Influence of Surface Finishing on Corrosion Behaviour of 3D Printed TiAlV Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Surface Finishing
2.3. Electrochemical Measurement
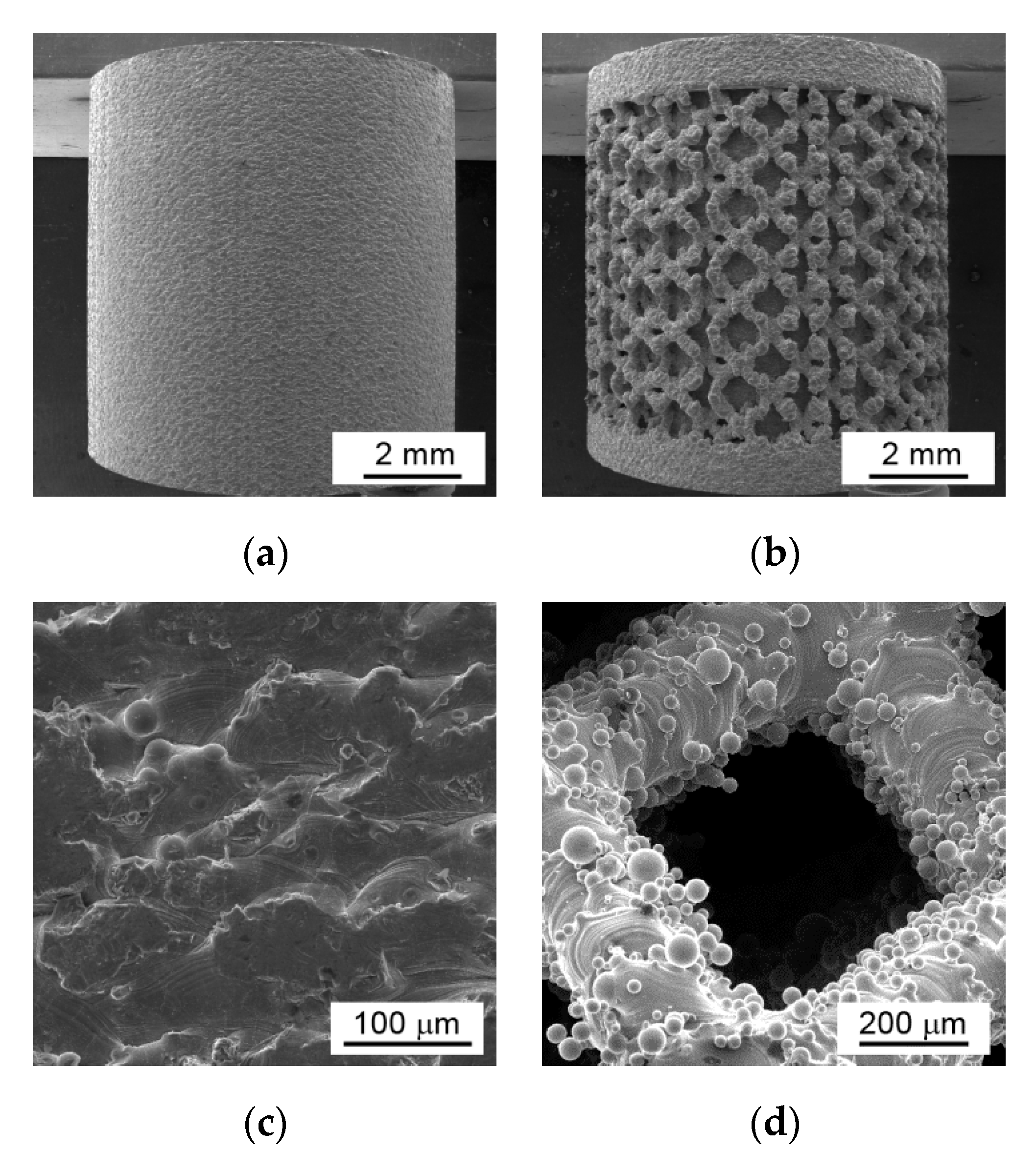
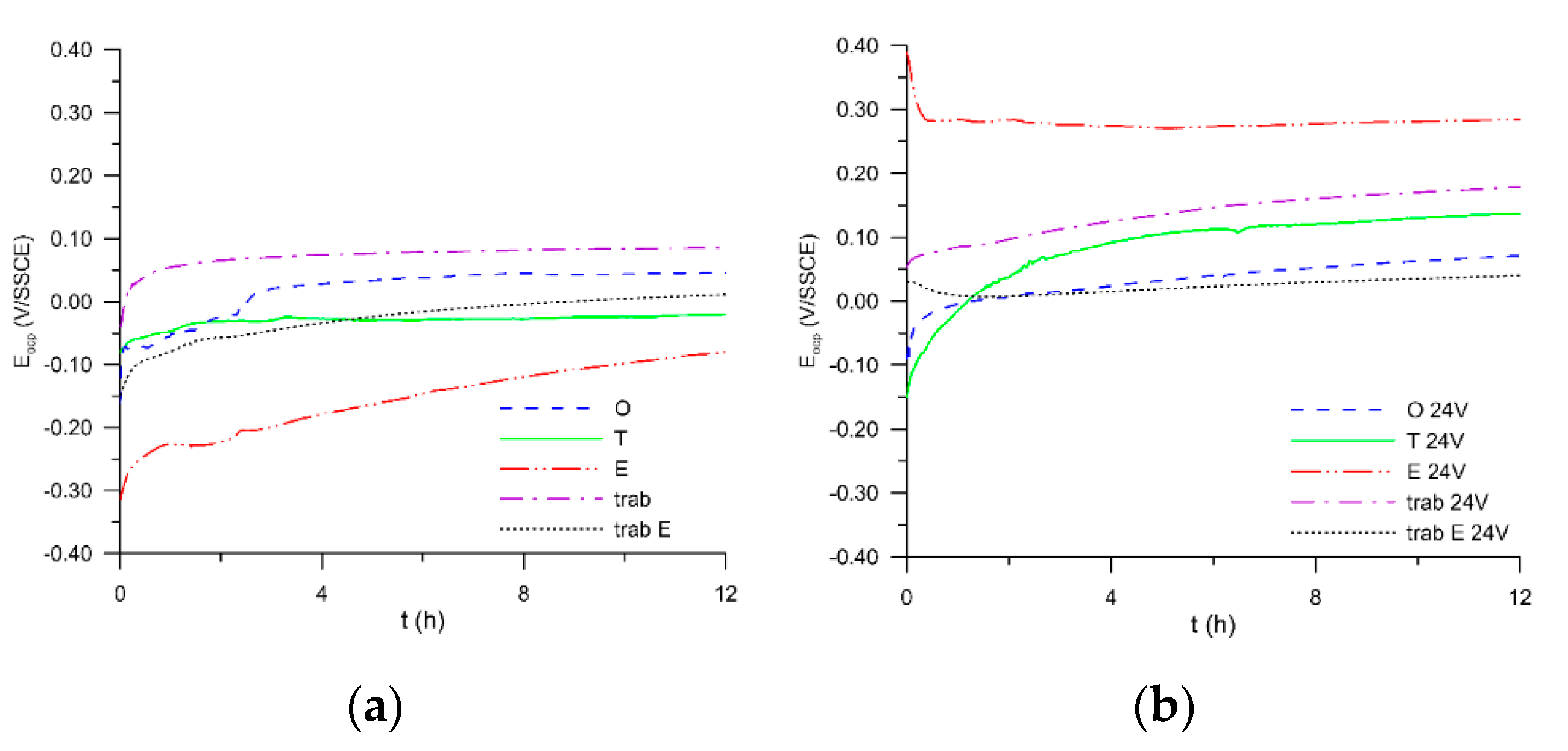
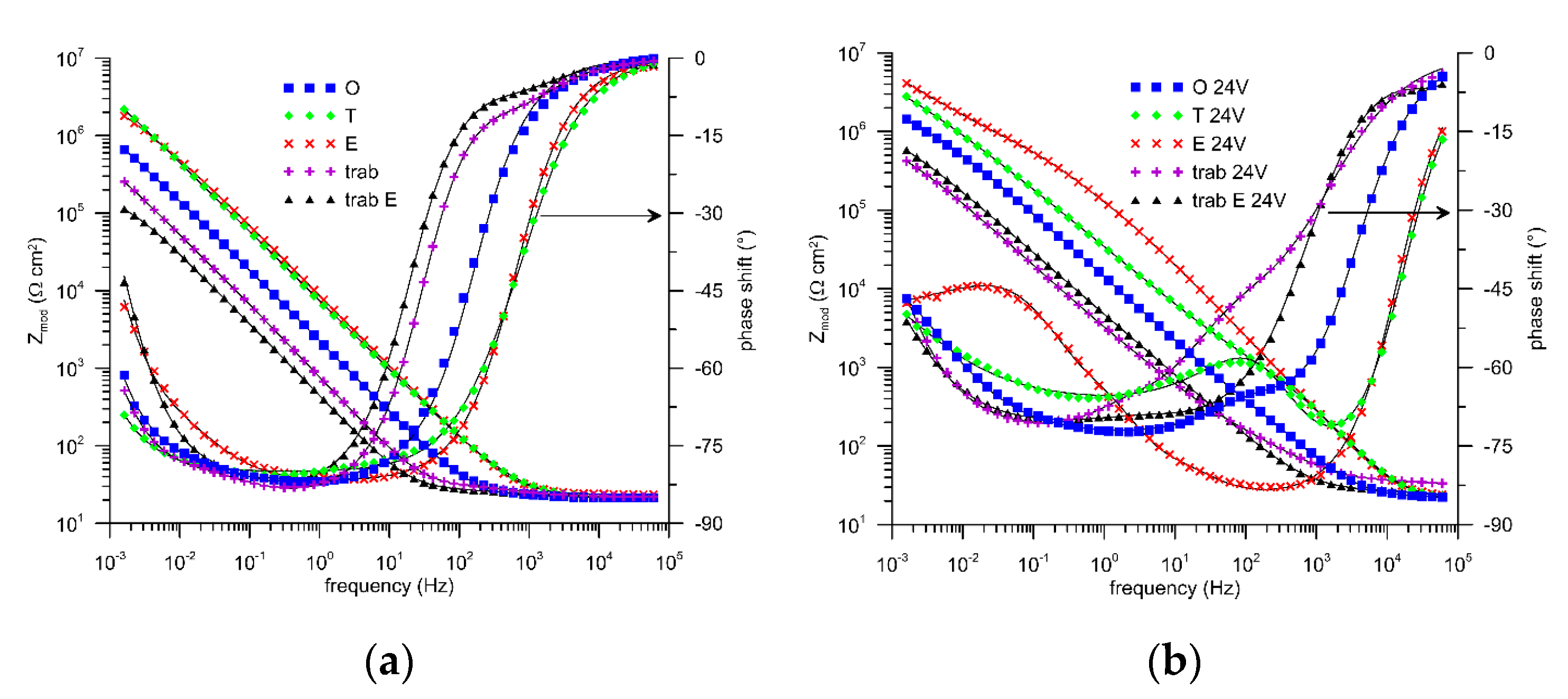
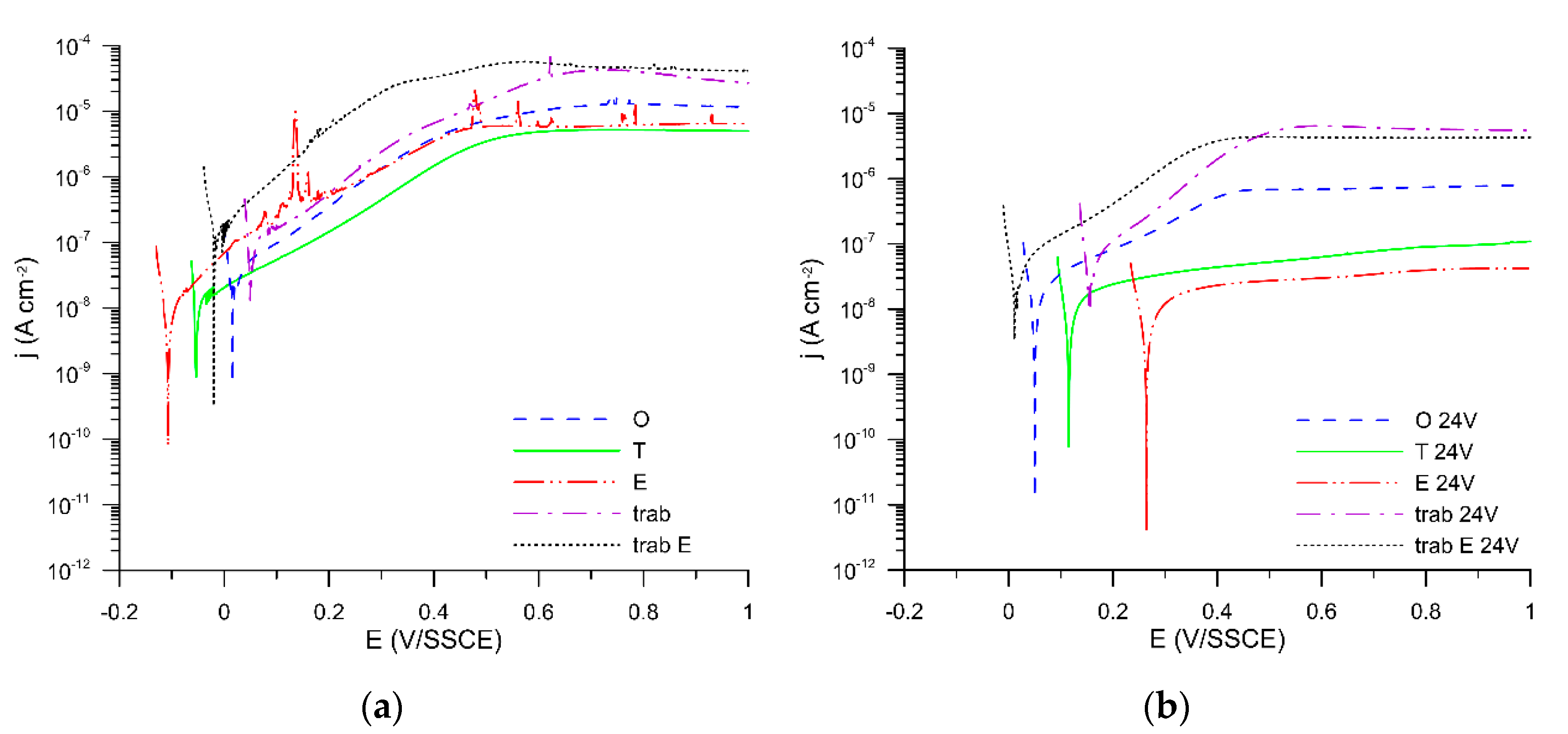
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Lin, H.H.; Lonic, D.; Lo, L.J. 3D printing in orthognathic surgery—A literature review. J. Formos. Med. Assoc. 2018, 117, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Mah, D.; Pelletier, M.H.; Lovric, V.; Walsh, W.R. Corrosion of 3D-Printed Orthopaedic Implant Materials. Ann. Biomed. Eng. 2019, 47, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Chambrone, L.; van Noort, R.; Miller, C.; Hatton, P.; Mangano, C. Direct metal laser sintering titanium dental implants: A review of the current literature. Int. J. Biomater. 2014, 2014, 461534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Van Noort, R. Titanium: The implant material of today. J. Mater. Sci. 1987, 22, 3801–3811. [Google Scholar] [CrossRef]
