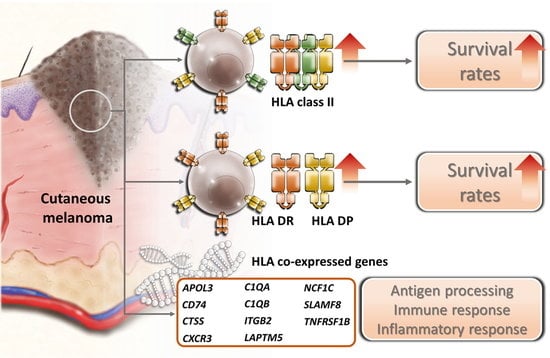
Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Expression Analysis
2.2. Co-Expression Gene Analysis
2.3. Survival Analysis
2.4. OncoLnc Tool
2.5. Gene Ontology Enrichment Analysis
2.6. Statistical Analysis
3. Results
3.1. The Expressions of HLA Class II Genes Were Up-Regulated in Cutaneous Melanoma
3.2. High HLA Class II mRNA Expression Levels Were Associated with a Good Prognosis in Cutaneous Melanoma Patients
3.3. The Expressions of HLA Class II Genes in Cutaneous Melanoma Were Most Closely Correlated with Patient Survival among 21 Common Cancer Types in TCGA Database
3.4. Top 11 Genes Co-Expressed with HLA-DPA1 and HLA-DRA in Clinical Cutaneous Melanoma Samples
3.5. Gene Ontology Enrichment Analysis of HLA-DPA1 and -DRA Co-Expressed Genes Identified the Association of These Genes with Antigen Processing, Immune and Inflammatory Response in Skin Cutaneous Melanoma
3.6. Genes Co-Expressed with HLA-DPA1 or HLA-DRA Positively Associated with Clinical Outcome in Patients with Cutaneous Melanoma
4. Discussion
5. Conclusions
Public Databases
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fecher, L.A.; Cummings, S.D.; Keefe, M.J.; Alani, R.M. Toward a molecular classification of melanoma. J. Clin. Oncol. 2007, 25, 1606–1620. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Kloor, M.; Ferrone, S. HLA class II antigen-processing pathway in tumors: Molecular defects and clinical relevance. Oncoimmunology 2017, 6, e1171447. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.S.; Livingston, P.O. HLA and DR antigen frequencies in melanoma patients: Possible relation to disease prognosis. Tissue Antigens 1985, 26, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, M.; Fonsatti, E.; Visintin, A.; Maio, M. Targeted therapy of solid malignancies via HLA class II antigens: A new biotherapeutic approach? Oncogene 2003, 22, 6564–6569. [Google Scholar] [CrossRef]
- Novellino, L.; Castelli, C.; Parmiani, G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol. Immunother. CII 2005, 54, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T.; Kierstead, L.S.; Ranieri, E.; Gesualdo, L.; Schena, F.P.; Finke, J.H.; Bukowski, R.M.; Brusic, V.; Sidney, J.; Sette, A.; et al. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin. Cancer Res. 2003, 9, 947–954. [Google Scholar] [PubMed]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Paco, L.; Romero, I.; Berruguilla, E.; Stefansky, J.; Collado, A.; Algarra, I.; Garrido, F.; Garcia-Lora, A.M. MHC class I molecules act as tumor suppressor genes regulating the cell cycle gene expression, invasion and intrinsic tumorigenicity of melanoma cells. Carcinogenesis 2012, 33, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, Y.; Huang, J.; Greshock, J.; Horiates, G.; Nathanson, K.; Yang, X.; Herlyn, M.; Weber, B. Distinct MHC gene expression patterns during progression of melanoma. Genes Chromosom. Cancer 2010, 49, 144–154. [Google Scholar] [PubMed]
- Taramelli, D.; Fossati, G.; Mazzocchi, A.; Delia, D.; Ferrone, S.; Parmiani, G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res. 1986, 46, 433–439. [Google Scholar] [PubMed]
