Characteristics of in Vivo Model Systems for Ovarian Cancer Studies
Abstract
:1. Introduction
2. Animal Models for Ovarian Cancer Studies
2.1. Fruit Fly Model
2.2. Mouse Models
2.2.1. Xenograft Models
Xenografts Using Established Cell Lines
Patient Derived Xenografts (PDXs)
2.2.2. Syngeneic Model
2.2.3. Genetically Modified Mice
2.3. Xenopus Model
2.4. Laying Hen Model
Chorioallantoic Membrane Model
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubeau, L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008, 9, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, K.A.; Lisowska, K.M. Ovarian cancer—From biology to clinic. Postepy Higieny i Medycyny Doswiadczalnej 2015, 69, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Basta, A.; Bidzinski, M.; Bienkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J.; et al. Recommendation of the Polish Society of Oncological Gynaecology on the diagnosis and treatment of epithelial ovarian cancer. Oncol. Clin. Pract. 2015, 11, 233–243. [Google Scholar]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Goyos, A.; Robert, J. Tumorigenesis and anti-tumor immune responses in Xenopus. Front. Biosci. 2009, 14, 167–176. [Google Scholar] [CrossRef]
- Bernardo, A.M.; Thorsteinsdottir, S.; Mummery, C.L. Advantages of the avian model for human ovarian cancer. Mol. Clin. Oncol. 2015, 3, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Cho, K.R. Preclinical Models of Ovarian Cancer: Pathogenesis, Problems, and Implications for Prevention. Clin. Obstet. Gynecol. 2017, 60, 789–800. [Google Scholar] [CrossRef]
- Naora, H.; Montell, D.J. Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nat. Rev. Cancer 2005, 5, 355–366. [Google Scholar] [CrossRef]
- Rosales-Nieves, A.E.; Gonzalez-Reyes, A. Genetics and mechanisms of ovarian cancer: Parallels between Drosophila and humans. Semin. Cell Dev. Biol. 2014, 28, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Beccari, S.; Teixeira, L.; Rorth, P. The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech. Dev. 2002, 111, 115–123. [Google Scholar] [CrossRef]
- Ghiglione, C.; Devergne, O.; Georgenthum, E.; Carballes, F.; Medioni, C.; Cerezo, D.; Noselli, S. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 2002, 129, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.L.; Geisbrecht, E.R.; Montell, D.J. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 2005, 132, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.L.; Montell, D.J. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell 2001, 107, 831–841. [Google Scholar] [CrossRef]
- Xi, R.W.; McGregor, J.R.; Harrison, D.A. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 2003, 4, 167–177. [Google Scholar] [CrossRef]
- Bai, J.W.; Uehara, Y.; Montell, D.J. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AlB1, a steroid receptor coactivator amplified in breast cancer. Cell 2000, 103, 1047–1058. [Google Scholar] [CrossRef]
- Anzick, S.L.; Kononen, J.; Walker, R.L.; Azorsa, D.O.; Tanner, M.M.; Guan, X.Y.; Sauter, G.; Kallioniemi, O.P.; Trent, J.M.; Meltzer, P.S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 1997, 277, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Domanitskaya, E.; Anllo, L.; Schuepbach, T. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev. Biol. 2014, 386, 408–418. [Google Scholar] [CrossRef]
- Torres-Arzayus, M.I.; de Mora, J.F.; Yuan, J.; Vazquez, F.; Branson, R.; Rue, M.; Sellers, W.R.; Brown, M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 2004, 6, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Tanner, M.M.; Grenman, S.; Koul, A.; Johannsson, O.; Meltzer, P.; Pejovic, T.; Borg, A.; Isola, J.J. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin. Cancer Res. 2000, 6, 1833–1839. [Google Scholar] [PubMed]