- Grosgogeat, B.; Boinet, M.; Dalard, F.; Lissac, M. Electrochemical studies of the corrosion behaviour of titanium and the Ti-6Al-4V alloy using electrochemical impedance spectroscopy. Bio-Med. Mater. Eng. 2004, 14, 323–331. [Google Scholar]
- Karambakhsh, A.; Afshar, A.; Malekinejad, P. Corrosion Resistance and Color Properties of Anodized Ti-6Al-4V. J. Mater. Eng. Perform. 2012, 21, 121–127. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 832–841. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, R.L.; Majumdar, T.; Mantri, S.A.; Ravi, V.A.; Banerjee, R.; Birbilis, N. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using in-situ methods. Acta Biomater. 2017, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Zhang, X.; Ni, Q.; Chen, Y.; Wu, M.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Toptan, F.; Alves, A.C.; Carvalho, Ó.; Bartolomeu, F.; Pinto, A.M.P.; Silva, F.; Miranda, G. Corrosion and tribocorrosion behaviour of Ti6Al4V produced by selective laser melting and hot pressing in comparison with the commercial alloy. J. Mater. Process. Technol. 2019, 266, 239–245. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wang, J.; Zheng, M.; Guo, P.; Zhang, Y.; Ren, Y.; Liu, J.; Huang, W. Effect of stress-relief annealing on anodic dissolution behaviour of additive manufactured Ti-6Al-4V via laser solid forming. Corros. Sci. 2019, 153, 314–326. [Google Scholar] [CrossRef]
- Fojt, J.; Fousova, M.; Jablonska, E.; Joska, L.; Hybasek, V.; Pruchova, E.; Vojtech, D.; Ruml, T. Corrosion behaviour and cell interaction of Ti-6Al-4V alloy prepared by two techniques of 3D printing. Mater. Sci. Eng. C 2018, 93, 911–920. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Manam, N.S.; Kamariah, M.S.I.N.; Sharif, S.; Zulkifly, A.H.; Ahmad, I.; Miura, H. A review of powdered additive manufacturing techniques for Ti-6al-4v biomedical applications. Powder Technol. 2018, 331, 74–97. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Buhagiar, J.; Szlazak, K.; Brynk, T.; Kurzydlowski, K.J.; Swieszkowski, W. The influence of chemical polishing of titanium scaffolds on their mechanical strength and in-vitro cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 428–439. [Google Scholar] [CrossRef]