- Bernsen, M.R.; Hakansson, L.; Gustafsson, B.; Krysander, L.; Rettrup, B.; Ruiter, D.; Hakansson, A. On the biological relevance of MHC class II and B7 expression by tumour cells in melanoma metastases. Br. J. Cancer 2003, 88, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyn, J.; Jaber-Hijazi, F.; MacKenzie, D.; Cole, J.J.; Mann, E.; Pawlikowski, J.S.; Rai, T.S.; Nelson, D.M.; McBryan, T.; Ivanov, A.; et al. Oncogene-expressing senescent melanocytes up-regulate MHC class II, a candidate melanoma suppressor function. J. Invest. Derm. 2017, 137, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Bordeaux, J.; Kim, J.Y.; Vaupel, C.; Rimm, D.L.; Ho, T.H.; Joseph, R.W.; Daud, A.I.; Conry, R.M.; Gaughan, E.M.; et al. Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of Anti-PD-1 therapies in metastatic melanoma. Clin. Cancer Res. 2018, 24, 5250–5260. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, I.; Sylla, K.; Deshayes, F.; Lauriol, J.; Ghislin, S.; Dieu-Nosjean, M.C.; Viguier, M.; Verola, O.; Charron, D.; Alcaide-Loridan, C.; et al. Coexpression of major histocompatibility complex class II with chemokines and nuclear NFkappaB p50 in melanoma: A rational for their association with poor prognosis. Melanoma Res. 2009, 19, 226–237. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Genomic classification of cutaneous melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Human Genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Shaul, Y.D.; Yuan, B.; Thiru, P.; Nutter-Upham, A.; McCallum, S.; Lanzkron, C.; Bell, G.W.; Sabatini, D.M. MERAV: A tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016, 44, D560–D566. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef]
- Bogunovic, D.; O’Neill, D.W.; Belitskaya-Levy, I.; Vacic, V.; Yu, Y.L.; Adams, S.; Darvishian, F.; Berman, R.; Shapiro, R.; Pavlick, A.C.; et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc. Natl. Acad. Sci. USA 2009, 106, 20429–20434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. Peer J. Comp. Sci. 2016, 2, e67. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Zhou, G.; Xia, J. OmicsNet: A web-based tool for creation and visual analysis of biological networks in 3D space. Nucleic Acids Res. 2018, 46, W514–W522. [Google Scholar] [CrossRef] [PubMed]
- Rimsza, L.M.; Roberts, R.A.; Miller, T.P.; Unger, J.M.; LeBlanc, M.; Braziel, R.M.; Weisenberger, D.D.; Chan, W.C.; Muller-Hermelink, H.K.; Jaffe, E.S.; et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: A follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 2004, 103, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Takenouchi, T.; Shimada, H.; Tomonaga, T.; Hayashi, H.; Shioya, A.; Komatsu, A.; Matsubara, H.; Ochiai, T. Strong HLA-DR antigen expression on cancer cells relates to better prognosis of colorectal cancer patients: Possible involvement of c-myc suppression by interferon-gamma in situ. Cancer Sci. 2006, 97, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, M.; Tanaka, S.; Haruma, K.; Yoshihara, M.; Sumii, K.; Kajiyama, G.; Shimamoto, F. Combined expression of HLA-DR antigen and proliferating cell nuclear antigen correlate with colorectal cancer prognosis. Oncology 1998, 55, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lazaris, A.C.; Theodoropoulos, G.E.; Davaris, P.S.; Panoussopoulos, D.; Nakopoulou, L.; Kittas, C.; Golematis, B.C. Heat shock protein 70 and HLA-DR molecules tissue expression. Prognostic implications in colorectal cancer. Dis. Colon Rectum 1995, 38, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Oldford, S.A.; Robb, J.D.; Codner, D.; Gadag, V.; Watson, P.H.; Drover, S. Tumor cell expression of HLA-DM associates with a Th1 profile and predicts improved survival in breast carcinoma patients. Int. Immunol. 