- Chuffa, L.G.D.; Lupi, L.A.; Costa, A.B.; Amorim, J.P.D.; Seiva, F.R.F. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Knipprath-Meszaros, A.; Heinzelmann-Schwarz, V.; Vetter, M. Endocrine therapy in epithelial ovarian cancer (EOC) New insights in an old target: A mini review. J. Cancer Clin. Trials 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Modugno, F.; Laskey, R.; Smith, A.L.; Andersen, C.L.; Haluska, P.; Oesterreich, S. Hormone response in ovarian cancer: Time to reconsider as a clinical target? Endocr.-Relat. Cancer 2012, 19, R255–R279. [Google Scholar] [CrossRef] [PubMed]
- Stanley, B.; Hollis, R.L.; Nunes, H.; Towler, J.D.; Yan, X.; Rye, T.; Dawson, C.; Mackean, M.J.; Nussey, F.; Churchman, M.; et al. Endocrine treatment of high grade serous ovarian carcinoma; quantification of efficacy and identification of response predictors. Gynecol. Oncol. 2019, 152, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J., 3rd; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.L.; et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef]
- Lin, T.H.; Yeh, T.H.; Wang, T.W.; Yu, J.Y. The Hippo Pathway Controls Border Cell Migration Through Distinct Mechanisms in Outer Border Cells and Polar Cells of the Drosophila Ovary. Genetics 2014, 198, 1087–1099. [Google Scholar] [CrossRef]
- Lucas, E.P.; Khanal, I.; Gaspar, P.; Fletcher, G.C.; Polesello, C.; Tapon, N.; Thompson, B.J. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 2013, 201, 875–885. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.J. The Hippo Signaling Pathway in Development and Cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Snigdha, K.; Gangwani, K.S.; Lapalikar, G.V.; Singh, A.; Kango-Singh, M. Hippo Signaling in Cancer: Lessons From Drosophila Models. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.A.; Wang, R.S.; Miao, J.Y.; Oliva, E.; Shen, X.Y.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; George, J.; Deb, S.; Degoutin, J.L.; Takano, E.A.; Fox, S.B.; AOCS Study Group; Bowtell, D.D.L.; Harvey, K.F. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011, 30, 2810–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montell, D.J.; Yoon, W.H.; Starz-Gaiano, M. Group choreography: Mechanisms orchestrating the collective movement of border cells. Nat. Rev. Mol. Cell Biol. 2012, 13, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Ye, D.F.; Xie, X.; Chen, B.Y.; Lu, W.G. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol. Oncol. 2004, 94, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.A.; Kim, T.J.; Landen, C.N., Jr.; Lu, C.; Han, L.Y.; Lin, Y.G.; Merritt, W.M.; Thaker, P.H.; Gershenson, D.M.; Bischoff, F.Z.; et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007, 67, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Yazici, S.; Nilsson, M.B.; Yokoi, K.; Tsan, R.Z.; He, J.; Kim, S.J.; Fidler, I.J.; Sood, A.K. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin. Cancer Res. 2005, 11, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Klovstad, M.; Abdu, U.; Schupbach, T. Drosophila BRCA2 is required for mitotic and meiotic DNA repair and efficient activation of the meiotic recombination checkpoint. PLoS Genet. 2008, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Han, Y.; Xi, R.W. Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Gene. Dev. 2010, 24, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Kakar, S.S. Ovarian cancer mouse models: A summary of current models and their limitations. J. Ovarian Res. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- House, C.D.; Hernandez, L.; Annunziata, C.M. Recent technological advances in using mouse models to study ovarian cancer. Front. Oncol. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bobbs, A.S.; Cole, J.M.; Cowden Dahl, K.D. Emerging and Evolving Ovarian Cancer Animal Models. Cancer Growth Metastasis 2015, 8, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Garson, K.; Shaw, T.J.; Clark, K.V.; Yao, D.S.; Vanderhyden, B.C. Models of ovarian cancer—Are we there yet? Mol. Cell. Endocrinol. 2005, 239, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, E.; Marasco, W.A. The latest animal models of ovarian cancer for novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Hippo, Y. Current Status of Patient-Derived Ovarian Cancer Models. Cells 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Tudrej, P.; Kujawa, K.A.; Cortez, A.J.; Lisowska, K.M. Characteristics of in vitro model systems for ovarian cancer studies. Oncol. Clin. Pract. 2019, 15. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beaufort, C.M.; Helmijr, J.C.A.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van IJcken, W.F.J.; Heine, A.A.J.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef] [PubMed]
- Tudrej, P.; Olbryt, M.; Zembala-Nozynska, E.; Kujawa, K.A.; Cortez, A.J.; Fiszer-Kierzkowska, A.; Piglowski, W.; Nikiel, B.; Glowala-Kosinska, M.; Bartkowska-Chrobok, A.; et al. Establishment and characterization of the novel high-grade serous ovarian cancer cell line OVPA8. Int. J. Mol. Sci. 2018, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.C.; Young, R.C.; Ozols, R.F. Experimental-model systems of ovarian cancer—Applications to the design and evaluation of new treatment approaches. Semin. Oncol. 1984, 11, 285–298. [Google Scholar] [PubMed]
- Molthoff, C.F.M.; Calame, J.J.; Pinedo, H.M.; Boven, E. Human Ovarian-Cancer Xenografts in nude mice: Characterization and Analysis of Antigen Expression. Int. J. Cancer 1991, 47, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Matulonis, U.A. Current status and evolution of preclinical drug development models of epithelial ovarian cancer. Front. Oncol. 2013, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Kim, M.K.; Lyle, L.T.; Bunch, K.P.; House, C.D.; Ning, F.; Noonan, A.M.; Annunziata, C.M. Characterization of ovarian cancer cell lines as in vivo models for preclinical studies. Gynecol. Oncol. 2016, 142, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letourneau, I.J.; Quinn, M.C.; Wang, L.L.; Portelance, L.; Caceres, K.Y.; Cyr, L.; Delvoye, N.; Meunier, L.; de Ladurantaye, M.; Shen, Z.; et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 2012, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Chanana, P.; Davila, J.I.; Hou, X.N.; Zanfagnin, V.; McGehee, C.D.; Goode, E.L.; Polley, E.C.; Haluska, P.; Weroha, S.J.; et al. Gene expression differences between matched pairs of ovarian cancer patient tumors and patient-derived xenografts. Sci. Rep. 2019, 9, 6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zietarska, M.; Maugard, C.M.; Filali-Mouhim, A.; Alam-Fahmy, M.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.M. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol. Carcinog. 2007, 46, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Ha, G.; Tseng, Y.Y.; Greenwald, N.F.; Oh, C.; Shih, J.; McFarland, J.M.; Wong, B.; Boehm, J.S.; Beroukhim, R.; et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 2017, 49, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolfschoten, G.M.; Pinedo, H.M.; Scheffer, P.G.; Schluper, H.M.M.; Erkelens, C.A.M.; Boven, E. Development of a panel of 15 human ovarian cancer xenografts for drug screening and determination of the role of the glutathione detoxification system. Gynecol. Oncol. 