Ecological Interactions Affecting the Efficacy of Aphidius colemani in Greenhouse Crops
Abstract
:1. Introduction
| Factors Affecting A. colemani | Direct or Indirect | Type of Effect | Positive or Negative for Biological Control | Ways Biological Control by A. colemani is Negatively or Positively Affected | Example References for A. colemani |
|---|---|---|---|---|---|
| Plants | Direct | Morphological defenses (e.g., trichomes, spines, waxy layers) | Negative | Increase A. colemani grooming time, and may impede movement on plant | [25] |
| Increased aphid handling time | [24] | ||||
| Non-defensive morphological traits (e.g., PGR effects on plant architecture) | Negative | Host-finding is more difficult | [26] | ||
| May negatively affects mummy abundance, percent emergence, female parasitoid size, and sex ratio | [27] | ||||
| Volatile organic compounds (e.g., plant species cues alone) | Variable | Could affect host plant choice | [28,29,30,31,32] | ||
| Volatile organic compounds (e.g., natal-host effects) | Positive | A. colemani may prefer host complex on which it is reared | [29,30] | ||
| Resource provisioning (e.g., Flower nectar) | Positive | Can increase fecundity, percent emergence, female sex ratio, and longevity | [33] | ||
| Negative | Could benefit pests and hyperparasitoids | NA | |||
| Indirect | Good plant quality | Positive | Can increase fecundity, percent emergence, female sex ratio, and longevity | [33] | |
| Herbivore resistance traits (e.g., toxic allelochemicals) | Negative | May negatively affects life history traits | [32] | ||
| Fertilizers | Positive | Increase percent emergence, mummy weight, male longevity and adult size | [34] | ||
| Negative | Could benefit herbivore pests | NA | |||
| Could decrease parasitism | NA | ||||
| Could affect plant defensive compounds, which can affect herbivores and their natural enemies | NA | ||||
| Plant symbionts (e.g., rhizobacteria) | Positive | Could increase crop vigor and resistance to pests | NA | ||
| Negative | Could alter the volatile composition, which may make plants less attractive to A. colemani | NA | |||
| Endophytes | Negative | Could affect reproductive ability of the F1 generation | NA | ||
| Could increase development times | NA | ||||
| Could reduce female abundance | NA | ||||
| Varieties/species effects | Variable | Percent emergence may be reduced on some species, compared to other. | [35] | ||
| Variance in female development time | [36] | ||||
| Variance in number of mummies | [36] | ||||
| Aphid hosts | Direct | Aphid species | Variable | Offspring survival, female ratio, and size were lower on R. padi than M. persicae, A. gossypii, and S. graminum | [37] |
| Using a poor quality aphid as an alternate host on a banker plant can benefit biological control of higher quality aphid hosts on crop plant | [38] | ||||
| If multiple pest aphid species are present in a greenhouse, there could be variable levels of control | NA | ||||
| Endosymbionts (e.g., Regiella insecticola) | Negative | Infected clones may be resistant to A. colemani | [39] | ||
| Parasitoids could be equally attracted to infected and uninfected hosts, so they may waste their eggs and energy | NA | ||||
| Preference for A. gossypii | Positive | Good control if target pest is A. gossypii | [1] | ||
| Negative | May experience reduced life history traits on A. gossypii compared to M. persicae | [37] | |||
| May not perform well in multi-pest environment | NA | ||||
| Clones | Variable | Parasitism levels vary with clone (red clone > light green > dark green) | [40] | ||
| Negative | Less effective against insecticide-resistance clones | [41] | |||
| Host instar | Variable | Prefers 1st and 2nd instars of A. gossypii and M. persicae on eggplant | [41,42] | ||
| Prefers 2nd and 3rd instars of M. persicae on pepper | [42] | ||||
| Prefers 4th and 5th instars of A. glycines on soybean | [43] | ||||
| Defensive behavior | Negative | Increase handling time and risk of injury | [44] | ||
| Small parasitoids have narrower host range than large ones | [45] | ||||
| Host density | Positive | Density is positively correlated with foraging time and ovipositions | [4,46] | ||
| Variable | Type II functional response at high-densities; Type III functional response at low-densities; Type II functional response at low-densities; Type III functional response at high-densities | [4] | |||
| Honeydew production | Positive | Benefits A. colemani longevity | [47] | ||
| Could help host finding | NA | ||||
| Indirect | NA | NA | NA | NA | |
| Third and fourth trophic levels | Direct | Multiparasitism (i.e., multiple parasitoids species parasitizing same host) | Negative | Other aphid parasitoids can outcompete A. colemani larvae | [48] |
| Predators | Neutral | Does not avoid predator-infested plants | [49] | ||
| Negative | Predators can reduce parasitoid abundance by eating the parasitized aphids | [50] | |||
| Positive | Additive and synergistic effects from a diversity of natural enemies | [51] | |||
| Entomopathogenic fungi | Negative | Beauveria bassiana can infect and kill adult A. colemani at high rates (>55% of the population) | [52,53] | ||
| Can also infect parasitized aphids and reduce mummy formation and adult emergence | [54] | ||||
| A. colemani does not detect infected hosts, so wastes eggs/energy | [55] | ||||
| Neutral | Verticillium lecanii is safe for A. colemani in mummy form (5 days post-parasitism) | [54] | |||
| Hyperparasitoids (e.g., Alloxysta victrix and Dendrocerus aphidum) | Negative | Parasitize A. colemani | [56,57] | ||
| In the summer, when hyperparasitoid population is high, aphid control can fail | [14] | ||||
| Can affect parasitoid population on banker plants | [20] | ||||
| Indirect | NA | NA | NA | NA | |
| Abiotic factors in greenhouses | Direct | Pesticides | Negative | Can lead to direct mortality of A. colemani | [58,59,60,61,62] |
| Temperature | Variable | Temperatures could exceed development threshold for A. colemani (e.g., larvae generally cease development at 30 or 31 ºC) | [63,64] | ||
| Development is roughly fastest between 22 °C and 28 °C | [17,64,65] | ||||
| Faster development can result in smaller parasitoids, with shorter lifespans and reduced fecundity | [66] | ||||