2006, 18, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.A.; Hwang, S.H.; Song, I.H.; Heo, S.H.; Kim, Y.A.; Bang, W.S.; Park, H.S.; Lee, M.; Gong, G.; Lee, H.J. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. PLoS ONE 2017, 12, e0182786. [Google Scholar] [CrossRef]
- Callahan, M.J.; Nagymanyoki, Z.; Bonome, T.; Johnson, M.E.; Litkouhi, B.; Sullivan, E.H.; Hirsch, M.S.; Matulonis, U.A.; Liu, J.; Birrer, M.J.; et al. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin. Cancer Res. 2008, 14, 7667–7673. [Google Scholar] [CrossRef]
- Esteban, F.; Ruiz-Cabello, F.; Concha, A.; Perez-Ayala, M.; Sanchez-Rozas, J.A.; Garrido, F. HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin. Exp. Metastasis 1990, 8, 319–328. [Google Scholar] [CrossRef]
- Anichini, A.; Mortarini, R.; Nonaka, D.; Molla, A.; Vegetti, C.; Montaldi, E.; Wang, X.; Ferrone, S. Association of antigen-processing machinery and HLA antigen phenotype of melanoma cells with survival in American Joint Committee on Cancer stage III and IV melanoma patients. Cancer Res. 2006, 66, 6405–6411. [Google Scholar] [CrossRef]
- Oble, D.A.; Loewe, R.; Yu, P.; Mihm, M.C., Jr. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009, 9, 3. [Google Scholar] [PubMed]
- Zikich, D.; Schachter, J.; Besser, M.J. Predictors of tumor-infiltrating lymphocyte efficacy in melanoma. Immunotherapy 2016, 8, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, S.G.; Ruiter, D.J.; Broecker, E.B.; van der Velde, E.A.; Sorg, C.; Welvaart, K.; Ferrone, S. Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res. 1988, 48, 1019–1025. [Google Scholar] [PubMed]
- Goodwin, B.L.; Xi, H.; Tejiram, R.; Eason, D.D.; Ghosh, N.; Wright, K.L.; Nagarajan, U.; Boss, J.M.; Blanck, G. Varying functions of specific major histocompatibility class II transactivator promoter III and IV elements in melanoma cell lines. Cell Growth Differ. 2001, 12, 327–335. [Google Scholar] [PubMed]
- Deffrennes, V.; Vedrenne, J.; Stolzenberg, M.C.; Piskurich, J.; Barbieri, G.; Ting, J.P.; Charron, D.; Alcaïde-Loridan, C. Constitutive expression of MHC class II genes in melanoma cell lines results from the transcription of class II transactivator abnormally initiated from its B cell-specific promoter. J. Immunol. 2001, 167, 98–106. [Google Scholar] [CrossRef]
- Page, N.M.; Butlin, D.J.; Lomthaisong, K.; Lowry, P.J. The human apolipoprotein L gene cluster: Identification, classification, and sites of distribution. Genomics 2001, 74, 71–78. [Google Scholar] [CrossRef]
- Lv, C.; Sun, L.; Guo, Z.; Li, H.; Kong, D.; Xu, B.; Lin, L.; Liu, T.; Guo, D.; Zhou, J.; et al. Circular RNA regulatory network reveals cell-cell crosstalk in acute myeloid leukemia extramedullary infiltration. J. Transl. Med. 2018, 16, 361. [Google Scholar] [CrossRef]