- Sutter, E.M.M.; Goetz-Grandmont, G.J. The behaviour of titanium in nitric-hydrofluoric acid solutions. Corros. Sci. 1990, 30, 461–476. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Szlazak, K.; Strzelczyk, K.; Brynk, T.; Kurzydlowski, K.J.; Swieszkowski, W. Post Processing and Biological Evaluation of the Titanium Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 197. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE: Houston, TX, USA, 1974; p. 644. [Google Scholar]
- Milošev, I.; Kosec, T.; Strehblow, H.H. XPS and EIS study of the passive film formed on orthopaedic Ti–6Al–7Nb alloy in Hank’s physiological solution. Electrochim. Acta 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Milosev, I.; Metikos-Hukovic, M.; Strehblow, H.H. Passive film on orthopedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications. In Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O.M., White, R.E., Eds.; Springer: Boston, MA, USA, 2002; pp. 143–248. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 523. [Google Scholar]
- Lewis, G.; Vejerla, R.; Mishra, S. One equivalent electrical circuit is applicable to model the interface between the passive surface layer on an orthopaedic alloy and a biosimulating aqueous solution. Bio-Med. Mater. Eng. 2007, 17, 97–108. [Google Scholar]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of titanium in artificial saliva. J. Appl. Electrochem. 2007, 37, 511–517. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Y.; Gao, R.; Yang, J.; Wei, K.; Tang, D.; Fu, T. The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode. Materials 2019, 12, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Zhou, X.; Mohanty, P. Corrosion behaviour of cold sprayed titanium coatings in simulated body fluid. Corros. Eng. Sci. Technol. 2012, 47, 145–154. [Google Scholar] [CrossRef]
- Garsivaz jazi, M.R.; Golozar, M.A.; Raeissi, K.; Fazel, M. Surface Characteristics and Electrochemical Impedance Investigation of Spark-Anodized Ti-6Al-4V Alloy. J. Mater. Eng. Perform. 2014, 23, 1270–1278. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Guo, P.; Song, M.; Huang, W. Electrochemical behaviour of laser solid formed Ti–6Al–4V alloy in a highly concentrated NaCl solution. Corros. Sci. 2018, 142, 161–174. [Google Scholar] [CrossRef]
