-ERW. Mater. Charact. 2016, 118, 14–21. [Google Scholar]
- Torganchuk, V.; Vysotskiy, I.; Malopheyev, S.; Mironov, S.; Kaibyshev, R. Microstructure evolution and strengthening mechanisms in friction-stir welded TWIP steel. Mater. Sci. Eng. A 2019, 746, 248–258. [Google Scholar]
- Kannan, M.B.; Raman, R.K.S.; Khoddam, S.; Liyanaarachchi, S. Corrosion behavior of twinning-induced plasticity (TWIP) steel. Mater. Corros. 2013, 64, 231–235. [Google Scholar]
- Fajardo, S.; Llorente, I.; Jiménez, J.A.; Bastidas, J.M.; Bastidas, D.M. Effect of Mn additions on the corrosion behaviour of TWIP Fe-Mn-Al-Si austenitic steel in chloride solution. Corros. Sci. 2019, 154, 246–253. [Google Scholar]
- Fujii, H.; Cui, L.; Tsuji, N.; Maeda, M.; Nakata, K.; Nogi, K. Friction stir welding of carbon steels. Mater. Sci. Eng. A 2006, 429, 50–57. [Google Scholar]
- Mújica Roncery, L.; Weber, S.; Theisen, W. Welding of twinning-induced plasticity steels. Scripta Mater. 2012, 66, 997–1001. [Google Scholar]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005–075005-13. [Google Scholar]
- Orłowska, M.; Ura-Bińczyk, E.; Olejnik, L.; Lewandowska, M. The effect of grain size and grain boundary misorientation on the corrosion resistance of commercially pure aluminium. Corros. Sci. 2019, 148, 57–70. [Google Scholar]
- Hao, Y.W.; Deng, B.; Zhong, C.; Jiang, Y.M.; Li, J. Effect of Surface Mechanical Attrition Treatment on Corrosion Behavior of 316 Stainless Steel. J. Iron Steel Res. Int. 2009, 16, 68–72. [Google Scholar]
- Wang, X.Y.; Li, D.Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel. Electrochim. Acta 2002, 47, 3939–3947. [Google Scholar]
- Tiamiyu, A.A.; Eduok, U.; Szpunar, J.A.; Odeshi, A.G. Corrosion behavior of metastable AISI 321 austenitic stainless steel: Investigating the effect of grain size and prior plastic deformation on its degradation pattern in saline media. Sci. Rep. 2019, 9, 12116. [Google Scholar]
- Kim, H.-J.; Jeon, S.-H.; Kim, S.-T.; Lee, I.-S.; Park, Y.-S.; Kim, K.-T.; Kim, Y.-S. Investigation of the sensitization and intergranular corrosion of tube-to-tubesheet welds of hyper duplex stainless steel using an electrochemical reactivation method. Corros. Sci. 2014, 87, 60–70. [Google Scholar]
- Wu, W.; Hu, S.; Shen, J. Microstructure, mechanical properties and corrosion behavior of laser welded dissimilar joints between ferritic stainless steel and carbon steel. Mater. Des. 2015, 65, 855–861. [Google Scholar]
- Huang, L.-Y.; Wang, K.-S.; Wang, W.; Zhao, K.; Yuan, J.; Qiao, K.; Zhang, B.; Cai, J. Mechanical and corrosion properties of low-carbon steel prepared by friction stir processing. Int. J. Miner. Metall. Mater. 2019, 26, 202–209. [Google Scholar]
- ASTM. ASTM G 5-04, Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM. ASTM G-106, Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Dudzik, K.; Charchalis, A. EIS Research of AW-7020 Alloy Joints Welded by FSW. Solid State Phenom. 2013, 199, 412–417. [Google Scholar]
- Starosta, R. Corrosion of Ni-Al and Ni-Al-Al2O3 Flame Sprayed Coatings of “CastoDyn 8000” System in 0.01 M H2SO4 and 3.5% NaCl Solutions. Solid State Phenom. 2012, 183, 185–192. [Google Scholar]
- Brandon, D.G. The structure of high-angle grain boundaries. Acta Metall. 1966, 14, 1479–1484. [Google Scholar]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and post-dynamic recrystallization under hot, cold and severe plastic deformation conditions. Progress Mater. Sci. 2014, 60, 130–207. [Google Scholar]
- Liu, X.C.; Sun, Y.F.; Nagira, T.; Fujii, H. Investigation of temperature dependent microstructure evolution of pure iron during friction stir welding using liquid CO2 rapid cooling. Mater. Charact. 2018, 137, 24–38. [Google Scholar]