- Nagel, G.; Bjorge, T.; Stocks, T.; Manjer, J.; Hallmans, G.; Edlinger, M.; Haggstrom, C.; Engeland, A.; Johansen, D.; Kleiner, A.; et al. Metabolic risk factors and skin cancer in the Metabolic Syndrome and Cancer Project (Me-Can). Br. J. Derm. 2012, 167, 59–67. [Google Scholar] [CrossRef]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef]
- Scalera, A.; Tarantino, G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 9217–9228. [Google Scholar] [CrossRef]
- Koch, K.S.; Leffert, H.L. Ectopic expression of CD74 in Ikkbeta-deleted mouse hepatocytes. Acta Histochem. 2011, 113, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, S.; Davies, M.A.; Tanese, K.; Roszik, J.; Shin-Sim, M.; Bassett, R.L., Jr.; Milton, D.R.; Woodman, S.E.; Prieto, V.G.; Gershenwald, J.E.; et al. Inflammatory marker testing identifies CD74 expression in melanoma tumor cells, and its expression associates with favorable survival for stage III melanoma. Clin. Cancer Res. 2016, 22, 3016–3024. [Google Scholar] [CrossRef] [PubMed]
- Tanese, K.; Hashimoto, Y.; Berkova, Z.; Wang, Y.; Samaniego, F.; Lee, J.E.; Ekmekcioglu, S.; Grimm, E.A. Cell surface CD74-MIF interactions drive melanoma survival in response to interferon-γ. J. Invest. Derm. 2015, 135, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, P.S.; Zinke, J.; Kowalewski, D.J.; Bernatz, S.; Tichy, J.; Ronellenfitsch, M.W.; Thorsen, F.; Berger, A.; Forster, M.T.; Muller, A.; et al. CD74 regulates complexity of tumor cell HLA class II peptidome in brain metastasis and is a positive prognostic marker for patient survival. Acta Neuropathol. Commun. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.R.; Azevedo, R.A.; Mousdell, S.; Resende-Lara, P.T.; Ireland, L.; Santos, A.; Girola, N.; Cunha, R.; Schmid, M.C.; Polonelli, L.; et al. Blockade of MIF-CD74 signalling on macrophages and dendritic cells restores the antitumour immune response against metastatic melanoma. Front. Immunol. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Csomor, E.; Bajtay, Z.; Sandor, N.; Kristof, K.; Arlaud, G.J.; Thiel, S.; Erdei, A. Complement protein C1q induces maturation of human dendritic cells. Mol. Immunol. 2007, 44, 3389–3397. [Google Scholar] [CrossRef] [PubMed]
- Mangogna, A.; Agostinis, C.; Bonazza, D.; Belmonte, B.; Zacchi, P.; Zito, G.; Romano, A.; Zanconati, F.; Ricci, G.; Kishore, U.; et al. Is the complement protein C1q a pro- or anti-tumorigenic factor? Bioinformatics analysis involving human carcinomas. Front. Immunol. 2019, 10, 865. [Google Scholar] [CrossRef]
- Hong, Q.; Sze, C.I.; Lin, S.R.; Lee, M.H.; He, R.Y.; Schultz, L.; Chang, J.Y.; Chen, S.J.; Boackle, R.J.; Hsu, L.J.; et al. Complement C1q activates tumor suppressor WWOX to induce apoptosis in prostate cancer cells. PLoS ONE 2009, 4, e5755. [Google Scholar] [CrossRef]
- Bulla, R.; Tripodo, C.; Rami, D.; Ling, G.S.; Agostinis, C.; Guarnotta, C.; Zorzet, S.; Durigutto, P.; Botto, M.; Tedesco, F. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat. Commun. 2016, 7, 10346. [Google Scholar] [CrossRef] [Green Version]