- Fojt, J.; Kacenka, Z.; Jablonska, E.; Hybasek, V.; Pruchova, E. Influence of the surface etching on the corrosion behaviour of a three-dimensional printed Ti-6Al-4V alloy. Mater. Corros. 2020, 71, 1691–1696. [Google Scholar] [CrossRef]

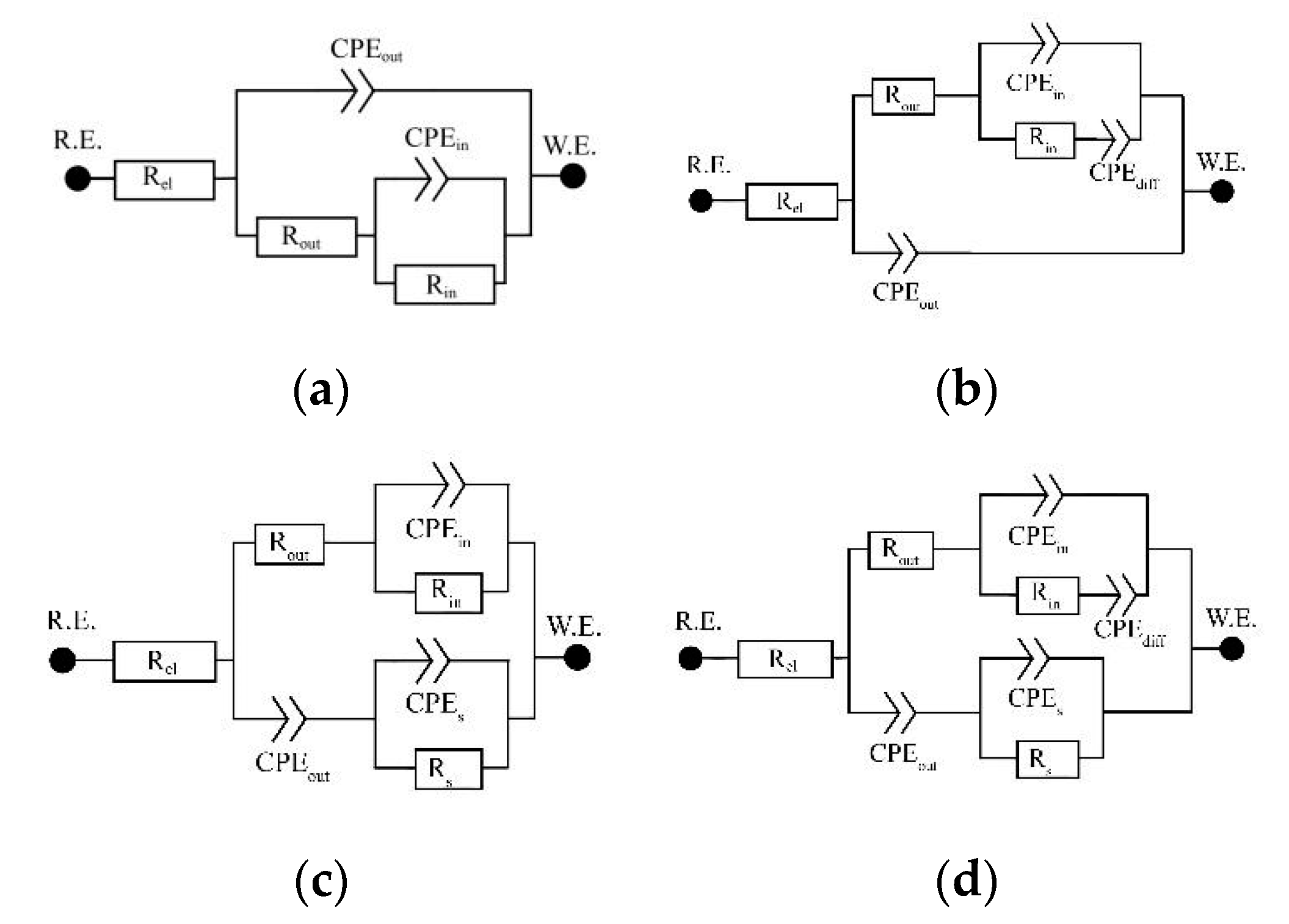
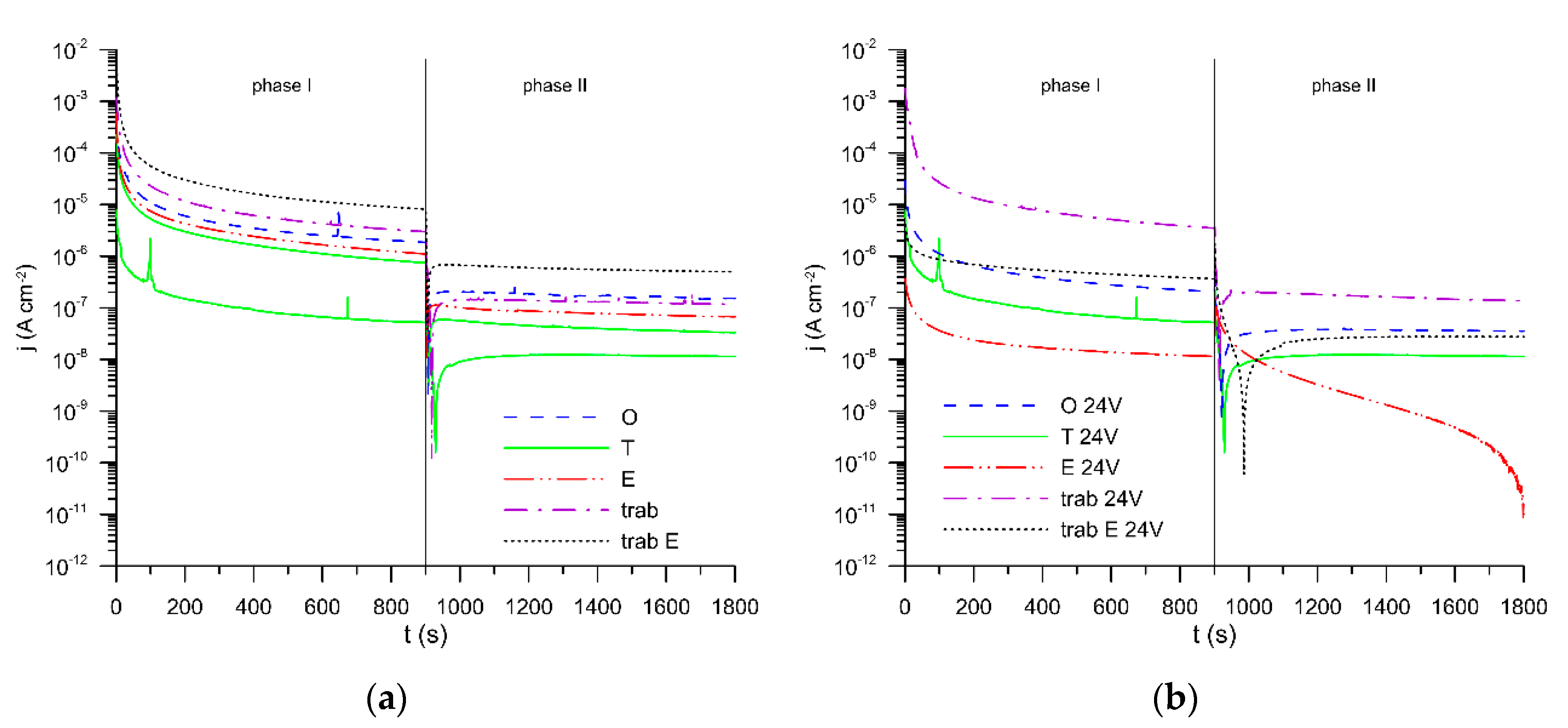
| Specimen | EC | Rel | Rout | Rin | Rs | CPEout | αout | CPEin | αin | CPEs | αs | CPEdiff | αdiff | Goodness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Ω cm2) | (Ω cm2) | (Ω cm2) | (Ω cm2) | (S sα cm−2) | (S sα cm−2) | (S sα cm−2) | (S sα cm−2) | of Fit | ||||||
| original | A | 21.9 | 7.77 × 105 | 1.18 × 106 | - | 4.64 × 10−5 | 0.999 | 8.32 × 10−5 | 0.910 | - | - | - | - | 8 × 10−4 |
| turned | A | 21.6 | 226.4 | 1.12 × 107 | - | 2.12 × 10−5 | 0.893 | 4.84 × 10−6 | 0.878 | - | - | - | - | 2 × 10−4 |
| etched | A | 23.6 | 1.08 × 106 | 2.24 × 106 | - | 2.16 × 10−5 | 0.912 | 9.57 × 10−6 | 0.825 | - | - | - | - | 1 × 10−4 |
| original + 24 V | B | 22.0 | 714.4 | 1.54 × 106 | - | 7.73 × 10−6 | 0.869 | 8.42 × 10−6 | 0.745 | - | - | 6.36 × 10−6 | 0.474 | 4 × 10−5 |
