The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy
Abstract
:1. Introduction
2. Immune-Surveillance and Immune-Support of CTCs
2.1. Natural Killer (NK) Cells
2.2. CD8+ and CD4+ T Cells
2.3. Regulatory T Lymphocytes (Tregs)
2.4. Other Lymphocytic Subsets
2.5. Neutrophils
2.6. Monocytes
2.7. Myeloid-Derived Suppressor Cells (MDSCs)
2.8. Macrophages
2.9. Dendritic Cells (DCs)
2.10. Other Circulating Immune Cells Interacting with CTCs
3. CTC Evasion from Immune Surveillance
- The downregulation or loss of surface MHCI expression to escape the action of CTLs (an event that, in fact, makes them susceptible to the action of NK cells) [144];
- the acquisition of a ‘pseudonormal’ phenotype by the transfer of MHCI molecules from the surface of platelets to escape NK cell-mediated cytotoxicity [145];
- the expression of cytokeratins (CK8, CK18, CK19) that interfere with the recognition of MHCI complexes by T cell receptors (TCRs) on CTLs [146].
- the association of CTCs inside CTMs, where they are hidden and protected from immune attacks [155];
3.1. CTC Surface Markers Involved in Immune Escape and Metastatic Dissemination
3.1.1. CD44
3.1.2. CD47
3.1.3. PD-L1
3.1.4. EpCAM
4. CTCs as Biomarkers in Cancer Immunotherapy
4.1. Blocking of Oncogenic Pathways
4.1.1. EGFR
4.1.2. HER2
4.2. Osteoclast Regulation
4.3. Immune Checkpoint Inhibitors
4.3.1. CTLA-4
4.3.2. The PD-1/PD-L1 Axis
4.4. Adoptive Cell Transfer (ACT)
4.5. Cancer Vaccines
4.6. Immunotherapies Targeting CTCs
5. Future Opportunities
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | adoptive cell transfer |
| ADCC | antibody-dependent cellular cytotoxicity |
| APC | antigen-presenting cell |
| BC | breast cancer |
| CAML | cancer-associated macrophage-like cell |
| CAR | chimeric antigen receptor |
| CDC | complement-dependent cytotoxicity |
| CMC | circulating melanoma cell |
| CRC | colorectal cancer |
| CS | CellSearch |
| CSC | cancer stem cell |
| CTC | circulating tumor cell |
| CTL | cytotoxic T lymphocyte |
| CTM | circulating tumor microemboli |
| DC | dendritic cell |
| DCreg | regulatory dendritic cell |
| DFS | disease-free survival |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-to-mesenchymal transition |
| EpCAM | epithelial cell adhesion molecule |
| FAS | first apoptosis signal receptor |
| FDA | Food and Drug Administration |
| G-CSF | granulocyte-colony stimulating factor |
| HER2 | human epidermal growth factor receptor 2 |
| ILC2 | type 2 innate lymphoid cell |
| mAb | monoclonal antibody |
| MAM | metastasis-associated macrophage |
| MHC | major histocompatibility complex |
| MDSC | myeloid-derived suppressor cell |
| MET | mesenchymal-to-epithelial transition |
| MTF | macrophage–tumor cell fusion |
| NET | neutrophil extracellular trap |
| NK (cell) | natural killer (cell) |
| NSCLC | non-small cell lung cancer |
| OS | overall survival |
| PBMC | peripheral blood mononuclear cell |
| PD-L1 | programmed death-ligand 1 |
| PFS | progression-free survival |
| PMo | patrolling monocyte |
| RANKL | nuclear factor kappa-B ligand |
| TAM | tumor-associated macrophage |
| tBreg | tumor-evoked regulatory B cell |
| TCR | T cell receptor |
| TDSF | tumor-derived soluble factor |
| Th (cell) | T helper (cell) |
| TIL | tumor-infiltrating lymphocyte |
| TLR | Toll-like receptor |
| Treg | regulatory T lymphocyte |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Lange, P.H.; Porter, M.P.; Lin, D.W.; Ellis, W.J.; Gallaher, I.S.; Vessella, R.L. Disseminated Tumor Cells in Prostate Cancer Patients after Radical Prostatectomy and without Evidence of Disease Predicts Biochemical Recurrence. Clin. Cancer Res. 2009, 15, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G.; et al. Systemic Spread Is an Early Step in Breast Cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Amadori, A.; Rossi, E.; Zamarchi, R.; Carli, P.; Pastorelli, D.; Jirillo, A. Circulating and Disseminated Tumor Cells in the Clinical Management of Breast Cancer Patients: Unanswered Questions. Oncology 2009, 76, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Metastasis: Quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl. Cancer Inst. 1970, 45, 773–782. [Google Scholar] [PubMed]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, T.; Qian, B.-Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratsuka, S.; Watanabe, A.; Aburatani, H.; Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006, 8, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Takahashi, H.; Lin, W.-W.; Descargues, P.; Grivennikov, S.; Kim, Y.; Luo, J.-L.; Karin, M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabioglu, N.; Sahin, A.A.; Morandi, P.; Meric-Bernstam, F.; Islam, R.; Lin, H.Y.; Bucana, C.D.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Cristofanilli, M. Chemokine receptors in advanced breast cancer: Differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann. Oncol. 2009, 20, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.; Vančurová, I.; Becht, E.; Palata, O.; Strnad, P.; Tesařová, P.; Čabiňaková, M.; Švec, D.; Kubista, M.; Bartůňková, J.; et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. OncoImmunology 2015, 5, e1102827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.F.; Mannam, V.K.R.; Craft, B.S.; Puneky, L.V.; Sheehan, N.T.; Lewis, R.E.; Cruse, J.M. Comparative analysis of innate immune system function in metastatic breast, colorectal, and prostate cancer patients with circulating tumor cells. Exp. Mol. Pathol. 2014, 96, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N. Role of natural killer cells in control of cancer metastasis. Cancer Metastasis Rev. 1982, 1, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, T.; Nehmann, N.; Bethge, A.; Wedemann, G.; Schumacher, U. Perforin-dependent direct cytotoxicity in natural killer cells induces considerable knockdown of spontaneous lung metastases and computer modelling-proven tumor cell dormancy in a HT29 human colon cancer xenograft mouse model. Mol. Cancer 2014, 13, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.L.; Cruse, J.M.; Lewis, R.E. Circulating tumor cells (CTCs) from metastatic breast cancer patients linked to decreased immune function and response to treatment. Exp. Mol. Pathol. 2013, 95, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2009, 29, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Mego, M.; Scarpi, E.; Giuliano, M.; Giordano, A.; Reuben, J.M.; Valero, V.; Ueno, N.T.; Hortobagyi, G.N.; Cristofanilli, M. Relationship Between Lymphocytopenia and Circulating Tumor Cells as Prognostic Factors for Overall Survival in Metastatic Breast Cancer. Clin. Breast Cancer 2012, 12, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Gao, H.; Cohen, E.N.; Anfossi, S.; Giordano, A.; Sanda, T.; Fouad, T.M.; De Giorgi, U.; Giuliano, M.; Woodward, W.A.; et al. Circulating Tumor Cells (CTC) Are Associated with Defects in Adaptive Immunity in Patients with Inflammatory Breast Cancer. J. Cancer 2016, 7, 1095–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Zhang, F.; Li, H.; Yang, L.; Lv, T.; Gu, W.; Song, Y. Circulating Tumor Cells Were Associated with the Number of T Lymphocyte Subsets and NK Cells in Peripheral Blood in Advanced Non-Small-Cell Lung Cancer. Dis. Mark. 2017, 2017, 5727815. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-W.; Xu, Z.-H.; Lian, P.; Gao, B.-L.; Hu, J.-A. Characteristics of circulating tumor cells in organ metastases, prognosis, and T lymphocyte mediated immune response. OncoTargets Ther. 2017, 10, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Gruber, I.; Landenberger, N.; Staebler, A.; Hahn, M.; Wallwiener, D.; Fehm, T. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res. 2013, 33, 2233–2238. [Google Scholar] [PubMed]
- De Lafaille, M.A.C.; Lafaille, J.J. Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity 2009, 30, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Mendez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting Galectin-1 Overcomes Breast Cancer-Associated Immunosuppression and Prevents Metastatic Disease. Cancer Res. 2012, 73, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Gebhardt, C.; Umansky, L.; Beckhove, P.; Schulze, T.J.; Utikal, J.; Umansky, V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int. J. Cancer 2015, 136, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Sakakibara, M.; Nagashima, T.; Sangai, T.; Arai, M.; Fujimori, T.; Takano, S.; Shida, T.; Nakatani, Y.; Miyazaki, M. Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur. J. Cancer 2009, 45, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.L.