- Mandruzzato, S.; Callegaro, A.; Turcatel, G.; Francescato, S.; Montesco, M.C.; Chiarion-Sileni, V.; Mocellin, S.; Rossi, C.R.; Bicciato, S.; Wang, E.; et al. A gene expression signature associated with survival in metastatic melanoma. J. Transl. Med. 2006, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Pinzi, C.; Berti, E.; Spallanzani, A.; Chiarugi, A.; Boddi, V.; Reali, U.M.; Giannotti, B. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res. 1997, 7, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Orentas, R.J.; Yang, J.J.; Wen, X.; Wei, J.S.; Mackall, C.L.; Khan, J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front. Oncol. 2012, 2, 194. [Google Scholar] [CrossRef] [PubMed]









| Gene | Hazard Ratio | p-Value | Log Rank p-Value | Fold Change |
|---|---|---|---|---|
| HLA-DPA1 | 0.50 | 7.1 × 10−7 | 4.3 × 10−7 | 8.01 |
| HLA-DPB1 | 0.54 | 7.4 × 10−6 | 5.4 × 10−6 | 7.38 |
| HLA-DPB2 | 0.67 | 0.0039 | 0.0037 | 20.28 |
| HLA-DQA1 | 0.60 | 1.7 × 10−4 | 1.4 × 10−4 | 11.37 |
| HLA-DQA2 | 0.74 | 0.027 | 0.027 | 13.26 |
| HLA-DQB1 | 0.59 | 1.3 × 10−4 | 1.1 × 10−4 | 9.81 |
| HLA-DQB2 | 0.56 | 2.0 × 10−5 | 1.5 × 10−5 | 13.51 |
| HLA-DRA | 0.52 | 1.9 × 10−6 | 1.2 × 10−6 | 8.28 |
| HLA-DRB1 | 0.62 | 4.7 × 10−4 | 4.1 × 10−4 | 8.35 |
| HLA-DRB5 | 0.70 | 0.009 | 0.0087 | 10.63 |
| HLA-DRB6 | 0.65 | 0.0014 | 0.0013 | 11.05 |
| HLA-DMA | 0.62 | 3.9 × 10−4 | 3.5 × 10−4 | 6.06 |
| HLA-DMB | 0.53 | 4.3 × 10−6 | 3.0 × 10−6 | 6.73 |
| HLA-DOA | 0.54 | 9.2 × 10−6 | 6.8 × 10−6 | 11.39 |
| HLA-DOB | 0.52 | 2.2 × 10−6 | 1.4 × 10−6 | 17.20 |
| Gene | Cox Coefficient | p-Value | FDR Corrected | Rank | Median Expression | Mean Expression |
|---|---|---|---|---|---|---|
| HLA-DPA1 | −0.337 | 5.40 × 10−7 | 1.08 × 10−4 | 80 | 4585.31 | 9121.78 |
| HLA-DPB1 | −0.358 | 2.30 × 10−7 | 8.21 × 10−5 | 45 | 3117.18 | 5914.74 |
| HLA-DPB2 | −0.393 | 5.20 × 10−8 | 3.34 × 10−5 | 25 | 4.14 | 13.99 |
| HLA-DQA1 | −0.302 | 4.70 × 10−6 | 3.51 × 10−4 | 214 | 1170.1 | 2855.2 |
| HLA-DQA2 | −0.29 | 1.60 × 10−5 | 7.65 × 10−4 | 335 | 283.68 | 690.69 |
| HLA-DQB1 | −0.28 | 1.10 × 10−5 | 6.16 × 10−4 | 285 | 1368.12 | 3053.13 |
| HLA-DQB2 | −0.296 | 2.20 × 10−5 | 9.45 × 10−4 | 370 | 84.35 | 196.98 |
| HLA-DRA | −0.344 | 2.50 × 10−7 | 8.73 × 10−5 | 46 | 9839.46 | 18,668.64 |
| HLA-DRB1 | −0.373 | 6.60 × 10−8 | 3.52 × 10−5 | 30 | 3544.56 | 7634.49 |
| HLA-DRB5 | −0.296 | 6.90 × 10−6 | 4.53 × 10−4 | 244 | 1079.7 | 2645.34 |
| HLA-DRB6 | −0.304 | 8.50 × 10−6 | 5.19 × 10−4 | 262 | 263.9 | 636.43 |
| HLA-DMA | −0.335 | 1.90 × 10−6 | 2.01 × 10−4 | 152 | 1236.6 | 2101.88 |
| HLA-DMB | −0.341 | 4.60 × 10−7 | 1.04 × 10−4 | 70 | 771.47 | 1417.4 |
| HLA-DOA | −0.348 | 3.20 × 10−7 | 9.52 × 10−5 | 53 | 440.2 | 1098.24 |
| HLA-DOB | −0.322 | 2.60 × 10−6 | 2.43 × 10−4 | 172 | 31.68 | 107.89 |
| Gene | Cancer | Cox Coefficient | p-Value | FDR Corrected | Rank | Median Expression | Mean Expression |
|---|---|---|---|---|---|---|---|
| HLA-DPA1 | SKCM | −0.337 | 5.40 × 10−7 | 1.08 × 10−4 | 80 | 4585.31 | 9121.78 |
| LUAD | −0.235 | 1.50 × 10−3 | 0.031 | 797 | 9849.34 | 12,162.44 | |
| SARC | −0.289 | 8.70 × 10−3 | 0.096 | 1466 | 4027.86 | 7692.16 | |
| BRCA | −0.183 | 0.038 | 0.342 | 1834 | 5003.01 | 6437.27 | |
| LGG | 0.429 | 2.30 × 10−5 | 1.53 × 10−4 | 2513 | 1388.13 | 2947.54 | |
| LUSC | 0.106 | 0.120 | 0.636 | 3073 | 4131.19 | 5702.61 | |
| CESC | −0.231 | 0.072 | 0.381 | 3079 | 3826.45 | 6061.82 | |