| Can develop at temperatures as low as 10 °C | [63,65] | ||||
| Elevated temperature can increase parasitoid performance | NA | ||||
| Dynamic climate regimes | Variable | Dynamic climate regimes could affect efficacy | NA | ||
| Humidity | Variable | Could affect fecundity, hatching and predation | NA | ||
| Could affect flight and dispersal | NA | ||||
| Parasitoid eclosion and adult longevity could decrease at high humidity levels | NA | ||||
| Low humidity levels could have negligible effects on foraging | NA | ||||
| “Precipitation” | Negative | Could reduce foraging and increase parasitoid cleaning time | NA | ||
| Light (e.g., light emitting diodes (LED), photoselective screens (e.g., UV absorbing), and changes in photoperiod) | Neutral | Reduced UV light has no effects on A. colemani performance | [67] | ||
| Wind | Negative | Could reduce oviposition and increase resting behavior of parasitoid | NA | ||
| Indirect | Pesticides (including residual effects) | Negative | Can be exposed to insecticides even through honeydew and nectaries | [59,68,69] | |
| Could experience decreased attraction to aphids on treated plants | NA | ||||
| Reduced re-invasion of areas treated with pesticides | [70] | ||||
| Could cause a reduction in foraging behavior | NA | ||||
| Can reduce oviposition and fecundity | [59,62,71] | ||||
| Could impact development time and sex ratio | NA | ||||
| Temperature | Negative | Can increase A. gossypii populations | [72] | ||
| Variable | Populations of A. gossypii and M. persicae can still increase at 30 ºC–33 ºC | [67,73] | |||
| Light (e.g., light emitting diodes (LED), photoselective screens (e.g., UV absorbing), and changes in photoperiod) | Variable | Changes in lighting can alter plant nutritional quality, physical or chemical defenses, and/or volatile emissions or profiles, which in turn could affect A. colemani | NA | ||
| Reduction of UV light does not negatively affect performance of A. colemani | [74] | ||||
| Wind | Negative | Could interfere with male mating flights | NA |
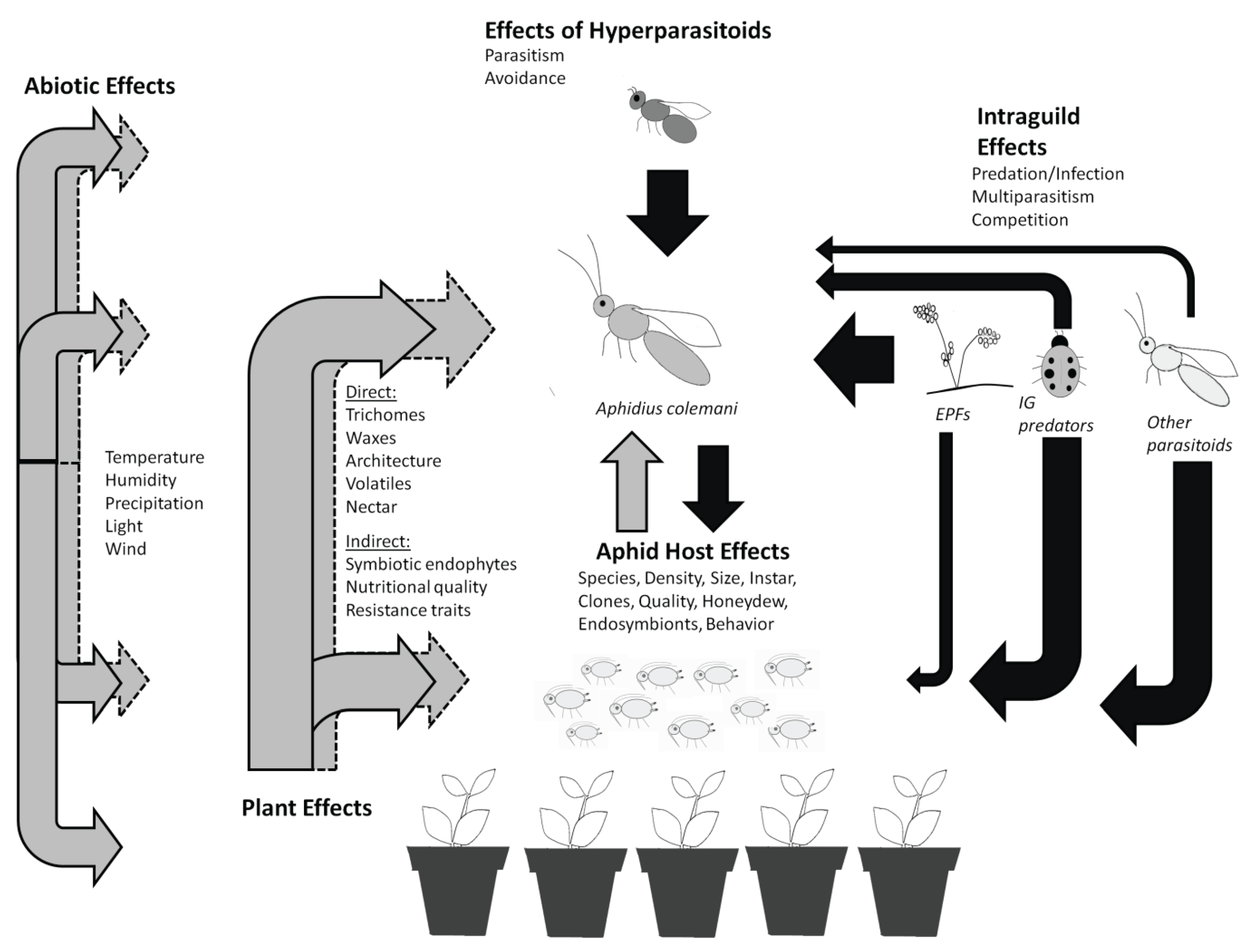
2. Plant Effects on Pest Suppression by A. colemani
2.1. Direct Plant Effects
2.2. Indirect Plant Effects
3. Host Aphid Effects on Pest Suppression by A. colemani
3.1. Host Aphid Effects on Parasitoid Development and Fitness
3.2. Host Effects on Aphid Acceptance and Suppression by A. colemani
3.3. Host Density Effects on Aphid Suppression by A. colemani
4. Considerations at the 3rd and 4th Trophic Levels: Effects of Competition, Intraguild Predation, Hyperparasitism and Multiparasitism
5. Abiotic Considerations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Messing, R.; Rabasse, J.M. Oviposition behaviour of the polyphagous aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiidae). Agric. Ecosyst. Environ. 1995, 52, 13–17. [Google Scholar] [CrossRef]
- Starý, P. Aphidius colemani Viereck: its taxonomy, distribution and host range (Hymenoptera, Aphidiidae). Acta Entomol. Bohemoslov. 1975, 72, 156–163. [Google Scholar]
- Benelli, G.; Messing, R.H.; Wright, M.G.; Giunti, G.; Kavallieratos, N.G.; Canale, A. Cues triggering mating and host-seeking behavior in the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae: Aphidiinae): Implications for biological control. J. Econ. Entomol. 2014, 107, 2005–2022. [Google Scholar] [CrossRef]
- Van Steenis, M.J.; El-Khawass, K.A.M.H. Behaviour of Aphidius colemani searching for aphis gossypii: Functional response and reaction to previously searched aphid colonies. Biocontrol Sci. Technol. 1995, 5, 339–348. [Google Scholar] [CrossRef]
- Bilu, E.; Hopper, K.R.; Coll, M. Host choice by Aphidius colemani: Effects of plants, plant-aphid combinations and the presence of intra-guild predators. Ecol. Entomol. 2006, 31, 331–336. [Google Scholar] [CrossRef]
- Vásquez, G.M.; Orr, D.B.; Baker, J.R. Efficacy assessment of Aphidius colemani (Hymenoptera: Braconidae) for suppression of Aphis gossypii (Homoptera: Aphididae) in greenhouse-grown chrysanthemum. J. Econ. Entomol. 2006, 99, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Van Schelt, J.; Hoogerbrugge, H.; Becker, N.; Messelink, G.J.; Blockmans, K. Comparing Aphidius colemani and Aphidius matricariae on Myzus persicae ssp. nicotianae in sweet pepper. IOBC/WPRS Bull. 2011, 68, 169–172. [Google Scholar]
- Chau, A.; Heinz, K. Biological control of aphids on ornamental crops. In Biocontrol in Protected Culture; Heinz, K.M., van Driesche, R.G., Parella, M.P., Eds.; Ball Publishing: Batavia, Illinois, 2001; p. 277. [Google Scholar]
- Yano, E. Ecological considerations for biological control of aphids in protected culture. Popul. Ecol. 2006, 48, 333–339. [Google Scholar] [CrossRef]
- Van Lenteren, J. Biological and integrated pest control in greenhouses. Annu. Rev. Entomol. 1988, 33, 239–269. [Google Scholar] [CrossRef]
- Heinz, K.M. Dispersal and dispersion of aphids (Homoptera: Aphididae) and selected natural enemies in spatially subdivided greenhouse environments. Environ. Entomol. 1998, 27, 1029–1038. [Google Scholar] [CrossRef]
- Bennison, J.A.; Corless, S.P. Biological control of aphids on cucumbers: further development of open rearing units or “banker plants” to aid the establishment of aphid natural enemies. IOBC/WPRS Bull. 1992, 16, 5–8. [Google Scholar]
- Van Steenis, M.J. Evaluation of four aphidiine parasitoids for biological control of Aphis gossypii. Entomol. Exp. Appl. Appl. 1995, 75, 151–157. [Google Scholar] [CrossRef]
- Van Driesche, R.G.; Lyon, S.; Sanderson, J.P.S.; Bennett, K.C.; Stanek, E.J.I.; Zhang, R. Greenhouse Trials of Aphidius colemani (Hymenoptera: Braconidae) banker plants for control of aphids (Hemiptera: Aphididae) in greenhouse spring floral crops. Florida Entomol. 2008, 91, 583–591. [Google Scholar]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Van Steenis, M.J. Intrinsic rate of increase of Aphidius colemani Vier. (Hym., Braconidae), a parasitoid of Aphis gossypii Glov. (Hom., Aphididae), at different temperatures. J. Appl. Entomol. 1993, 116, 192–198. [Google Scholar]
- Burgio, G.; Ferrari, R.; Giorgio, N. Biological and integrated control of Aphis gossypii Glove (Hom., Aphidide) in protected cucumber and melon. Available online: http://www.bulletinofinsectology.org/pdfarticles/vol51-1997-171-178burgio.pdf (accessed on 2 Februay 2015).