2000, 76, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, P.; Stewart, J.M.; Sayad, A.; Virtanen, C.; Shaw, P.A.; Clarke, B.; Stickle, N.; Bernardini, M.Q.; Neel, B.G. A Genomically Characterized Collection of High-Grade Serous Ovarian Cancer Xenografts for Preclinical Testing. Am. J. Pathol. 2018, 188, 1120–1131. [Google Scholar] [CrossRef] [Green Version]
- Harris, F.R.; Zhang, P.Y.; Yang, L.; Hou, X.N.; Leventakos, K.; Weroha, S.J.; Vasmatzis, G.; Kovtun, I.V. Targeting HER2 in patient-derived xenograft ovarian cancer models sensitizes tumors to chemotherapy. Mol. Oncol. 2019, 13, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Kenney, L.L.; Jangalwe, S.; Aryee, K.E.; Greiner, D.L.; Brehm, M.A.; Shultz, L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017, 12, 187–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2015, 34, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, C.W.; Goldberg, R.L.; Carter, L.E.; Gamwell, L.F.; Al-Hujaily, E.M.; Collins, O.; Macdonald, E.A.; Garson, K.; Daneshmand, M.; Carmona, E.; et al. A new spontaneously transformed syngeneic model of high-grade serous ovarian cancer with a tumor-initiating cell population. Front. Oncol. 2014, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 2005, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mullany, L.K.; Richards, J.S. Minireview: Animal Models and Mechanisms of Ovarian Cancer Development. Endocrinology 2012, 153, 1585–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsulic, S.; Li, Y.; Soslow, R.A.; Vitale-Cross, L.A.; Gutkind, J.S.; Varmus, H.E. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 2002, 1, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.Y.; Orsulic, S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6936–6941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, D.; Orsulic, S. A mouse model for the molecular characterization of brca1-associated ovarian carcinoma. Cancer Res. 2006, 66, 8949–8953. [Google Scholar] [CrossRef]
- Flesken-Nikitin, A.; Choi, K.C.; Eng, J.P.; Shmidt, E.N.; Nikitin, A.Y. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003, 63, 3459–3463. [Google Scholar] [PubMed]
- Flesken-Nikitin, A.; Hwang, C.-I.; Cheng, C.-Y.; Michurina, T.V.; Enikolopov, G.; Nikitin, A.Y. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 2013, 495, 241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Coffey, D.M.; Creighton, C.J.; Yu, Z.F.; Hawkins, S.M.; Matzuk, M.M. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 3921–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.; Ohman, A.W.; Stepule, C.D.; Kwak, S.; et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.C.; Bao, R.; Nikitin, A.Y.; Stephens, K.C.; Poole, T.W.; Hua, X.; Harris, S.S.; Vanderhyden, B.C.; Hamilton, T.C. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003, 63, 1389–1397. [Google Scholar] [PubMed]