| LAML | 0.141 | 0.200 | 0.595 | 5017 | 4888.65 | 6455.32 | |
| KIRC | −0.193 | 0.011 | 0.034 | 5394 | 13,119.52 | 15,657.27 | |
| LIHC | −0.138 | 0.140 | 0.379 | 5721 | 2345.75 | 3679.56 | |
| HNSC | −0.083 | 0.240 | 0.586 | 6709 | 3054.79 | 5091.7 | |
| STAD | −0.094 | 0.260 | 0.641 | 6756 | 5108.6 | 7427.25 | |
| OV | −0.068 | 0.380 | 0.851 | 7462 | 5886.04 | 8121.03 | |
| UCEC | −0.069 | 0.510 | 0.995 | 8405 | 2478.44 | 3839.0 | |
| BLCA | −0.072 | 0.320 | 0.585 | 8873 | 2159.67 | 4356.95 | |
| GBM | 0.055 | 0.560 | 0.931 | 10,033 | 5543.04 | 6987.86 | |
| KIRP | −0.135 | 0.370 | 0.558 | 10,855 | 4783.41 | 6319.32 | |
| READ | 0.015 | 0.940 | 0.993 | 15,531 | 1829.92 | 2538.31 | |
| COAD | 0.0 | 1.00 | 1.00 | 16,386 | 2198.83 | 3337.41 | |
| ESCA | −0.004 | 0.980 | 0.995 | 16,471 | 1974.74 | 3355.17 | |
| PAAD | −0.003 | 0.980 | 0.988 | 17,035 | 5814.37 | 6925.07 |
| Gene | Cancer | Cox Coefficient | p-Value | FDR Corrected | Rank | Median Expression | Mean Expression |
|---|---|---|---|---|---|---|---|
| HLA-DRA | SKCM | −0.344 | 2.50 × 10−7 | 8.73 × 10−5 | 46 | 9839.46 | 18,668.64 |
| LUAD | −0.24 | 1.20 × 10−7 | 0.028 | 706 | 27,710.37 | 34,117.21 | |
| SARC | −0.305 | 5.80 × 10−3 | 0.078 | 1200 | 8466.06 | 16,968.25 | |
| BRCA | −0.191 | 0.0292 | 0.313 | 1525 | 11,992.61 | 15,710.87 | |
| UCEC | −0.168 | 0.120 | 0.958 | 1988 | 8596.84 | 11,817.06 | |
| LGG | 0.449 | 8.00 × 10−6 | 6.45 × 10−5 | 2084 | 2897.56 | 6102.37 | |
| CESC | −0.242 | 0.064 | 0.362 | 2878 | 12,787.47 | 18,592.32 | |
| KIRC | −0.218 | 3.80 × 10−3 | 0.015 | 4304 | 29,068.65 | 32,924.27 | |
| LIHC | −0.156 | 0.100 | 0.316 | 4941 | 5590.88 | 9443.03 | |
| HNSC | −0.095 | 0.180 | 0.520 | 5661 | 9416.73 | 14,015.01 | |
| STAD | −0.107 | 0.210 | 0.590 | 5926 | 12,673.01 | 18,011.42 | |
| LAML | 0.116 | 0.270 | 0.659 | 6152 | 12,957.72 | 18,731.49 | |
| LUSC | 0.076 | 0.290 | 0.770 | 6291 | 11,713.15 | 18,081.96 | |
| ESCA | −0.124 | 0.380 | 0.977 | 6402 | 5720.11 | 9112.51 | |
| BLCA | −0.097 | 0.180 | 0.430 | 6735 | 5928.98 | 11,505.42 | |
| OV | −0.058 | 0.440 | 0.873 | 8442 | 15,570.23 | 20,289.93 | |
| COAD | −0.082 | 0.410 | 0.767 | 8685 | 6279.5 | 9455.2 | |
| KIRP | −0.157 | 0.300 | 0.487 | 10,064 | 10,478.68 | 14,076.0 | |
| GBM | 0.036 | 0.690 | 0.958 | 12,029 | 14,344.39 | 19,440.02 | |
| READ | −0.021 | 0.920 | 0.992 | 15,189 | 5247.53 | 7508.25 | |
| PAAD | 0.026 | 0.800 | 0.895 | 15,331 | 16,654.63 | 18,258.57 |
| Gene | Gene Name | |
|---|---|---|
| Co-expressed with both HLA-DPA1 and HLA-DRA | APOL3 | apolipoprotein L3 |
| CD74 | CD74 molecule | |
| CXCR3 | C-X-C motif chemokine receptor 3 | |
| HLA-DMA | major histocompatibility complex, class II, DM alpha | |
| HLA-DPB1 | major histocompatibility complex, class II, DP beta 1 | |
| HLA-DQA1 | major histocompatibility complex, class II, DQ alpha 1 | |
| HLA-DQB1 | major histocompatibility complex, class II, DQ beta 1 | |
| ITGB2 | integrin subunit beta 2 | |
| LAPTM5 | lysosomal protein transmembrane 5 | |
| TNFRSF1B | TNF receptor superfamily member 1B | |
| Co-expressed with HLA-DPA1 | C1QA | complement C1q A chain |
| C1QB | complement C1q B chain | |
| HLA-DRA | major histocompatibility complex, class II, DR alpha | |
| NCF1C | neutrophil cytosolic factor 1C pseudogene | |