- Jacobson, R.J.; Croft, P. Strategies for the control of Aphis gossypii Glover (Hom.: Aphididae) with Aphidius colemani Viereck (Hym.: Braconidae) in protected cucumbers. Biocontrol Sci. Technol. 1998, 8, 377–387. [Google Scholar]
- Nagasaka, K.; Takahasi, N.; Okabayashi, T. Impact of secondary parasitism on Aphidius colemani in the banker plant system on aphid control in commercial greenhouses in Kochi, Japan. Appl. Entomol. Zool. 2010, 45, 541–550. [Google Scholar] [CrossRef]
- Acheampong, S.; Gillespie, D.R.; Quiring, D. Survey of parasitoids and hyperparasitoids (Hymenoptera) of the green peach aphid, Myzus persicae and the foxglove aphid, Aulacorthum solani (Hemiptera: Aphididae) in British Columbia. J. Entomol. Soc. Br. Columbia 2012, 109, 12–22. [Google Scholar]
- Jandricic, S.E.; Ontario Ministry of Agriculture and Food and Rural Affair: Vineland, Canada. Personal Observation, 2015.
- Aalbers, J.; Flowers Canada Growers: Guelph, Canada. Personal communication, 2015.
- Holt, R. Predation, apparent competition, and the structure of prey communities. Theor. Popul. Biol. 1977, 12, 197–229. [Google Scholar] [CrossRef]
- Desneux, N.; Ramirez-Romero, R. Plant characteristics mediated by growing conditions can impact parasitoid’s ability to attack host aphids in winter canola. J. Pest Sci. 2009, 82, 335–342. [Google Scholar] [CrossRef]
- Prado, S.G.; Frank, S.D. Compact plants reduce biological control of Myzus persicae by Aphidius colemani. Biol. Control 2013, 65, 184–189. [Google Scholar] [CrossRef]
- Prado, S.G.; Frank, S.D. Tritrophic effects of plant growth regulators in an aphid-parasitoid system. Biol. Control 2013, 66, 72–76. [Google Scholar] [CrossRef]
- Grasswitz, T.R. Effect of adult experience on the host-location behavior of the aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiidae). Biol. Control 1998, 12, 177–181. [Google Scholar] [CrossRef]
- Storeck, A.; Poppy, G.M.; van Emden, H.F.; Powell, W. The role of plant chemical cues in determining host preference in the generalist aphid parasitoid Aphidius colemani. Entomol. Exp. Appl. 2000, 97, 41–46. [Google Scholar] [CrossRef]
- Douloumpaka, S.; van Emden, H.F. A maternal influence on the conditioning to plant cues of Aphidius colemani Viereck, parasitizing the aphid Myzus persicae Sulzer. Physiol. Entomol. 2003, 28, 108–113. [Google Scholar] [CrossRef]
- Lo Pinto, M.; Wajnberg, E.; Colazza, S.; Curty, C.; Fauvergue, X. Olfactory response of two aphid parasitoids, Lysiphlebus testaceipes and Aphidius colemani, to aphid-infested plants from a distance. Entomol. Exp. Appl. 2004, 110, 159–164. [Google Scholar] [CrossRef]
- Kalule, T.; Wright, D.J. The influence of cultivar and cultivar-aphid odours on the olfactory response of the parasitoid Aphidius colemani. J. Appl. Entomol. 2004, 128, 120–125. [Google Scholar] [CrossRef]
- Charles-Tollerup, J.J. Resource Provisioning as a Habitat Manipulation Tactic to Enhance the Aphid Parasitoid, Aphidius colemani Viereck (Hymenoptera: Braconidae: Aphidiinae),and the Plant-Mediated Effects of a Systemic Insecticide, Imidacloprid. Available online: https://escholarship.org/uc/item/97w046gw#page-1 (accessed on 3 March 2014).
- Aqueel, M.A.; Raza, A.M.; Balal, R.M.; Shahid, M.A.; Mustafa, I.; Javaid, M.M.; Leather, S.R. Tritrophic interactions between parasitoids and cereal aphids are mediated by nitrogen fertilizer. Insect Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Jandricic, S.E.; Dale, A.G.; Bader, A.; Frank, S.D. The effect of banker plant species on the fitness of Aphidius colemani Viereck and its aphid host (Rhopalosiphum padi L.). Biol. Control 2014, 76, 28–35. [Google Scholar] [CrossRef]
- Schädler, M.; Brandl, R.; Kempel, A. Host plant genotype determines bottom-up effects in an aphid-parasitoid-predator system. Entomol. Exp. Appl. 2010, 135, 162–169. [Google Scholar] [CrossRef]
- Ode, P.J.; Hopper, K.R.; Coll, M. Oviposition vs. offspring fitness in Aphidius colemani parasitizing different aphid species. Entomol. Exp. Appl. 2005, 115, 303–310. [Google Scholar]
- Prado, S.G.; Frank, S.D. Optimal foraging by an aphid parasitoid affects the outcome of apparent competition. Ecol. Entomol. 2014, 39, 236–244. [Google Scholar] [CrossRef]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. 2009, 1–3. [Google Scholar]
- Gillespie, D.R.; Quiring, D.J.M.; Foottit, R.G.; Foster, S.P.; Acheampong, S. Implications of phenotypic variation of Myzus persicae (Hemiptera: Aphididae) for biological control on greenhouse pepper plants. J. Appl. Entomol. 2009, 133, 505–511. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P.; Garantonakis, N.G.; Iatrou, S.A. Instar preference and parasitization of Aphis gossypii and Myzus persicae (Hemiptera: Aphididae) by the parasitoid Aphidius colemani (Hymenoptera: Aphidiidae). Eur. J. Entomol. 2004, 101, 333–336. [Google Scholar] [CrossRef]
- Martinou, A.F.; Wright, D.J. Host instar and host plant effects on Aphidius colemani. J. Appl. Entomol. 2007, 131, 621–624. [Google Scholar] [CrossRef]
- Lin, L.A.; Ives, A.R. The effect of parasitoid host-size preference on host population growth rates: An example of Aphidius colemani and Aphis glycines. Ecol. Entomol. 2003, 28, 542–550. [Google Scholar] [CrossRef]
- Wu, G.-M.; Barrette, M.; Boivin, G.; Brodeur, J.; Giraldeau, L.; Hance, T.; Wu, A.G. Temperature influences the handling efficiency of an aphid parasitoid through body size-mediated effects. Environ. Entomol. 2011, 40, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Lykouressis, D.; Garantonakis, N.; Perdikis, D.; Fantinou, A.; Mauromoustakos, A. Effect of female size on host selection by a koinobiont insect parasitoid (Hymenoptera: Braconidae: Aphidiinae). Eur. J. Entomol. 2009, 106, 363–367. [Google Scholar] [CrossRef]
- Stadler, B.; Volkl, W. Foraging patterns of two aphid parasitoids, Lysiphlebus testaceipes and Aphidius colemani on banana. Entomol. Exp. Appl. 1991, 58, 221–229. [Google Scholar] [CrossRef]
- Wäckers, F.L.; van Rijn, P.C.J.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Sampaio, M.V.; Bueno, V.H.P.; Soglia, M.; da, C.; de, M.; de Conti, B.F.; Rodrigues, S.M.M. Larval competition between Aphidius colemani and Lysiphlebus testaceipes after multiparasitism of the host Aphis gossypii. Bull. Insectology 2006, 59, 147–151. [Google Scholar]
- Brodeur, J.; Rosenheim, J.A. Intraguild interactions in aphid parasitoids. Entomol. Exp. Appl. 2000, 97, 93–108. [Google Scholar] [CrossRef]
- Bilu, E.; Coll, M. The importance of intraguild interactions to the combined effect of a parasitoid and a predator on aphid population suppression. BioControl 2007, 52, 753–763. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Sabelis, M.W.; Janssen, A. Biological control of aphids in the presence of thrips and their enemies. BioControl 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Shipp, J.L.; Zhang, Y.; Hunt, D.W.A.; Ferguson, G. Influence of humidity and greenhouse Microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pests. Environ. Entomol. 2003, 32, 1154–1163. [Google Scholar] [CrossRef]
- Ludwig, S.W.; Oetting, R.D. Susceptibility of natural enemies to infection by Beauveria bassiana and impact of insecticides on Ipheseius degenerans Acari: Phytoseiidae. J. Agric. Urban Entomol. 2001, 18, 169–178. [Google Scholar]