- Mabuchi, S.; Altomare, D.A.; Connolly, D.C.; Klein-Szanto, A.; Litwin, S.; Hoelzle, M.K.; Hensley, H.H.; Hamilton, T.C.; Testa, J.R. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007, 67, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, I.; Takahashi, K.; Kon, Y.; Okamura, T.; Mototani, Y.; Araki, Y.; Kasai, N. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large T-antigen: Tumor formation and its hormonal regulation. Mol. Reprod. Dev. 2002, 63, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Mullany, L.K.; Fan, H.Y.; Liu, Z.; White, L.D.; Marshall, A.; Gunaratne, P.; Anderson, M.L.; Creighton, C.J.; Xin, L.; Deavers, M.; et al. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene 2011, 30, 3522–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Hendrix-Lucas, N.; Kuick, R.; Zhai, Y.L.; Schwartz, D.R.; Akyol, A.; Hanash, S.; Misek, D.E.; Katabuchi, H.; Williams, B.O.; et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 2007, 11, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, J.; Yoshida, H.; Rosen, D.; Naora, H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat. Med. 2005, 11, 531–537. [Google Scholar] [CrossRef]
- Clark-Knowles, K.V.; Senterman, M.K.; Collins, O.; Vanderhyden, B.C. Conditional Inactivation of Brca1, p53 and Rb in Mouse Ovaries Results in the Development of Leiomyosarcomas. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Fan, H.Y.; Liu, Z.L.; Paquet, M.; Wang, J.R.; Lydon, J.P.; DeMayo, F.J.; Richards, J.S. Cell Type-Specific Targeted Mutations of Kras and Pten Document Proliferation Arrest in Granulosa Cells versus Oncogenic Insult to Ovarian Surface Epithelial Cells. Cancer Res 2009, 69, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.A.; Brake, T.; Hua, X.; Baxter-Jones, K.; Litwin, S.; Ellenson, L.H.; Connolly, D.C. Induction of Ovarian Leiomyosarcomas in Mice by Conditional Inactivation of Brca1 and p53. PLoS ONE 2009, 4, e8404. [Google Scholar] [CrossRef] [PubMed]
- Szabova, L.; Yin, C.Y.; Bupp, S.; Guerin, T.M.; Schlomer, J.J.; Householder, D.B.; Baran, M.L.; Yi, M.; Song, Y.R.; Sun, W.P.; et al. Perturbation of Rb, p53, and Brca1 or Brca2 Cooperate in Inducing Metastatic Serous Epithelial Ovarian Cancer. Cancer Res. 2012, 72, 4141–4153. [Google Scholar] [CrossRef] [PubMed]
- Sherman-Baust, C.A.; Kuhn, E.; Valle, B.L.; Shih, L.M.; Kurman, R.J.; Wang, T.L.; Amano, T.; Ko, M.S.H.; Miyoshi, I.; Araki, Y.; et al. A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development. J. Pathol. 2014, 233, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Mohapatra, G.; Chiang, S.; Engler, D.A.; Zhang, L.H.; Kaneko-Tarui, T.; Ohguchi, Y.; Birrer, M.J.; Teixeira, J.M. Loss of LKB1 and PTEN tumor suppressor genes in the ovarian surface epithelium induces papillary serous ovarian cancer. Carcinogenesis 2014, 35, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Tirodkar, T.S.; Budiu, R.A.; Elishaev, E.; Zhang, L.X.; Mony, J.T.; Brozick, J.; Edwards, R.P.; Vlad, A.M. MUC1 Positive, Kras and Pten Driven Mouse Gynecologic Tumors Replicate Human Tumors and Vary in Survival and Nuclear Grade Based on Anatomical Location. PLoS ONE 2014, 9, e102409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Coffey, D.M.; Ma, L.; Matzuk, M.M. The Ovary Is an Alternative Site of Origin for High-Grade Serous Ovarian Cancer in Mice. Endocrinology 2015, 156, 1975–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.A.; Mullany, L.K.; Liu, Z.L.; Herron, A.J.; Wong, K.K.; Richards, J.S. Mutant p53 Promotes Epithelial Ovarian Cancer by Regulating Tumor Differentiation, Metastasis, and Responsiveness to Steroid Hormones. Cancer Res. 2016, 76, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhai, Y.L.; Kuick, R.; Karnezis, A.N.; Garcia, P.; Naseem, A.; Hu, T.C.; Fearon, E.R.; Cho, K.R. Impact of oviductal versus ovarian epithelial cell of origin on ovarian endometrioid carcinoma phenotype in the mouse. J. Pathol. 2016, 240, 341–351. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Burdette, J.E. Evaluating the progenitor cells of ovarian cancer: Analysis of current animal models. BMB Rep. 2011, 44, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Polack, S.S. The xenopus pregnancy test. Can. Med Assoc. J. 1949, 60, 159–161. [Google Scholar] [PubMed]