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast Cancer Lung Metastasis Requires Expression of Chemokine Receptor CCR4 and Regulatory T Cells. Cancer Res. 2009, 69, 5996–6004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, M.J.; Teng, M.W.L.; Swann, J.; Kyparissoudis, K.; Godfrey, D.I.; Hayakawa, Y. CD4+CD25+ T Regulatory Cells Suppress NK Cell-Mediated Immunotherapy of Cancer. J. Immunol. 2006, 176, 1582–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Li, Q.-J.; Feng, Y.; Zhang, Y.; Markowitz, G.J.; Ning, S.; Deng, Y.; Zhao, J.; Jiang, S.; Yuan, Y.; et al. TGF-β-miR-34a-CCL22 Signaling-Induced Treg Cell Recruitment Promotes Venous Metastases of HBV-Positive Hepatocellular Carcinoma. Cancer Cell 2012, 22, 291–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, M.; Riedel, S.S.; Biehl, M.; Krieger, S.; von Krosigk, V.; Bäuerlein, C.A.; Brede, C.; Jordan Garrote, A.-L.; Kraus, S.; Schäfer, V.; et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis 2013, 34, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanzer, S.; Dandachi, N.; Balic, M.; Resel, M.; Samonigg, H.; Bauernhofer, T. Resistance to Apoptosis and Expansion of Regulatory T Cells in Relation to the Detection of Circulating Tumor Cells in Patients with Metastatic Epithelial Cancer. J. Clin. Immunol. 2007, 28, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, B.; Wu, J.; Zhang, C.; Zhou, Y.; Yang, X.; Zhou, J.; Guo, W.; Fan, J. Association of preoperative EpCAM Circulating Tumor Cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer 2016, 16, 506. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 2011, 470, 548–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, E.S.; Reid, A.L.; Bowyer, S.; Calapre, L.; Siew, K.; Pearce, R.; Cowell, L.; Frank, M.H.; Millward, M.; Ziman, M. Circulating Melanoma Cell Subpopulations: Their Heterogeneity and Differential Responses to Treatment. J. Investig. Dermatol. 2015, 135, 2040–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, K.; Coffelt, S.B.; Hoogstraat, M.; Verstegen, N.J.M.; Vrijland, K.; Ciampricotti, M.; Doornebal, C.W.; Hau, C.-S.; Wellenstein, M.D.; Salvagno, C.; et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. OncoImmunology 2017, 6, e1334744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011, 1, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.Y.; Yang, C.Y.; Liang, S.C.; Liu, R.S.; Yang, S.H.; Lin, J.K.; Chen, Y.M.; Wu, Y.C.; Jiang, J.K.; Lin, C.H. Interleukin-17A Modulates Circulating Tumor Cells in Tumor Draining Vein of Colorectal Cancers and Affects Metastases. Clin. Cancer Res. 2014, 20, 2885–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.E.I.; Wang, H.U.I.; Yang, F.E.N.; Ding, Q.I.; Zhao, J. Interleukin-17 potently increases non-small cell lung cancer growth. Mol. Med. Rep. 2016, 13, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Fei, G.; Guo, Z.; Ren, T.; Liu, Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol. Lett. 2012, 148, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Liao, Q.; Zhao, J.; Hu, Y.; You, L.; Lu, Z.; Jia, C.; Wei, Y.; Zhao, Y. High Expression of Interleukin-22 and Its Receptor Predicts Poor Prognosis in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2013, 21, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.C.; Leal, A.C.; Goncalves-Silva, T.; Mercadante, A.C.; Kestelman, F.; Chaves, S.B.; Azevedo, R.B.; Monteiro, J.P.; Bonomo, A. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 2013, 8, e68171. [Google Scholar] [CrossRef] [PubMed]
- Taranova, A.G.; Maldonado, D.; Vachon, C.M.; Jacobsen, E.A.; Abdala-Valencia, H.; McGarry, M.P.; Ochkur, S.I.; Protheroe, C.A.; Doyle, A.; Grant, C.S.; et al. Allergic Pulmonary Inflammation Promotes the Recruitment of Circulating Tumor Cells to the Lung. Cancer Res. 2008, 68, 8582–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodogai, M.; Moritoh, K.; Lee-Chang, C.; Hollander, C.M.; Sherman-Baust, C.A.; Wersto, R.P.; Araki, Y.; Miyoshi, I.; Yang, L.; Trinchieri, G.; et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015, 75, 3456–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saranchova, I.; Han, J.; Zaman, R.; Arora, H.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Welch, I.; et al. Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis. Sci. Rep. 2018, 8, 2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaei, F.; Wu, X.; Qu, X.; Kowanetz, M.; Yu, L.; Tan, M.; Meng, Y.G.; Ferrara, N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc. Natl. Acad. Sci. USA 2009, 106, 6742–6747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Spicer, J.; Giannais, B.; Fallavollita, L.; Brodt, P.; Ferri, L.E. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int. J. Cancer 2009, 125, 1298–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils Promote Liver Metastasis via Mac-1-Mediated Interactions with Circulating Tumor Cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently Entrapped Circulating Tumor Cells Interact with Neutrophils to Facilitate Lung Metastasis Development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strell, C.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp. Cell Res. 2010, 316, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; Loughran, P.; Gianfrate, G.C.; Ellis, J.T.; Singhi, A.D.; Kang, R.; Tang, D.; Lotze, M.T.; et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015, 22, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Sun, W.; Cui, W.; Li, X.; Yao, J.; Jia, X.; Li, C.; Wu, H.; Hu, Z.; Zou, X. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14075–14086. [Google Scholar] [PubMed]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.N.; Cekic, C.; Sag, D.; Tacke, R.; Thomas, G.D.; Nowyhed, H.; Herrley, E.; Rasquinha, N.; McArdle, S.; Wu, R.; et al. Patrolling monocytes control tumor metastasis to the lung. Science 2015, 350, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Zhang, B.; Wang, B.; Zhang, F.; Fan, K.-X.; Guo, Y.-J. Increased CD14+HLA-DR-/low myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunology, Immunotherapy 2013, 62, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunology, Immunotherapy 2008, 58, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Du, W.; Yan, F.; Wang, Y.; Li, H.; Cao, S.; Yu, W.; Shen, C.; Liu, J.; Ren, X. Myeloid-Derived Suppressor Cells Suppress Antitumor Immune Responses through IDO Expression and Correlate with Lymph Node Metastasis in Patients with Breast Cancer. J. Immunol. 2013, 190, 3783–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnoletti, J.P.; Zhu, X.; Almodovar, A.J.O.; Veldhuis, P.P.; Sause, R.; Griffith, E.; Corpus, G.; Chang, J.C.C.; Fanaian, N.; Litherland, S.A. Portal Venous Blood Circulation Supports Immunosuppressive Environment and Pancreatic Cancer Circulating Tumor Cell Activation. Pancreas 2017, 46, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Lee, O.Y.; Shon, S.Y.; Nam, O.; Ryu, P.M.; Seo, M.W.; Lee, D.S. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013, 15, R79. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004, 6, 409–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Huang, J.; Ren, X.; Gorska, A.E.; Chytil, A.; Aakre, M.; Carbone, D.P.; Matrisian, L.M.; Richmond, A.; Lin, P.C.; et al. Abrogation of TGFβ Signaling in Mammary Carcinomas Recruits Gr-1+CD11b+ Myeloid Cells that Promote Metastasis. Cancer Cell 2008, 13, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.H.; Pickup, M.; Pang, Y.; Gorska, A.E.; Li, Z.; Chytil, A.; Geng, Y.; Gray, J.W.; Moses, H.L.; Yang, L. Gr-1+CD11b+ Myeloid Cells Tip the Balance of Immune Protection to Tumor Promotion in the Premetastatic Lung. Cancer Res. 2010, 70, 6139–6149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, B.; Wang, X.; Keeble, J.; Sim, W.J.; Khoo, K.; Wong, W.C.; Kato, M.; Prevost-Blondel, A.; Thiery, J.P.; Abastado, J.P. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011, 9, e1001162. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayon, L.G.; Izquierdo, M.A.; Sirovich, I.; van Rooijen, N.; Beelen, R.H.; Meijer, S. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology 1996, 23, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deneve, E.; Riethdorf, S.; Ramos, J.; Nocca, D.; Coffy, A.; Daures, J.P.; Maudelonde, T.; Fabre, J.M.; Pantel, K.; Alix-Panabieres, C. Capture of Viable Circulating Tumor Cells in the Liver of Colorectal Cancer Patients. Clin. Chem. 2013, 59, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gül, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bögels, M.; Braster, R.; Vidarsson, G.; ten Hagen, T.L.M.; Kubes, P.; van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Investig. 