- Pierce, J.; Jiménez, D.T.A.; Bentley, J.; Raabe, D.; Oskay, C.; Wittig, J.E. The influence of manganese content on the stacking fault and austenite/ε-martensite interfacial energies in Fe–Mn–(Al–Si) steels investigated by experiment and theory. Acta Mater. 2014, 68, 238–253. [Google Scholar]
- Bhattacharyya, M.; Langelier, B.; Purdy, G.R.; Zurob, H.S. Effect of Mn and C on Grain Growth in Mn Steels. Metall. Mater. Trans. A 2019, 50, 905–914. [Google Scholar]
- Cahoon, J.R.; Li, Q.; Richards, N.L. Microstructural and processing factors influencing the formation of annealing twins. Mater. Sci. Eng. A 2009, 526, 56–61. [Google Scholar]
- Gleiter, H. The formation of annealing twins. Acta Metall. 1969, 17, 1421–1428. [Google Scholar]
- Li, H.; Wang, Y.; Peng, Q. High degradation rate of Fe-20Mn-based bio-alloys by accumulative cryo-rolling and annealing. Mater. Sci. Eng. C 2017, 79, 37–44. [Google Scholar]
- Masroor, S.; Mobin, M.; Alam, M.J.; Ahmad, S. The novel iminium surfactant p-benzylidene benzyldodecyl iminium chloride as a corrosion inhibitor for plain carbon steel in 1 M HCl: Electrochemical and DFT evaluation. RSC Adv. 2017, 7, 23182–23196. [Google Scholar]
- Lee, S.-J.; Park, T.M.; Nam, J.-H.; Choi, W.S.; Sun, Y.; Fujii, H.; Han, J. The unexpected stress-strain response of medium Mn steel after friction stir welding. Mater. Sci. Eng. A 2019, 744, 340–348. [Google Scholar]
- Jia, Q.; Liu, L.; Guo, W.; Peng, Y.; Zou, G.; Tian, Z.; Zhou, Y.N. Microstructure and tensile-shear properties of resistance spot-welded medium Mn steels. Metals 2018, 8, 48. [Google Scholar]
- Casalino, G. Recent achievements in rotary, linear and friction stir welding of metals alloys. Metals 2020, 10, 80. [Google Scholar]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar]
- Miyamoto, H.; Harada, K.; Mimaki, T.; Vinogradov, A.; Hashimoto, S. Corrosion of ultra-fine grained copper fabricated by equal-channel angular pressing. Corros. Sci. 2008, 50, 1215–1220. [Google Scholar]
- Gollapudi, S. Grain size distribution effects on the corrosion behaviour of materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar]
- Kokawa, H.; Shimada, M.; Michiuchi, M.; Wang, Z.J.; Sato, Y.S. Arrest of weld-decay in 304 austenitic stainless steel by twin-induced grain boundary engineering. Acta Mater. 2007, 55, 5401–5407. [Google Scholar]
- Han, W.; Chen, D.; Ha, Y.; Kimura, A.; Serizawa, H.; Fujii, H.; Morisada, Y. Modifications of grain-boundary structure by friction stir welding in the joint of nano-structured oxide dispersion strengthened ferritic steel and reduced activation martensitic steel. Scr. Mater. 2015, 105, 2–5. [Google Scholar]


| Specimen | Chemical Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| Mn | Al | Si | C | S | Fe | |
| C-free TWIP | 29.9 | 2.95 | 3.09 | 0.032 | 0.010 | Bal. |
| Specimen | aEcorr (mV) | Corrosion Rate | Constant Tafel Value | ||
|---|---|---|---|---|---|
| bIcorr (μA/cm2) | cmpy (Mils/Year) | dβa (mV/Decade) | eβc (mV/Decade) | ||
| Base metal | −865 | 6.080 | 2.780 | 49.9 | 122.7 |
| Stir zone | −835 | 5.350 | 2.444 | 43.3 | 141.9 |
| Specimen | aRs (Ω) | bRct (Ω) | cCdl (μF) |
|---|---|---|---|
| Base metal | 16.46 | 467.6 | 439.3 |
| Stir zone | 15.97 | 495.9 | 387.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Fujii, H.; Lee, S.-J. Corrosion Behavior and Microstructure of Stir Zone in Fe-30Mn-3Al-3Si Twinning-Induced Plasticity Steel after Friction Stir Welding. Metals 2020, 10, 1557. https://doi.org/10.3390/met10111557
Kim H-J, Fujii H, Lee S-J. Corrosion Behavior and Microstructure of Stir Zone in Fe-30Mn-3Al-3Si Twinning-Induced Plasticity Steel after Friction Stir Welding. Metals. 2020; 10(11):1557. https://doi.org/10.3390/met10111557
Chicago/Turabian StyleKim, Hye-Jin, Hidetoshi Fujii, and Seung-Joon Lee. 2020. "Corrosion Behavior and Microstructure of Stir Zone in Fe-30Mn-3Al-3Si Twinning-Induced Plasticity Steel after Friction Stir Welding" Metals 10, no. 11: 1557. https://doi.org/10.3390/met10111557