- Hardwick, L.J.A.; Philpott, A. An oncologist’s friend: How Xenopus contributes to cancer research. Dev. Biol. 2015, 408, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Conlon, F.; Furlow, J.D.; Horb, M.E. Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Dev. Biol. 2017, 426, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Giles, J.R. The hen as a model of ovarian cancer. Nat. Rev. Cancer 2013, 13, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Bitterman, P.; Abramowicz, J.S.; Dirks, A.L.; Bahr, J.M.; Hales, D.B.; Bradaric, M.J.; Edassery, S.L.; Rotmensch, J.; Luborsky, J.L. Histopathology of ovarian tumors in laying hens a preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hawkridge, A.M. The chicken model of spontaneous ovarian cancer. Proteom. Clin. Appl. 2014, 8, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Trevino, L.S.; Giles, J.R.; Wang, W.; Urick, M.E.; Johnson, P.A. Gene expression profiling reveals differentially expressed genes in ovarian cancer of the hen: Support for oviductal origin? Horm. Cancer 2010, 1, 177–186. [Google Scholar] [CrossRef] [PubMed]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.C.; Harrington, D.A.; Yu, W.D.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Ribatti, D. Chick Embryo Chorioallantoic Membrane as a Useful Tool to Study Angiogenesis. Int. Rev. Cell Mol. Biol. 2008, 270, 181–224. [Google Scholar] [CrossRef]
- Aleksandrowicz, E.; Herr, I. Ethical Euthanasia and Short-Term Anesthesia of the Chick Embryo. ALTEX Altern. Anim. Exp. 2015, 32, 143–147. [Google Scholar] [CrossRef]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Doan, T.L.H.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick chorioallantoic membrane assay as an in vivo model to study the effect of nanoparticle-based anticancer drugs in ovarian cancer. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloskey, C.W.; Rodriguez, G.M.; Galpin, K.J.C.; Vanderhyden, B.C. Ovarian Cancer Immunotherapy: Preclinical Models and Emerging Therapeutics. Cancers 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]




| Features | Human | Fruit Fly | Clawed Frog | Mouse | Laying Hen | |
|---|---|---|---|---|---|---|
| Anatomic | Ovaries (number) | + (2) | + (2) | + (2) | + (2) | + (1) |
| Fallopian tubes (number) | + (2) | + (1) | + (2) | + (2) | + (1) | |
| Fallopian tube fimbriae | + | − | − | + | − | |
| Ovarian bursa | − | − | − | + | − | |
| Uterus | + | + | − 1 | + | + 2 | |
| Histological | Endometriosis | + | − | − | − 3 | − |
| Physiological | Menstrual cycle | + | − | − | − | − |
| Estrus cycle | − | − | − | + | − | |
| Clinical | Spontaneous cancer development | + | − | + | − | + |
| Pathological | Confirmed histotypes | + | − | − | + | − 4 |
| Experimental | Genetic modifications | NA | + | + | + | − |
| Injection Site | Features of the Model |
|---|---|
| Subcutaneously |
|
| Intraperitoneally |
|
| Orthotopically |
|
| Genotype(s) | Postulated Tissue of Tumor Origin | Histology of The Tumors | References |
|---|---|---|---|
| (p53−/−;cMyc+;Kras+) (p53−/−;Kras+;Akt+) (p53−/−;Akt+;cMyc+) (p53−/−;Akt+;cMyc+;Kras+) | OSE | primary tumors: poorly differentiated carcinomas; metastasis: poorly differentiated carcinomas and papillary serous carcinomas | Orsulic et al. (2002) [67] |
| (p53−/−;pRb−/−) | OSE | poorly differentiated carcinomas; | Connolly et al. (2003) [74] |