- Kim, J.J.; Kim, K.C.; Roberts, D.W. Impact of the entomopathogenic fungus Verticillium lecanii on development of an aphid parasitoid, Aphidius colemani. J. Invertebr. Pathol. 2005, 88, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Baverstock, J.; Alderson, P.G.; Pell, J. Influence of the aphid pathogen Pandora neoaphidis on the foraging behaviour of the aphid parasitoid Aphidius ervi. Ecol. Entomol. 2005, 30, 665–672. [Google Scholar] [CrossRef]
- Grasswitz, T.R.; Reese, B.D. Biology and host selection behaviour of the aphid hyperparasitoid Alloxysta victrix in association with the primary parasitoid Aphidius colemani and the host aphid Myzus persicae. BioControl 1998, 43, 261–271. [Google Scholar] [CrossRef]
- Bloemhard, C.M.J.; van der Wielen, M.; Messelink, G.J. Seasonal abundance of aphid hyperparasitoids in organic greenhouse crops in The Netherlands. IOBC/WPRS Bull. 2014, 102, 15–19. [Google Scholar]
- Dinter, A.; Wiles, J.A. Safety of the new DuPont insecticide indoxicarb to beneficial arthropods: An overview. IOBC/WPRS Bull. 2000, 23, 149–156. [Google Scholar]
- Bostanian, N.; Akalach, M.; Chiasson, H. Effects of a Chenopodium-based botanical insecticide/acaracide on Orius insidiosus (Hemiptera: Anthocoridae) and Aphidus colemani (Hymenoptera: Braconidae). Pest Manag. Sci. 2005, 61, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kojimoto, T.; Nagaoka, H.; Takagi, Y.; Oikawa, M. Evaluating the side effects of chlorothalonil (TPN) and spinosad on the parasitic wasp (Aphidius colemani). J. Pestic. Sci. 2005, 30, 11–16. [Google Scholar] [CrossRef]
- Tremblay, É.; Bélanger, A.; Brosseau, M.; Boivin, G. Toxicity and sublethal effects of an insecticidal soap on Aphidius colemani (Hymenoptera: Braconidae). Pest Manag. Sci. 2008, 64, 249–254. [Google Scholar]
- Stara, J.; Ourednickova, J.; Kocourek, F. Laboratory evaluation of the side effects of insecticides on Aphidius colemani (Hymenoptera: Aphidiidae), Aphidoletes aphidimyza (Diptera: Cecidomyiidae), and Neoseiulus cucumeris (Acari: Phytoseidae). J. Pest Sci. 2010, 84, 25–31. [Google Scholar] [CrossRef]
- Goh, H.G.; Kim, J.H.; Han, M.W. Application of Aphidius colemani viereck for control of the aphid in greenhouse. J. Asia. Pac. Entomol. 2001, 4, 171–174. [Google Scholar] [CrossRef]
- Bueno, V.H.P.; Sampaio, M.V.; van Lenteren, J.C.; De Conti, B.F.; Silva, R.J.; Rodrigues, S.M.M.; Carnevale, A.B. Evaluation of two aphid parasitoids as candidates for biocontrol of aphid pests in protected cultivation in Brazil. In Integrated Control in Protected Crops, Mediterranean Climate; Castañé, C., Sanchez, J.A., Eds.; IOBC/WPRS Bulletin: Darmstadt, Germany, 2006; Vol. 29, pp. 175–180. [Google Scholar]
- Zamani, A.A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Effect of temperature on life history of Aphidius colemani and Aphidius matricariae (Hymenoptera: Braconidae), two parasitoids of Aphis gossypii and Myzus persicae (Homoptera: Aphididae). Environ. Entomol. 2007, 36, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Boivin, G.; Hance, T. Manipulation of parasitoid size using the temperature-size rule: Fitness consequences. Oecologia 2007, 152, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W. Effects of high and fluctuating temperatures on Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2006, 35, 1461–1468. [Google Scholar] [CrossRef]
- Shipp, J.L.; Wang, K.; Ferguson, G. Residual toxicity of avermectin b1 and pyridaben to eight commercially produced beneficial arthropod species used for control of greenhouse pests. Biol. Control 2000, 17, 125–131. [Google Scholar] [CrossRef]
- Smith, T. Biological control: Pesticide compatibility, testing quality and storage. Available online: https://ag.umass.edu/fact-sheets/biological-control-pesticide-compatibility-testing-quality-storage (accessed on 3 February 2015).
- Langhof, M.; Gathmann, A.; Poehling, H.M.; Meyhöfer, R. Impact of insecticide drift on aphids and their parasitoids: Residual toxicity, persistence and recolonisation. Agric. Ecosyst. Environ. 2003, 94, 265–274. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Akalach, M. The contact toxicity of indoxacarb and five other insecticides to Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae), beneficials used in the greenhouse industry. Pest Manag. Sci. 2004, 60, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Brogaard, M.; Körner, O.; Enkegaard, A.; Aaslyng, J.M. The influence of a dynamic climate on pests, diseases and beneficial organisms: Recent research. IOBC WPRS Bull. 2005, 28, 127–134. [Google Scholar]
- Satar, S.; Kersting, U.; Uygun, N. Effect of temperature on development and fecundity of Aphis gossypii Glover (Homoptera: Aphididae) on cucumber. J. Pest Sci. 2005, 78, 133–137. [Google Scholar] [CrossRef]
- Chiel, E.; Messika, Y.; Steinberg, S.; Antignus, Y. The effect of UV-absorbing plastic sheet on the attraction and host location ability of three parasitoids: Aphidius colemani, Diglyphus isaea and Eretmocerus mundus. Biocontrol 2006, 51, 65–78. [Google Scholar] [CrossRef]
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar] [CrossRef]
- Kareiva, P.; Sahakian, R. Tritrophic effects of a simple architectural mutation in pea plants. Nature 1990, 345, 433–434. [Google Scholar] [CrossRef]
- Chang, G.C.; Neufeld, J.; Durr, D.; Duetting, P.S.; Eigenbrode, S.D. Waxy bloom in peas influences the performance and behavior of Aphidius ervi, a parasitoid of the pea aphid. Entomol. Exp. Appl. 2004, 110, 257–265. [Google Scholar] [CrossRef]
- Duetting, P.S.; Ding, H.; Neufeld, J.; Eigenbrode, S.D. Plant waxy bloom on peas affects infection of pea aphids by Pandora neoaphidis. J. Invertebr. Pathol. 2003, 84, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod Struct. Dev. 2004, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, D.C.; Bueno, V.H.P.; Auad, A.M. Efecto de los tricomas glandulares de Solanum berthaultii en el parasitismo de Aphidius colemani (Hymenoptera: Aphidiidae) sobre Myzus persicae (Homoptera: Aphididae). Vedalia 1997, 4, 21–24. [Google Scholar]
- Cloyd, R.A.; Sadof, C.S. (Hymenoptera: Encyrtidae), a Parasitoid of the Citrus Mealybug (Homoptera: Pseudococcidae). Environ. Entomol. 2000, 29, 535–541. [Google Scholar] [CrossRef]
- Gingras, D. Effect of plant structure on host finding capacity of lepidopterous pests of crucifers by two Trichogramma parasitoids. Biol. Control 2003, 27, 25–31. [Google Scholar] [CrossRef]
- Andow, D.A.; Prokrym, D.R. Plant structural complexity and host-finding by a parasitoid. Oecologia 1990, 82, 162–165. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Margolies, D.C.; Nechols, J.R.; Cloyd, R.A. Plant architecture, prey distribution and predator release strategy interact to affect foraging efficiency of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae) on cucumber. Biol. Control 2010, 53, 136–141. [Google Scholar] [CrossRef]
- Gingras, D.; Dutilleul, P.; Boivin, G. Modeling the impact of plant structure on host-finding behavior of parasitoids. Oecologia 2002, 130, 396–402. [Google Scholar] [CrossRef]
- Vet, L.E.M. Parasitoid searching efficiency links behaviour to population processes. Appl. Entomol. Zool. 2001, 36, 399–408. [Google Scholar] [CrossRef]
- Van Emden, H.F.; Eletherianos, I.; Rose, J.; Douloumpaka, S.; Pettersson, J. Aphid parasitoids detect that an alien plant was present nearby during their development. Physiol. Entomol. 2002, 27, 199–205. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Wratten, S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef]