2014, 124, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Asgharzadeh, S.; Salo, J.; Engell, K.; Wu, H.-w.; Sposto, R.; Ara, T.; Silverman, A.M.; DeClerck, Y.A.; Seeger, R.C.; et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Investig. 2009, 119, 1524–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, J.; Wang, W.; Lin, E.Y.; Wang, Y.; Pixley, F.; Stanley, E.R.; Graf, T.; Pollard, J.W.; Segall, J.; Condeelis, J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004, 64, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.F.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangaletti, S.; Di Carlo, E.; Gariboldi, S.; Miotti, S.; Cappetti, B.; Parenza, M.; Rumio, C.; Brekken, R.A.; Chiodoni, C.; Colombo, M.P. Macrophage-Derived SPARC Bridges Tumor Cell-Extracellular Matrix Interactions toward Metastasis. Cancer Res. 2008, 68, 9050–9059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Chen, J.; Su, F.; Yu, B.; Lin, L.; Liu, Y.; Huang, J.D.; Song, E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 2011, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Jia, Y.; Ma, J.; Wu, S.; Jiang, H.; Cao, Y.; Sun, X.; Yin, X.; Yan, S.; Shang, M.; et al. Tumor-associated macrophage-derived CCL20 enhances the growth and metastasis of pancreatic cancer. Acta Biochim. Biophys. Sin. 2016, 48, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Ören, B.; Urosevic, J.; Mertens, C.; Mora, J.; Guiu, M.; Gomis, R.R.; Weigert, A.; Schmid, T.; Grein, S.; Brüne, B.; et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J. Pathol. 2016, 239, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, M.Y.; Tang, S.J.; Chuang, M.J.; Cha, T.L.; Li, J.Y.; Sun, G.H.; Sun, K.H. TNF-Induces Epithelial-Mesenchymal Transition of Renal Cell Carcinoma Cells via a GSK3-Dependent Mechanism. Mol. Cancer Res. 2012, 10, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.T.; Dai, Z.; Song, K.; Zhang, Z.J.; Zhou, Z.J.; Zhou, S.L.; Zhao, Y.M.; Xiao, Y.S.; Sun, Q.M.; Ding, Z.B.; et al. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int. J. Oncol. 2015, 46, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Liu, S.-Y.; Chou, K.-C.; Yeh, C.-T.; Shiah, S.-G.; Huang, R.-Y.; Cheng, J.-C.; Yen, C.-Y.; Shieh, Y.-S. Tumor-Associated Macrophages Promote Oral Cancer Progression Through Activation of the Axl Signaling Pathway. Ann. Surg. Oncol. 2013, 21, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 induces an epithelial–mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawata, M.; Koinuma, D.; Ogami, T.; Umezawa, K.; Iwata, C.; Watabe, T.; Miyazono, K. TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J. Biochem. 2012, 151, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.-M.; Jing, Y.-Y.; Yu, G.-F.; Kou, X.-R.; Ye, F.; Gao, L.; Li, R.; Zhao, Q.-D.; Yang, Y.; Lu, Z.-H.; et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014, 352, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Roh-Johnson, M.; Bravo-Cordero, J.J.; Patsialou, A.; Sharma, V.P.; Guo, P.; Liu, H.; Hodgson, L.; Condeelis, J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 2013, 33, 4203–4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatelli, J.; Bravo-Cordero, J.J.; Roh-Johnson, M.; Gandhi, S.J.; Wang, Y.; Chen, X.; Eddy, R.J.; Xue, A.; Singer, R.H.; Hodgson, L.; et al. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci. Rep. 2016, 6, 37874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.; Rath, B.; Klameth, L.; Hochmair, M.J. Small cell lung cancer: Recruitment of macrophages by circulating tumor cells. OncoImmunology 2015, 5, e1093277. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Lim, S.Y.; Gordon-Weeks, A.N.; Tapmeier, T.T.; Im, J.H.; Cao, Y.; Beech, J.; Allen, D.; Smart, S.; Muschel, R.J. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 2013, 57, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 2009, 4, e6562. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar] [CrossRef]