| (Hoxa9+) (Hoxa10+) (Hoxa11+) | OSE | Hoxa9+: papillary serous carcinomas; Hoxa10+: endometrioid carcinomas; Hoxa11+: mucinous carcinomas | Cheng et al. (2005) [79] |
| (Kras+;Pten−/−) | OSE | endometrioid carcinomas | Dinulescu et al. (2005) [65] |
| (Brca1−/−;p53−/−;cMyc+) | OSE | papillary serous carcinomas | Xing and Orsulic (2006) [69] |
| (Apc−/−;Pten −/−) | OSE | endometrioid carcinomas | Wu et al. (2007) [78] |
| (p53−/−) (Brca1−/−;p53−/−) (pRb−/−;p53−/−) (pRb−/−;p53−/−;Brca1−/−) | OSE | malignant leiomyosarcomas | Clark-Knowles et al. (2009) [80] |
| (K-ras−/−;Pten−) | OSE | low-grade serous carcinomas (LGSC) | Fan et al. (2009) [81] |
| (Brca1−/−;p53−/−) | OSE | high-grade leiomyosarcomas | Quinn et al. (2009) [82] |
| (Dicer−/−;Pten −/−) | FTE | high-grade serous carcinomas (HGSC) | Kim et al. (2012) [72] |
| (Brca1−/−;p53 −/−;pRb−/−) (Brca2−/−;p53−/−;pRb−/−) | OSE | serous carcinomas | Szabova et al. (2012) [83] |
| (Brca1−/−;p53mut;Pten−/−) (Brca1+/−;p53mut;Pten−/−) (Brca2−/−;p53mut;Pten−/−) (Brca2+/−;p53mut;Pten−/−) | FTE | serous tubal intra-epithelial carcinomas (STIC) | Perets et al. (2013) [73] |
| (p53−/−) (pRb−/−) (p53−/−;pRb−/−) | OSE | p53−/−: poorly differentiated CK8-positive neoplasms; p53−/−;pRb−/−: well-differentiated serous epithelial neoplasms, poorly differentiated CK8-positive neoplasms, undifferentiated neoplasms | Flesken-Nikitin et al. (2013) [70] |
| (p53−/−;Top2a+) | FTE | serous tubal intra-epithelial carcinomas (STIC) | Sherman-Baust et al. (2014) [84] |
| (Lkb1−/−;Pten−/−) | OSE | high-grade serous carcinomas (HGSC) | Tanwar et al. (2014) [85] |
| (Kras+;Pten−/−) (Muc1+;Kras+;Pten−/−) | OSE, FTE | endometrioid carcinomas | Tirodkar et al. (2014) [86] |
| (p53mut;Dicer−;Pten−) | OSE | high-grade serous carcinomas (HGSC) | Kim et al. (2015) [87] |
| (p53+/+;Pten−/−;Kras+) (p53+/−;Pten−/−;Kras+) (p53−/−;Pten−/−;Kras+) | OSE | p53+/+;Pten−/−;Kras+: low-grade serous carcinomas (LGSC); p53+/−;Pten−/−;Kras+: invasive serous carcinomas, mucinous carcinomas; p53−/−;Pten−/−;Kras+: likely early serous carcinomas | Ren et al. (2016) [88] |
| (Apc−/−;Pten−/−) | FTE, OSE | OSE: poorly differentiated carcinomas; FTE: well-differentiated tumors | Wu et al. (2016) [89] |
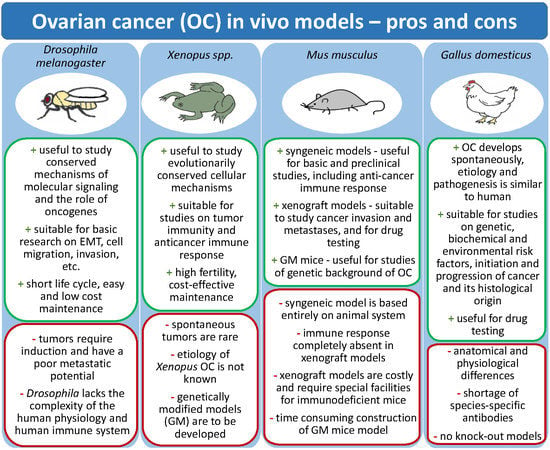
| Ovarian Cancer Models | Advantages | Limitations |
|---|---|---|
| Fruit fly(Drosophila melanogaster) |
|
|
| Clawed frog(Xenopus laevis, Xenopus tropicallis) |
|
|
| Mouse (Mus musculus) | ||
| Xenograft mouse models |
|
|
| PDX (patient-derived xenografts) mouse models |
|
|
| Syngeneic mouse models |
|
|
| Genetically-engineered mouse models |
|
|
| Hen (Gallus domesticus) | ||
| Laying hen |
|
|
| Chicken chorioallantoic membrane (CAM) |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudrej, P.; Kujawa, K.A.; Cortez, A.J.; Lisowska, K.M. Characteristics of in Vivo Model Systems for Ovarian Cancer Studies. Diagnostics 2019, 9, 120. https://doi.org/10.3390/diagnostics9030120
Tudrej P, Kujawa KA, Cortez AJ, Lisowska KM. Characteristics of in Vivo Model Systems for Ovarian Cancer Studies. Diagnostics. 2019; 9(3):120. https://doi.org/10.3390/diagnostics9030120
Chicago/Turabian StyleTudrej, Patrycja, Katarzyna Aleksandra Kujawa, Alexander Jorge Cortez, and Katarzyna Marta Lisowska. 2019. "Characteristics of in Vivo Model Systems for Ovarian Cancer Studies" Diagnostics 9, no. 3: 120. https://doi.org/10.3390/diagnostics9030120
APA StyleTudrej, P., Kujawa, K. A., Cortez, A. J., & Lisowska, K. M. (2019). Characteristics of in Vivo Model Systems for Ovarian Cancer Studies. Diagnostics, 9(3), 120. https://doi.org/10.3390/diagnostics9030120