| turned + 24 V | B | 21.1 | 1.44 × 103 | 4.76 × 106 | - | 9.56 × 10−7 | 0.934 | 6.68 × 10−6 | 0.705 | - | - | 1.64 × 10−6 | 0.404 | 2 × 10−4 |
| trabecular | C | 22.0 | 1.03 × 105 | 7.31 × 105 | 7.6 | 2.29 × 10−4 | 0.945 | 3.23 × 10−5 | 0.792 | 1.58 × 10−4 | 0.813 | - | - | 1 × 10−5 |
| trabecular etched | C | 21.4 | 7.90 × 104 | 8.85 × 104 | 5.3 | 4.09 × 10−4 | 0.932 | 1.09 × 10−4 | 0.924 | 2.89 × 10−4 | 0.775 | - | - | 4 × 10−5 |
| trabecular + 24 V | C | 32.9 | 2.45 × 104 | 9.12 × 105 | 105.8 | 6.72 × 10−5 | 0.792 | 5.48 × 10−6 | 0.952 | 1.44 × 10−4 | 0.661 | - | - | 6 × 10−5 |
| etched 24 V | D | 22.6 | 2.16 × 105 | 4.05 × 105 | 1.29 × 103 | 1.00 × 10−6 | 0.945 | 1.09 × 10−6 | 0.900 | 8.28 × 10−6 | 0.960 | 2.89 × 10−6 | 0.542 | 1 × 10−4 |
| trabecular etched 24 V | D | 22.1 | 1.40 × 104 | 1.26 × 106 | 3.8 | 4.38 × 10−5 | 0.803 | 6.67 × 10−6 | 0.718 | 9.52 × 10−7 | 1.000 | 3.13 × 10−5 | 0.487 | 5 × 10−5 |
| Specimen | Anodic Slope (mV/dec) | jcor (A/cm2) | jpas (A/cm2) |
|---|---|---|---|
| original | 199 | 3.05 × 10−8 | 1.3 × 10−5 |