| Co-expressed with HLA-DRA | CTSS | cathepsin S |
| HLA-DPA1 | major histocompatibility complex, class II, DP alpha 1 | |
| SLAMF8 | SLAM family member 8 |
| GO Term and Function | Count | Gene | p-Value | Fold Enrichment | FDR |
|---|---|---|---|---|---|
| GOTERM_BP | |||||
| GO:0019886, antigen processing and presentation of exogenous peptide antigen via MHC class II | 8 | HLA-DQB1, HLA-DPA1, CTSS, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1, HLA-DRA | 7.29 × 10−13 | 91.26 | 8.58 × 10−10 |
| GO:0002504, antigen processing and presentation of peptide or polysaccharide antigen via MHC class II | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DMA, HLA-DQA1, HLA-DRA | 1.66 × 10−12 | 370.41 | 1.96 × 10−9 |
| GO:0019882, antigen processing and presentation | 7 | HLA-DQB1, HLA-DPA1, CTSS, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 4.56 × 10−12 | 133.5727 | 5.37 × 10−9 |
| GO:0006955, immune response | 9 | HLA-DQB1, TNFRSF1B, HLA-DPA1, CTSS, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1, HLA-DRA | 8.06 × 10−10 | 22.44 | 9.49 × 10−7 |
| GO:0060333, interferon-gamma-mediated signaling pathway | 5 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA | 3.87 × 10−7 | 73.91 | 4.55 × 10−4 |
| GO:0031295, T cell costimulation | 5 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA | 5.66 × 10−7 | 67.28 | 6.66 × 10−4 |
| GO:0050852, T cell receptor signaling pathway | 5 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA | 7.33 × 10−6 | 35.46 | 0.009 |
| GO:0006954, inflammatory response | 4 | APOL3, TNFRSF1B, ITGB2, CXCR3 | 0.004 | 11.08 | 4.88 |
| GO:0002503, peptide antigen assembly with MHC class II protein complex | 2 | HLA-DMA, HLA-DRA | 0.004 | 419.80 | 5.12 |
| GO:0050870, positive regulation of T cell activation | 2 | HLA-DPA1, HLA-DPB1 | 0.016 | 116.61 | 17.26 |
| GOTERM_CC | |||||
| GO:0042613, MHC class II protein complex | 7 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1, HLA-DRA | 1.17 × 10−14 | 341.09 | 1.10 × 10−11 |
| GO:0071556, integral component of lumenal side of endoplasmic reticulum membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 3.06 × 10−11 | 221.79 | 2.88 × 10−8 |
| GO:0030658, transport vesicle membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 1.29 × 10−10 | 169.26 | 1.21 × 10−7 |
| GO:0030669, clathrin-coated endocytic vesicle membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 1.92 × 10−10 | 156.88 | 1.81 × 10−7 |
| GO:0012507, ER to Golgi transport vesicle membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 6.62 × 10−10 | 123.69 | 6.23 × 10−7 |
| GO:0005765, lysosomal membrane | 8 | HLA-DQB1, LAPTM5, HLA-DPA1, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1, HLA-DRA | 1.64 × 10−9 | 31.299 | 1.54 × 10−6 |
| GO:0030666, endocytic vesicle membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 2.26 × 10−9 | 97.45 | 2.13 × 10−6 |
| GO:0032588, trans-Golgi network membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 7.28 × 10−9 | 77.49 | 6.85 × 10−6 |
| GO:0009986, cell surface | 6 | HLA-DPA1, ITGB2, HLA-DPB1, HLA-DMA, CD74, HLA-DRA | 7.60 × 10−5 | 11.86716 | 0.071 |
| GO:0000139, Golgi membrane | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 1.14 × 10−4 | 10.88 | 0.108 |