- Araj, S.A.; Wratten, S.D.; Lister, A.J.; Buckley, H.L. Floral nectar affects longevity of the aphid parasitoid Aphidius ervi and its hyperparasitoid Dedrocerys aphidium. New Zeal. Plant Prot. 2006, 59, 178–183. [Google Scholar]
- Berndt, L.A.; Wratten, S.D.; Scarratt, S.L. The influence of floral resource subsidies on parasitism rates of leafrollers (Lepidoptera: Tortricidae) in New Zealand vineyards. Biol. Control 2006, 37, 50–55. [Google Scholar] [CrossRef]
- Helenius, J. Effect of epigeal predators on infestation by the aphid Rhopalosiphum padi and on grain yield of oats in monocrops and mixed intercrops. Entomol. Exp. Appl. 1990, 54, 225–236. [Google Scholar] [CrossRef]
- Baggen, L.R.; Gurr, G.M.; Meats, A. Flowers in tri-trophic systems: Mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. Entomol. Exp. Appl. 1999, 91, 155–161. [Google Scholar] [CrossRef]
- Baggen, L.R.; Gurr, G.M. The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Biol. Control 1998, 11, 9–17. [Google Scholar] [CrossRef]
- Romeis, J.; Wäckers, F.L. Feeding responses by female Pieris brassicae butterflies to carbohydrates and amino acids. Physiol. Entomol. 2000, 25, 247–253. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Trumble, J.T. Host plant resistance to insects in integrated pest management in vegetable crops. J. Agric. Entomol. 1994, 11, 201–224. [Google Scholar]
- Ode, P.J. Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annu. Rev. Entomol. 2006, 51, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, D.G.; Barbosa, P.; Gould, F. Manipulating natural enemies by plant variety selection and modification: A realistic strategy? Annu. Rev. Entomol. 1998, 43, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, W.C.; Flanders, R.V.; Edwards, C.R. Effects of variably resistant soybean and lima bean cultivars on Pediobius foveolatus, a parasitoid of the Mexican bean beetle, Epilachna varivestis. Environ. Entomol. 1985, 14, 678–682. [Google Scholar] [CrossRef]
- Ode, P.J.; Berenbaum, M.R.; Zangerl, A.R.; Hardy, I.C.W. Host plant, host plant chemistry and the polyembryonic parasitoid Copidosoma sosares: Indirect effects in a tritrophic interaction. Oikos 2004, 104, 388–400. [Google Scholar] [CrossRef]
- Chen, Y.; Olson, D.M.; Ruberson, J.R. Effects of nitrogen fertilization on tritrophic interactions. Arthropod. Plant. Interact. 2010, 4, 81–94. [Google Scholar] [CrossRef]
- Throop, H.L.; Lerdau, M.T. Effects of nitrogen deposition on insect herbivory: Implications for community and ecosystem processes. Ecosystems 2004, 7, 109–133. [Google Scholar] [CrossRef]
- Krauss, J.; Härri, S.A.; Bush, L.; Husi, R.; Bigler, L.; Power, S.A.; Müller, C.B. Effects of fertilizer, fungal endophytes and plant cultivar on the performance of insect herbivores and their natural enemies. Funct. Ecol. 2007, 21, 107–116. [Google Scholar] [CrossRef]
- Zarghami, S.; Allahyari, H.; Bagheri, M.R.; Saboori, A. Effect of nitrogen fertilization on life table parameters and population growth of Brevicoryne brassicae. Bull. Insectology 2010, 63, 39–43. [Google Scholar]
- Couture, J.J.; Servi, J.S.; Lindroth, R.L. Increased nitrogen availability influences predator-prey interactions by altering host-plant quality. Chemoecology 2010, 20, 277–284. [Google Scholar] [CrossRef]
- Pope, T.W.; Girling, R.D.; Staley, J.T.; Trigodet, B.; Wright, D.J.; Leather, S.R.; van Emden, H.F.; Poppy, G.M. Effects of organic and conventional fertilizer treatments on host selection by the aphid parasitoid Diaeretiella rapae. J. Appl. Entomol. 2012, 136, 445–455. [Google Scholar] [CrossRef]
- Staley, J.T.; Girling, R.D.; Stewart-Jones, A.; Poppy, G.M.; Leather, S.R.; Wright, D.J. Organic and conventional fertilizer effects on a tritrophic interaction: Parasitism, performance and preference of Cotesia vestalis. J. Appl. Entomol. 2011, 135, 658–665. [Google Scholar] [CrossRef]
- Staley, J.T.; Stewart-Jones, A.; Poppy, G.M.; Leather, S.R.; Wright, D.J. Fertilizer affects the behaviour and performance of Plutella xylostella on brassicas. Agric. For. Entomol. 2009, 11, 275–282. [Google Scholar] [CrossRef]
- Kos, M.; Houshyani, B.; Achhami, B.B.; Wietsma, R.; Gols, R.; Weldegergis, B.T.; Kabouw, P.; Bouwmeester, H.J.; Vet, L.E.M.; Dicke, M.; van Loon, J.J. Herbivore-mediated effects of glucosinolates on different natural enemies of a specialist aphid. J. Chem. Ecol. 2012, 38, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Chau, A.; Heinz, K.M. Reducing fertilization for cut roses: Effect on crop productivity and twospotted spider mite abundance, distribution, and management. J. Econ. Entomol. 2009, 102, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Chau, A.; Heinz, K.M. Reducing fertilization: A management tactic against western flower thrips on roses. J. Appl. Entomol. 2012, 136, 520–529. [Google Scholar] [CrossRef]
- Härri, S.A.; Krauss, J.; Müller, C.B. Trophic cascades initiated by fungal plant endosymbionts impair reproductive performance of parasitoids in the second generation. Oecologia 2008, 157, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, M.A.B.; Nault, B.A.; Smart, C.D. Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot. 2008, 27, 996–1002. [Google Scholar] [CrossRef]
- Boutard-Hunt, C.; Smart, C.D.; Thaler, J.; Nault, B.A. Impact of plant growth-promoting rhizobacteria and natural enemies on Myzus persicae (Hemiptera: Aphididae) infestations in pepper. J. Econ. Entomol. 2009, 102, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Soler, R.; Weldegergis, B.T.; Shimwela, M.M.; Van Loon, J.J.; Dicke, M. Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant. Cell Environ. 2013, 36, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Zytynska, S.E.; Fleming, S.; Tetard-Jones, C.; Kertesz, M.A.; Preziosi, R. Community genetic interactions mediate indirect ecological effects between a parasitoid wasp and rhizobacteria. Ecology 2010, 91, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Härri, S.A.; Krauss, J.; Müller, C.B. Extended larval development time for aphid parasitoids in the presence of plant endosymbionts. Ecol. Entomol. 2009, 34, 20–25. [Google Scholar] [CrossRef]
- Strand, M.R.; Obrycki, J.J. Host specificity of insect parasitoids and predators. Bioscience 1996, 46, 422–429. [Google Scholar] [CrossRef]
- Rehman, A.; Powell, W. Host selection behaviour of aphid parasitoids (Aphidiidae: Hymenoptera). J. Plant Breed. Crop Sci. 2010, 2, 299–311. [Google Scholar]
- Sampaio, M.V.; Bueno, V.H.P.; De Conti, B.F. The effect of the quality and size of host aphid species on the biological characteristics of Aphidius colemani (Hymenoptera: Braconidae: Aphidiinae). Eur. J. Entomol. 2008, 105, 489–494. [Google Scholar] [CrossRef]
- Visser, M.E. The importance of being large: The relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J. Anim. Ecol. 1994, 63, 963–978. [Google Scholar] [CrossRef]
- Eijs, I.E.M.; van Alphen, J.J.M. Life history correlations: Why are hymenopteran parasitoids an exception? Ecol. Lett. 1999, 2, 27–35. [Google Scholar] [CrossRef]
- Jandricic, S.E. Investigations of the Biology of the Pest Aphid Aulacorthum Solani (Kaltenbach) (Hemiptera: Aphididae) And Of Biological Control Agents For Control Of Multi-Species Aphid Outbreaks In Greenhouse Floriculture Crops. Available online: https://dspace.library.cornell.edu/bitstream/1813/34184/1/sej48.pdf (accessed on 2 February 2015).