| original + 24 V | 286 | 2.50 × 10−8 | 7.4 × 10−7 |
| turned | 246 | 1.30 × 10−8 | 5.3 × 10−6 |
| turned + 24 V | 1273 | 1.50 × 10−8 | 1.0 × 10−7 |
| etched | 243 | 3.05 × 10−8 | 6.6 × 10−6 |
| etched + 24 V | 2024 | 2.00 × 10−8 | 4.2 × 10−8 |
| trabecular | 220 | 9.70 × 10−8 | 2.9 × 10−5 |
| trabecular + 24 V | 177 | 6.50 × 10−8 | 5.8 × 10−6 |
| trabecular etched | 138 | 1.55 × 10−7 | 4.3 × 10−5 |
| trabecular etched + 24 V | 219 | 5.50 × 10−8 | 4.3 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fojt, J.; Hybášek, V.; Kačenka, Z.; Průchová, E. Influence of Surface Finishing on Corrosion Behaviour of 3D Printed TiAlV Alloy. Metals 2020, 10, 1547. https://doi.org/10.3390/met10111547
Fojt J, Hybášek V, Kačenka Z, Průchová E. Influence of Surface Finishing on Corrosion Behaviour of 3D Printed TiAlV Alloy. Metals. 2020; 10(11):1547. https://doi.org/10.3390/met10111547
Chicago/Turabian StyleFojt, Jaroslav, Vojtěch Hybášek, Zdeněk Kačenka, and Eva Průchová. 2020. "Influence of Surface Finishing on Corrosion Behaviour of 3D Printed TiAlV Alloy" Metals 10, no. 11: 1547. https://doi.org/10.3390/met10111547