| GO:0010008, endosome membrane | 4 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DQA1 | 5.23 × 10−4 | 23.178 | 0.491 |
| GO:0005602, complement component C1 complex | 2 | C1QA, C1QB | 0.002 | 1072 | 1.64 |
| GO:0005887, integral component of plasma membrane | 6 | TNFRSF1B, LAPTM5, HLA-DPA1, CXCR3, HLA-DQA1, HLA-DRA | 0.006 | 4.55 | 5.44 |
| GO:0016020, membrane | 7 | HLA-DQB1, APOL3, ITGB2, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1 | 0.008 | 3.41 | 7.62 |
| GO:0005886, plasma membrane | 9 | HLA-DQB1, TNFRSF1B, HLA-DPA1, ITGB2, CXCR3, HLA-DPB1, CD74, HLA-DQA1, HLA-DRA | 0.0149 | 2.34 | 13.19 |
| GO:0005764, lysosome | 3 | LAPTM5, CTSS, HLA-DRA | 0.0164 | 14.23 | 14.39 |
| GO:0016021, integral component of membrane | 10 | HLA-DQB1, TNFRSF1B, HLA-DPA1, SLAMF8, CXCR3, HLA-DPB1, HLA-DMA, CD74, HLA-DQA1, HLA-DRA | 0.0176 | 2.08 | 15.35 |
| GO:0005576, extracellular region | 5 | C1QA, APOL3, C1QB, TNFRSF1B, CTSS | 0.047 | 3.33 | 36.23 |
| GOTERM_MF | |||||
| GO:0032395, MHC class II receptor activity | 6 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DMA, HLA-DQA1, HLA-DRA | 7.86 × 10−13 | 422.03 | 7.31 × 10−10 |
| GO:0042605, peptide antigen binding | 5 | HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA | 8.16 × 10−9 | 188.40 | 7.59 × 10−6 |
| GO:0023026, MHC class II protein complex binding | 3 | HLA-DMA, CD74, HLA-DRA | 8.78 × 10−5 | 197.82 | 0.082 |
| GO:0004252, serine-type endopeptidase activity | 3 | C1QA, C1QB, CTSS | 0.021 | 12.41 | 17.90 |
| Gene | Cox Coefficient | p-Value | FDR Corrected | Rank | Median Expression | Mean Expression |
|---|---|---|---|---|---|---|
| APOL3 | −0.346 | 3.60 × 10−7 | 9.97 × 10−5 | 58 | 380.28 | 755.47 |
| C1QA | −0.346 | 5.30 × 10−7 | 1.08 × 10−4 | 79 | 2654.8 | 5159.04 |
| CD74 | −0.339 | 8.70 × 10−7 | 1.36 × 10−4 | 103 | 31,899.34 | 63,138.81 |
| C1QB | −0.337 | 1.10 × 10−6 | 1.45 × 10−4 | 114 | 2932.11 | 5671.22 |
| ITGB2 | −0.283 | 3.50 × 10−5 | 1.34 × 10−3 | 418 | 1577.14 | 2848.66 |
| NCF1C | −0.28 | 4.80 × 10−5 | 1.65 × 10−3 | 468 | 40.03 | 97.48 |
| CXCR3 | −0.262 | 5.00 × 10−5 | 1.69 × 10−3 | 474 | 69.42 | 194.29 |
| LAPTM5 | −0.271 | 5.10 × 10−5 | 1.71 × 10−3 | 478 | 2998.91 | 4868.46 |
| CTSS | −0.267 | 7.20 × 10−5 | 2.14 × 10−3 | 537 | 1627.91 | 2518.04 |
| TNFRSF1B | −0.263 | 1.50 × 10−4 | 3.64 × 10−3 | 661 | 805.72 | 1283.71 |
| SLAMF8 | −0.242 | 2.60 × 10−4 | 5.42 × 10−3 | 769 | 377.6 | 711.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chang, W.-A.; Lin, E.-S.; Chen, Y.-J.; Kuo, P.-L. Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets. Diagnostics 2019, 9, 59. https://doi.org/10.3390/diagnostics9020059
Chen Y-Y, Chang W-A, Lin E-S, Chen Y-J, Kuo P-L. Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets. Diagnostics. 2019; 9(2):59. https://doi.org/10.3390/diagnostics9020059
Chicago/Turabian StyleChen, Yang-Yi, Wei-An Chang, En-Shyh Lin, Yi-Jen Chen, and Po-Lin Kuo. 2019. "Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets" Diagnostics 9, no. 2: 59. https://doi.org/10.3390/diagnostics9020059
APA StyleChen, Y.-Y., Chang, W.-A., Lin, E.-S., Chen, Y.-J., & Kuo, P.-L. (2019). Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets. Diagnostics, 9(2), 59. https://doi.org/10.3390/diagnostics9020059