- Gil-Bernabe, A.M.; Ferjancic, S.; Tlalka, M.; Zhao, L.; Allen, P.D.; Im, J.H.; Watson, K.; Hill, S.A.; Amirkhosravi, A.; Francis, J.L.; et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012, 119, 3164–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headley, M.B.; Bins, A.; Nip, A.; Roberts, E.W.; Looney, M.R.; Gerard, A.; Krummel, M.F. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 2016, 531, 513–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhang, X.H.F.; Massagué, J. Macrophage Binding to Receptor VCAM-1 Transmits Survival Signals in Breast Cancer Cells that Invade the Lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic Cells in the Cancer Microenvironment. J. Cancer 2013, 4, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran Janco, J.M.; Lamichhane, P.; Karyampudi, L.; Knutson, K.L. Tumor-Infiltrating Dendritic Cells in Cancer Pathogenesis. J. Immunol. 2015, 194, 2985–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagawa, S.; Soeda, J.; Takagi, S.; Miwa, S.; Ichikawa, E.; Noike, T. Prognostic significance of mature dendritic cells and factors associated with their accumulation in metastatic liver tumors from colorectal cancer. Hum. Pathol. 2004, 35, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Lijun, Z.; Xin, Z.; Danhua, S.; Xiaoping, L.; Jianliu, W.; Huilan, W.; Lihui, W. Tumor-Infiltrating Dendritic Cells May Be Used as Clinicopathologic Prognostic Factors in Endometrial Carcinoma. Int. J. Gynecol. Cancer 2012, 22, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.L.; Santos, M.F.; Ejaeidi, A.A.; Craft, B.S.; Lewis, R.E.; Cruse, J.M. Toll-like receptor (TLR) expression of immune system cells from metastatic breast cancer patients with circulating tumor cells. Exp. Mol. Pathol. 2014, 97, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Herber, D.L.; Cao, W.; Nefedova, Y.; Novitskiy, S.V.; Nagaraj, S.; Tyurin, V.A.; Corzo, A.; Cho, H.-I.; Celis, E.; Lennox, B.; et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010, 16, 880–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mego, M.; Gao, H.; Cohen, E.N.; Anfossi, S.; Giordano, A.; Tin, S.; Fouad, T.M.; De Giorgi, U.; Giuliano, M.; Woodward, W.A.; et al. Circulating tumor cells (CTCs) are associated with abnormalities in peripheral blood dendritic cells in patients with inflammatory breast cancer. Oncotarget 2017, 8, 35656–35668. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Ohike, M.; Tsukamoto, N.; Kawakami, Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin. Exp. Metastasis 2012, 30, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, J.A.; Tseng, W.W.; Davidson, M.G.; Tolentino, L.L.; Choi, O.; Bhattacharya, N.; Seeley, E.S.; Winer, D.A.; Reticker-Flynn, N.E.; Engleman, E.G. An Immunosuppressive Dendritic Cell Subset Accumulates at Secondary Sites and Promotes Metastasis in Pancreatic Cancer. Cancer Res. 2017, 77, 4158–4170. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guo, D.; Weng, L.; Wang, S.; Ma, Z.; Yang, Y.; Wang, P.; Wang, J.; Cai, Z. Tumor-derived exosomes educate dendritic cells to promote tumor metastasis via HSP72/HSP105-TLR2/TLR4 pathway. OncoImmunology 2017, 6, e1362527. [Google Scholar] [CrossRef] [PubMed]
- Pryczynicz, A.; Cepowicz, D.; Zaręba, K.; Gryko, M.; Hołody-Zaręba, J.; Kędra, B.; Kemona, A.; Guzińska-Ustymowicz, K. Dysfunctions in the Mature Dendritic Cells Are Associated with the Presence of Metastases of Colorectal Cancer in the Surrounding Lymph Nodes. Gastroenterol. Res. Pract. 2016, 2016, 2405437. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Hensel, J.A.; Chanda, D.; Harris, B.A.; Siegal, G.P.; Maheshwari, A.; Ponnazhagan, S. Depletion of Plasmacytoid Dendritic Cells Inhibits Tumor Growth and Prevents Bone Metastasis of Breast Cancer Cells. J. Immunol. 2012, 189, 4258–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.; Gregorio, J.; Wang, Y.H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.J.; et al. Plasmacytoid Dendritic Cells Promote Immunosuppression in Ovarian Cancer via ICOS Costimulation of Foxp3+ T-Regulatory Cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlini, G.; Urso, C.; Mariotti, G.; Di Gennaro, P.; Palli, D.; Brandani, P.; Salvadori, A.; Pimpinelli, N.; Reali, U.M.; Borgognoni, L. Plasmacytoid dendritic cells represent a major dendritic cell subset in sentinel lymph nodes of melanoma patients and accumulate in metastatic nodes. Clin. Immunol. 