- Oliver, K.M.; Russell, J.; Moran, N.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Moran, N.; Hunter, M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, J.; Gaul, C.; Wolf, S.; Voisin, D.; Gerardo, N. The Effects of Endosymbionts Across Food Webs: How Aphid Endosymbionts Affect the Survival of the Predatory Invasive Lady Beetle Harmonia axyridis. Available online: http://www.xcdsystem.com/evolution2014/abstract/abstractforms/screen_view_abstract_public.cfm?ID=20067 (accessed on 2 February 2015).
- Costopoulos, K.; Kovacs, J.L.; Kamins, A.; Gerardo, N.M. Aphid facultative symbionts reduce survival of the predatory lady beetle Hippodamia convergens. BMC Ecol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Tsuchida, T.; Sakurai, M.; Fukatsu, T. Selective elimination of aphid endosymbionts: Effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 2007, 60, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bergeson, E.; Messina, F.J. Effect of a co-occurring aphid on the susceptibility of the Russian wheat aphid to lacewing predators. Entomol. Exp. Appl. 1998, 87, 103–108. [Google Scholar] [CrossRef]
- Sampaio, M.V.; Bueno, V.H.P.; Perez-Maluf, R. Parasitismo de Aphidius colemani Viereck (Hymenoptera: Aphidiidae) em Diferentes Densidades de Myzus persicae (Sulzer) (Hemiptera: Aphididae). Neotrop. Entomol. 2001, 30, 81–87. [Google Scholar] [CrossRef]
- Murphy, G.; Biological Control Solutions: Niagara, Ontario, Canada. Personal Communication, 2015.
- Benelli, G.; Kavallieratos, N.G.; Donati, E.; Mencattelli, M.; Bonsignori, G.; Stefanini, C.; Canale, A.; Messing, R.H. May the wild male loose? Male wing fanning performances and mating success in wild and mass-reared strains of the aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae: Aphidiinae). BioControl 2014, 59, 487–500. [Google Scholar]
- Jandricic, S.E.; Wraight, S.P.; Gillespie, D.R.; Sanderson, J.P. Oviposition behavior of the biological control agent Aphidoletes aphidimyza (Diptera: Cecidomyiidae) in environments with multiple pest aphid species (Hemiptera: Aphididae). Biol. Control 2013, 65, 235–245. [Google Scholar] [CrossRef]
- Meisner, M.; Harmon, J.P.; Ives, A.R. Presence of an unsuitable host diminishes the competitive superiority of an insect parasitoid: A distraction effect. Popul. Ecol. 2007, 49, 347–355. [Google Scholar] [CrossRef]
- Michaud, J.P.; Mackauer, M. The use of visual cues in host evaluation by aphidiid wasps—I. Comparison between three Aphidius parasitoids of the pea aphid. Entomol. Exp. Appl. 1994, 70, 273–283. [Google Scholar] [CrossRef]
- Foster, S.P.; Tomiczek, M.; Thompson, R.; Denholm, I.; Poppy, G.; Kraaijeveld, A.R.; Powell, W. Behavioural side-effects of insecticide resistance in aphids increase their vulnerability to parasitoid attack. Anim. Behav. 2007, 74, 621–632. [Google Scholar] [CrossRef]
- Foster, S.P.; Kift, N.B.; Baverstock, J.; Sime, S.; Reynolds, K.; Jones, J.E.; Thompson, R.; Tatchell, G.M. Association of MACE-based insecticide resistance in Myzus persicae with reproductive rate, response to alarm pheromone and vulnerability to attack by Aphidius colemani. Pest Manag. Sci. 2003, 59, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Karagounis, C.; Kourdoumbalos, K.; Margaritopoulos, J.T.; Nanos, G.D.; Tsitsipis, J. Organic farming-compatible insecticides against the aphid Myzus persicae (Sulzer) in peach orchards. J. Appl. Entomol. 2006, 130, 150–154. [Google Scholar] [CrossRef]
- Colinet, H.; Salin, C.; Boivin, G.; Hance, T. Host age and fitness-related traits in a koinobiont aphid parasitoid. Ecol. Entomol. 2005, 30, 473–479. [Google Scholar] [CrossRef]
- Le Ralec, A.; Anselme, C.; Outreman, Y.; Poirié, M.; van Baaren, J.; le Lann, C.; van Alphen, J.J.-M. Evolutionary ecology of the interactions between aphids and their parasitoids. C. R. Biol. 2010, 333, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Gerling, D.; Roitberg, B.D.; Mackauer, M. Instar-specific defense of the pea aphid, Acyrthosiphon pisum: Influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelmidae). J. Insect Behav. 1990, 3, 501–514. [Google Scholar] [CrossRef]
- Paul, G. Insect behavioral and morphological defenses against parasitoids. Annu. Rev. Entomol. 1993, 38, 251–273. [Google Scholar] [CrossRef]
- Henry, L.M.; Ma, B.O.; Roitberg, B.D. Size-mediated adaptive foraging: A host-selection strategy for insect parasitoids. Oecologia 2009, 161, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.P.; Mackauer, M. The use of visual cues in host evaluation by aphidiid wasps: II. Comparison between Ephedrus californicus, Monoctonus paulensis, and Praon pequodorum. Entomol. Exp. Appl. 1995, 74, 267–275. [Google Scholar] [CrossRef]
- Henry, L.M.; Roitberg, B.D.; Gillespie, D.R. Covariance of phenotypically plastic traits induces an adaptive shift in host selection behaviour. Proc. Biol. Sci. 2006, 273, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.W.; Tuda, M.; Kim, J.H.; Choi, M.Y. Functional responses of aphid parasitoids, Aphidius colemani (Hymenoptera: Braconidae) and Aphelinus asychis (Hymenoptera: Aphelinidae). Biocontrol Sci. Technol. 2011, 21, 57–70. [Google Scholar] [CrossRef]
- Bouchard, Y.; Cloutier, C. Honeydew as a source of host-searching kairomones for the aphid parasitoid Aphidius nigripes (Hymenoptera: Aphidiidae). Can. J. Zool. 1984, 62, 1513–1520. [Google Scholar] [CrossRef]
- Chow, F.J.; Mackauer, M. Inter- and intraspecific larval competion in Aphidius smithi and Praon pequodorum (Hymenoptera: Aphididae). Can. Entomol. 1984, 116, 1097–1107. [Google Scholar] [CrossRef]
- McBrien, H.; Mckauer, M. Heterospecific larval competition and host discrimination in two species of aphid parasitoids: Aphidius ervi and Aphidius smithi. Entomol. Exp. Appl. 1990, 56, 145–153. [Google Scholar] [CrossRef]
- Tomanović, T.Z.; Petrović, A.; Mitrović, M.; Kavallieratos, N.G.; Starý, P.; Rakhshani, E.; Rakhshanipour, M.; Popović, A.; Shukshu, A.H.; Ivanović, A. Molecular and morphological variability within the Aphidius colemani group with redescription of Aphidius platensis Brethes (Hymenoptera: Braconidae: Aphidiinae). Bull. Entomol. Res. 2014, 104, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Frewin, A.J.; Murphy, C.S.-D.G.; Hanner, R. DNA barcoding of commercial biological control agents: A quality management framework. IOBC WPRS Bull. 2014, 102, 71–76. [Google Scholar]