2007, 125, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhao, J.; Li, Q.; Wang, Q.; Zhou, Y.; Tong, Z. Gastric cancer patients have elevated plasmacytoid and CD1c+ dendritic cells in the peripheral blood. Oncol. Lett. 2018, 15, 5087–5092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küsters, B.; Kats, G.; Roodink, I.; Verrijp, K.; Wesseling, P.; Ruiter, D.J.; de Waal, R.M.W.; Leenders, W.P.J. Micronodular transformation as a novel mechanism of VEGF-A-induced metastasis. Oncogene 2007, 26, 5808–5815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsig, L.; Wong, R.; Hynes, R.O.; Varki, N.M.; Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl. Acad. Sci. USA 2002, 99, 2193–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda, D.G.; Duyverman, A.M.; Kohno, M.; Snuderl, M.; Steller, E.J.; Fukumura, D.; Jain, R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wong, K.H.K.; Khankhel, A.H.; Zeinali, M.; Reategui, E.; Phillips, M.J.; Luo, X.; Aceto, N.; Fachin, F.; Hoang, A.N.; et al. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 2017, 17, 3498–3503. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.L.; Adams, D.K.; Alpaugh, R.K.; Cristofanilli, M.; Martin, S.S.; Chumsri, S.; Tang, C.M.; Marks, J.R. Circulating Cancer-Associated Macrophage-Like Cells Differentiate Malignant Breast Cancer and Benign Breast Conditions. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Matters, G.L.; Xin, P.; Imamura-Kawasawa, Y.; Du, Z.; Thiboutot, D.M.; Helm, K.F.; Neves, R.I.; Abraham, T. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS ONE 2015, 10, e0134320. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Adams, D.K.; He, J.; Kalhor, N.; Zhang, M.; Xu, T.; Gao, H.; Reuben, J.M.; Qiao, Y.; Komaki, R.; et al. Sequential Tracking of PD-L1 Expression and RAD50 Induction in Circulating Tumor and Stromal Cells of Lung Cancer Patients Undergoing Radiotherapy. Clin. Cancer Res. 2017, 23, 5948–5958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Z.; Wang, C.; Ye, Z.; Rossi, G.; Sun, C.; Li, L.; Zhu, Z.; Yang, H.; Cristofanilli, M. Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer. Breast Cancer Res. Treat. 2017, 165, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2016, 14, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.S.; Ramage, J.M.; Madjd, Z.; Spendlove, I.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int. J. Cancer 2006, 118, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Orgel, M.; Schaller, M.; Jung, G.; Rammensee, H.G.; Kopp, H.G.; Salih, H.R. Platelet-Derived MHC Class I Confers a Pseudonormal Phenotype to Cancer Cells That Subverts the Antitumor Reactivity of Natural Killer Immune Cells. Cancer Res. 2011, 72, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.S.; Li, C.H.; Ruppert, J.G.; Chang, C.C. Cytokeratin 8-MHC class I interactions: A potential novel immune escape phenotype by a lymph node metastatic carcinoma cell line. Biochem. Biophys. Res. Commun. 2013, 441, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. OncoImmunology 2018, 7, e1438111. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Vetsika, E.-K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Tang, C.; Pachynski, R.K.; Chin, R.; Majeti, R.; Weissman, I.L. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011, 118, 4890–4901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Steinert, G.; Schölch, S.; Niemietz, T.; Iwata, N.; García, S.A.; Behrens, B.; Voigt, A.; Kloor, M.; Benner, A.; Bork, U.; et al. Immune Escape and Survival Mechanisms in Circulating Tumor Cells of Colorectal Cancer. Cancer Res. 2014, 74, 1694–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallermalm, K.; De Geer, A.; Kiessling, R.; Levitsky, V.; Levitskaya, J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. 2004, 64, 6775–6782. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fang, F.; Zhang, Q. Circulating tumor cell clusters: What we know and what we expect. Int. J. Oncol. 2016, 49, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.R.; Zuka, M.; Lorger, M.; Tschan, M.; Torbett, B.E.; Zijlstra, A.; Quigley, J.P.; Staflin, K.; Eliceiri, B.P.; Krueger, J.S.; et al. Activated tumor cell integrin αvβ3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb. Res. 2016, 140, S27–S36. [Google Scholar] [CrossRef] [Green Version]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Mannel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar] [PubMed]