- Meyhofer, R.; Klug, T. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera : Aphidiidae): Mortality risks and behavioral decisions made under the threats of predation. Biol. Control 2002, 25, 239–248. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Interactions between specialist and generalist natural enemies: Parasitoids, predators, and pea aphid biocontrol. Ecology 2003, 84, 91–107. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.J.; Francis, F.; Hance, T.; Haubruge, E. Discrimination of parasitized aphids by a hoverfly predator: effects on larval performance, foraging, and oviposition behavior. Entomol. Exp. Appl. 2008, 128, 73–80. [Google Scholar] [CrossRef]
- Martinou, A.F.; Raymond, B.; Milonas, P.G.; Wright, D.J. Impact of intraguild predation on parasitoid foraging behaviour. Ecol. Entomol. 2010, 35, 183–189. [Google Scholar] [CrossRef]
- Ferguson, K.I.; Stiling, P. Non-additive effects of multiple natural enemies on aphid populations. Oecologia 1996, 108, 375–379. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Generalist predators disrupt biological control by a specialist parasitoid. Ecology 2001, 82, 705. [Google Scholar] [CrossRef]
- Chacón, J.M.; Landis, D.; Heimpel, G.E. Potential for biotic interference of a classical biological control agent of the soybean aphid. Biol. Control 2008, 46, 216–225. [Google Scholar] [CrossRef]
- Colfer, R.G.; Rosenheim, J. Predation on immature parasitoids and its impact on aphid suppression. Oecologia 2001, 126, 292–304. [Google Scholar] [CrossRef]
- Costamagna, A.C.; Landis, D.A.; Difonzo, C.D. Suppression of soybean aphid by generalist predators results in a trophic cascade in soybeans. Ecol. Appl. 2007, 17, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.S.; Snyder, W.E. Increasing enemy biodiversity strengthens herbivore suppression on two plant species. Ecology 2008, 89, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Weisser, W.W. Additive effects of pea aphid natural enemies despite intraguild predation. In Proceedings of the 8th International Sympoisum on Ecolgoy of Aphidophaga: Biology, Ecology and Behavior of Aphidophagous Insects; Soares, A.O., Ventura, M.A., Garcia, V., Hemptinne, J.-L., Eds.; Life and Marine Sciences: Ponta Delgada, 2003; pp. 11–15. [Google Scholar]
- Snyder, W.E.; Ballard, S.N.; Yang, S.; Clevenger, G.M.; Miller, T.D.; Ahn, J.J.; Hatten, T.D.; Berryman, A. Complementary biocontrol of aphids by the ladybird beetle Harmonia axyridis and the parasitoid Aphelinus asychis on greenhouse roses. Biol. Control 2004, 30, 229–235. [Google Scholar] [CrossRef]
- Pilkington, L.J.; Messelink, G.; van Lenteren, J.C.; le Mottee, K. “Protected Biological Control”—Biological pest management in the greenhouse industry. Biol. Control 2010, 52, 216–220. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Zhang, Y. Impacts of Beauveria bassiana on life parameters and control efficiency of Aphidius gifuensis. Mycosystema 2007, 3, 433–439. (In Chinese) [Google Scholar]
- Fuentes-Contreras, E.; Pell, J.K.; Niemeyer, H.M. Influence of plant resistance at the third trophic level: Interactions between parasitoids and entomopathogenic fungi of cereal aphids. Oecologia 1998, 117, 426–432. [Google Scholar] [CrossRef]
- Powell, W.; Wilding, N.; Brobyn, P.J.; Clark, S.J. Interference between parasitoids (Hym.: Aphidiidae) and fungi (Entomophthorales) attacking cereal aphids. Entomophaga 1986, 31, 293–302. [Google Scholar]
- Jandricic, S.E.; Filotas, M.; Sanderson, J.P.; Wraight, S.P. Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol. 2014, 118, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Kanagaratnam, P.; Hall, R.A.; Burges, H.D. Control of glasshouse whitefly, Trialeurodes vaporariorum, by an “aphid” strain of the fungus Verticillium lecanii. Ann. Appl. Biol. 1982, 100, 213–219. [Google Scholar] [CrossRef]
- Osborne, L.S.; Landa, Z. Biological control of whiteflies with entomopathogenic fungi. Florida Entomol. 1992, 75, 456–471. [Google Scholar] [CrossRef]
- Rashki, M.; Kharazi-pakdel, A.; Allahyari, H.; van Alphen, J.J.M. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biol. Control 2009, 50, 324–328. [Google Scholar] [CrossRef]
- Holler, C.; Micha, S.; Schulz, S.; Francke, W.; Pickett, J.A. Enemy-induced dispersal in a parasitic wasp. Experientia 1994, 50, 182–185. [Google Scholar] [CrossRef]
- Petersen, G.; Matthiesen, C.; Francke, W.; Wyss, U. Hyperparasitoid volatiles as possible foraging behaviour determinants in the aphid parasitoid Aphidius uzbekistanicus (Hymenoptera: Aphididae). Eur. J. Entomol. 2000, 97, 545–550. [Google Scholar] [CrossRef]
- Buitenhuis, R.; McNeil, J.N.; Boivin, G.; Brodeuri, J. The role of honeydew in host searching of aphid hyperparasitoids. J. Chem. Ecol. 2004, 30, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, T.D.; Messing, R. Development and use of molecular diagnostic tools to determine trophic links and interspecific interactions in aphid-parasitoid communities in Hawaii. Biol. Control 2012, 60, 26–38. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Biological control of aphids on cucumer in plastic greenhouses using banker plants. Korean J. Appl. Entomol. 2003, 42, 81–84. [Google Scholar]
- Brodeur, J.; McNeil, N. Life history of the aphid hyperparasitoid Asaphes volgaris Walker (Pteromalidae): Possible consequences on the efficacy of the primary parasitoid Aphidius nigripes Ashmead (Aphidiidae). Can. Entomol. 1994, 126, 1493–1497. [Google Scholar] [CrossRef]
- Bennison, J. Biological control of aphids on cucumbers: Use of open rearing systems or “banker plants” to aid establishment of Aphidius matricariae and Aphidoletes aphidimyza. Meded. van Fac. Landbouwwet. Universteit Gent. 1992, 57, 457–466. [Google Scholar]
- McClure, T.J.; Frank, S.D. North Carolina State University: Raleigh, North Carolina, USA, Unpublished Data. 2014.
- Short, M.; Eco Habitat Agri Services: Toronto, Ontario, Canada. Personal Communication, 2015.
- Desneux, N.; Denoyelle, R.; Kaiser, L. A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere 2006, 65, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Boller, E.F.; Vogt, H.; Ternes, P.; Malavolta, C. Working Document on Selectivity of Pesticides (2005). Available online: https://www.iobc-wprs.org/ip_ipm/03021_IOBC_WorkingDocumentPesticides_Explanations.pdf (accessed on 5 February 2015).
- Cloyd, R.A. Indirect Effects of Pesticides on Natural Enemies. In Pesticides—Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Longley, M.; Jepson, P.C. Effects of honeydew and insecticide residues on the distribution of foraging aphid parasitoids under glasshouse and field conditions. Entomol. Exp. Appl. 1996, 81, 189–198. [Google Scholar] [CrossRef]
- Desneux, N.; Pham-Delègue, M.-H.; Kaiser, L. Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp, Aphidius ervi. Pest Manag. Sci. 2004, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.E.; Araya, M.; Guerrero, M.A. Effects of some insecticides applied in sublethal concentrations on the survival and longevity of Aphidius ervi (Haliday) (Hymenoptera: Aphidiidae) adults. Chil. J. Agric. Res. 2010, 70, 221–227. [Google Scholar] [CrossRef]
- Joseph, J.-R.; Ameline, A.; Couty, A. Effects on the aphid parasitoid Aphidius ervi of an insecticide (Plenum®, pymetrozine) specific to plant-sucking insects. Phytoparasitica 2010, 39, 35–41. [Google Scholar] [CrossRef]
- Zemek, R.; Jarosik, V.; Havelka, J.; Chihale, F.P. Effect of host plant and temperature on Aphidius Colemani (Hymenoptera: Braconidae) intrinsic rate of population increase. Available online: http://www.researchgate.net/publication/267386398_EFFECTS_OF_HOST_PLANT_AND_TEMPERATURE_ON_Aphidius_colemani_%28HYMENOPTERA_BRACONIDAE%29_INTRINSIC_RATE_OF_POPULATION_INCREASE (accessed on 3 February 2015).
- Henry, L.; May, N.; Acheampong, S.; Gillespie, D.R.; Roitberg, B.D. Host-adapted parasitoids in biological control: Does source matter? Ecol. Appl. 2010, 20, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Roux, O.; Le Lann, C.; van Alphen, J.J.M.; van Baaren, J. How does heat shock affect the life history traits of adults and progeny of the aphid parasitoid Aphidius avenae (Hymenoptera: Aphidiidae)? Bull. Entomol. Res. 2010, 100, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Temperature-dependent functional response of two aphid parasitoids, Aphidius colemani and Aphidius matricariae (Hymenoptera: Aphidiidae), on the cotton aphid. J. Pest Sci. 2006, 79, 183–188. [Google Scholar] [CrossRef]
- Kim, J.-H.; Byeon, Y.W.; Kim, H.-Y.; Park, C.-G.; Choi, M.-Y.; Han, M.-J. Biological control of insect pests with arthropod natural enemies on greenhouse sweet pepper in winter cropping system. Korean J. Appl. Entomol. 2010, 49, 385–391. [Google Scholar] [CrossRef]
- Birch, L.C. The Intrinsic Rate of Natural Increase of an Insect Population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Torres, A.; Bueno, V.; Sampaio, M.V.; de Conti, B.F. Fertility life table of Aphidius colemani Viereck (Hymenoptera: Brandonidae, Aphidiinae) on Aphis gossypii Glover (Hemiptera: Aphididae). Neotrop. Entomol. 2007, 35, 532–536. [Google Scholar] [CrossRef]
- Denis, D.; Pierre, J.S.; van Baaren, J.; van Alphen, J.J.M. How temperature and habitat quality affect parasitoid lifetime reproductive success-A simulation study. Ecol. Modell. 2011, 222, 1604–1613. [Google Scholar] [CrossRef]
- De Koning, A.N.M. Long-term temperature integration of tomato. Growth and development under alternating temperature regimes. Sci. Hortic. 1990, 45, 117–127. [Google Scholar]
- Van den Berg, G.A.; Butwalda, F.; Rijpsma, E.C. Practical Demonstration of Mult-Day Temperature Integration; Wageningen UR: Wageningen, The Netherlands, 2001; p. 501. [Google Scholar]
- Gilkeson, A. A note on fecundity of the aphid predator, Aphidolete aphidimyza (Rondani) (Diptera: Cecidomyiidae). Can. Entomol. 1987, 6, 1145–1146. [Google Scholar] [CrossRef]
- Mori, H.; Chant, D.A. The influence of prey density, relative humidity and starvation on the predacious behavior of Phytoseiulus persimilis Athias-Henriot (Acarina: Phytoseiidae). Can. J. Zool. 1966, 44, 483–491. [Google Scholar] [CrossRef]
- Van Houten, Y.M.; van Rijn, P.C.J.; Tanigoshi, L.K.; van Stratum, P.; Bruin, J. Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Entomol. Exp. Appl. 1995, 74, 225–234. [Google Scholar] [CrossRef]
- Shipp, J.L.; Ward, K.I.; Gillespie, T.J. Influence of temperature and vapor pressure deficit on the rate of predation by the predatory mite, Amblyseius cucumeris, on Frankliniella occidentalis. Entomol. Exp. Appl. 1996, 78, 31–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Shipp, J.L. Effect of temperature and vapour pressure deficit on the flight acitivity of Orius insidiosus (Hemiptera: Anthrocoridae). Environ. Entomol. 1998, 27, 736–742. [Google Scholar] [CrossRef]
- Tuda, M.; Shima, K. Relative importance of weather and density dependence on the dispersal and on-plant activity of the predator Orius minutus. Popul. Ecol. 2002, 44, 251–257. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, W. Effect of humidity on eclosion rate and life of adult Aphidius gifuensis. 2012, 3, 121–123. [Google Scholar]
- Fink, U.; Volkl, W. The effect of abiotic factors on foraging and oviposition success of the aphid parasitoid, Aphidius rosae. Oecologia 1995, 103, 371–378. [Google Scholar] [CrossRef]
- Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Shipp, J.L.; Johansen, N.S. Prospecting the use of artificial lighting for integrated pest management. Acta Hortic. 2012, 956, 593–608. [Google Scholar]
- Johansen, N.S.; Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Shipp, L. In the light of new greenhouse technologies: 2. Direct effects of artificial lighting on arthropods and integrated pest management in greenhouse crops. Ann. Appl. Biol. 2011, 159, 1–27. [Google Scholar]
- Coombe, P. Wavelength specific behaviour of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J. Comp. Physiol. A 1981, 144, 83–90. [Google Scholar] [CrossRef]
- Antignus, Y.; Mor, N.; Ben, J.R.; Lapidot, M.; Cohen, S. Ultraviolet-absorbing plastic sheets protect crops from insect pests and from virus diseases vectored by insects. Environ. Entomol. 1996, 25, 919–924. [Google Scholar] [CrossRef]
- Doukas, D.; Payne, C.C. The use of ultraviolet-blocking films in insect pest management in the UK; effects on naturally occurring arthropod pest and natural enemy populations in a protected cucumber crop. Ann. Appl. Biol. 2007, 151, 221–231. [Google Scholar] [CrossRef]
- Shipp, J.L.; Johansen, N.; Vänninen, I.; Jacobson, R. Greenhouse climate: An important consideration when developing pest management programs for greenhouse crops. Acta Hortic. 2011, 893, 133–143. [Google Scholar]
- Vanninen, I.; Pinto, D.; Nissinen, A.; Johnansen, N.S.; Shipp, L. Prospecting the use of artifical lighting for integrated pest management. ISHS Acta Horticulturae 2010, 1, 593–608. [Google Scholar]
- McClure, M.; McNeil, J.N. The effect of abiotic factors on the male mate searching behavior and the mating success of Aphidius ervi (Hymenoptera: Aphidiidae). J. Insect Behav. 2009, 22, 101–110. [Google Scholar] [CrossRef]
- Marchand, D.; McNeil, J.N. Effects of wind speed and atmospheric pressure on mate searching behavior in the aphid parasitoid Aphidius nigripes (Hymenoptera: Aphidiidae). J. Insect Behav. 2000, 13, 187–199. [Google Scholar] [CrossRef]
- Ventilation for Greenhouses. Available online: https://ag.umass.edu/fact-sheets/ventilation-for-greenhouses (accessed on 3 February 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, S.G.; Jandricic, S.E.; Frank, S.D. Ecological Interactions Affecting the Efficacy of Aphidius colemani in Greenhouse Crops. Insects 2015, 6, 538-575. https://doi.org/10.3390/insects6020538
Prado SG, Jandricic SE, Frank SD. Ecological Interactions Affecting the Efficacy of Aphidius colemani in Greenhouse Crops. Insects. 2015; 6(2):538-575. https://doi.org/10.3390/insects6020538
Chicago/Turabian StylePrado, Sara G., Sarah E. Jandricic, and Steven D. Frank. 2015. "Ecological Interactions Affecting the Efficacy of Aphidius colemani in Greenhouse Crops" Insects 6, no. 2: 538-575. https://doi.org/10.3390/insects6020538