- Sneath, R.J.; Mangham, D.C. The normal structure and function of CD44 and its role in neoplasia. Mol. Pathol. 1998, 51, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zoller, M.; Haussmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Katoh, S.; Goi, T.; Naruse, T.; Ueda, Y.; Kurebayashi, H.; Nakazawa, T.; Kimura, Y.; Hirono, Y.; Yamaguchi, A. Cancer stem cell marker in circulating tumor cells: Expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res. 2015, 35, 239–244. [Google Scholar] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Ann. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023–5039. [Google Scholar]
- Anantharaman, A.; Friedlander, T.; Lu, D.; Krupa, R.; Premasekharan, G.; Hough, J.; Edwards, M.; Paz, R.; Lindquist, K.; Graf, R.; et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016, 16, 744. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Wong, J.; Che, J.; Matsumoto, M.; Grogan, T.; Elashoff, D.; Garon, E.B.; Goldman, J.W.; Sollier Christen, E.; Di Carlo, D.; et al. Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci. Rep. 2018, 8, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.; Mirenska, A.; Tan, M.; Lee, Y.; Oh, J.; Hong, L.Z.; Wnek, R.; Yap, Y.S.; Shih, S.J.; AA, S.B.; et al. A preliminary study for the assessment of PD-L1 and PD-L2 on circulating tumor cells by microfluidic-based chipcytometry. Future Sci. OA 2017, 3, FSO244. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Schnell, U.; Cirulli, V.; Giepmans, B.N.G. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Manicone, M.; Poggiana, C.; Facchinetti, A.; Zamarchi, R. Critical issues in the clinical application of liquid biopsy in non-small cell lung cancer. J. Thorac. Dis. 2017, 9, S1346–S1358. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.R.; Dracopoli, N.; Petty, B.G.; Compton, C.; Cristofanilli, M.; Deisseroth, A.; Hayes, D.F.; Kapke, G.; Kumar, P.; Lee, J.S.H.; et al. Considerations in the development of circulating tumor cell technology for clinical use. J. Transl. Med. 2012, 10, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Gun, B.T.F.; Melchers, L.J.; Ruiters, M.H.J.; de Leij, L.F.M.H.; McLaughlin, P.M.J.; Rots, M.G. EpCAM in carcinogenesis: The good, the bad or the ugly. Carcinogenesis 2010, 31, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Rose, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.-P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akalay, I.; Janji, B.; Hasmim, M.; Noman, M.Z.; Andre, F.; De Cremoux, P.; Bertheau, P.; Badoual, C.; Vielh, P.; Larsen, A.K.; et al. Epithelial-to-Mesenchymal Transition and Autophagy Induction in Breast Carcinoma Promote Escape from T-cell-Mediated Lysis. Cancer Res. 2013, 73, 2418–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satelli, A.; Mitra, A.; Brownlee, Z.; Xia, X.; Bellister, S.; Overman, M.J.; Kopetz, S.; Ellis, L.M.; Meng, Q.H.; Li, S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. 2015, 21, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Lampignano, R.; Schneck, H.; Neumann, M.; Fehm, T.; Neubauer, H. Enrichment, Isolation and Molecular Characterization of EpCAM-Negative Circulating Tumor Cells. Adv. Exp. Med. Biol. 2017, 994, 181–203. [Google Scholar] [PubMed]
- Papaioannou, N.E.; Beniata, O.V.; Vitsos, P.; Tsitsilonis, O.; Samara, P. Harnessing the immune system to improve cancer therapy. Ann. Transl. Med. 2016, 4, 261. [Google Scholar] [CrossRef] [PubMed]
- Madic, J.; Kiialainen, A.; Bidard, F.C.; Birzele, F.; Ramey, G.; Leroy, Q.; Rio Frio, T.; Vaucher, I.; Raynal, V.; Bernard, V.; et al. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int. J. Cancer 2015, 136, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Chiavenna, S.M.; Jaworski, J.P.; Vendrell, A. State of the art in anti-cancer mAbs. J. Biomed. Sci. 2017, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Zhang, B.; Gleason, M.K.; Oh, S.; Weiner, L.M.; Kaufman, D.S.; McCullar, V.; Miller, J.S.; Verneris, M.R. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother. Radiopharm. 2013, 28, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Prang, N.; Preithner, S.; Brischwein, K.; Goster, P.; Woppel, A.; Muller, J.; Steiger, C.; Peters, M.; Baeuerle, P.A.; da Silva, A.J. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br. J. Cancer 2005, 92, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaltriti, M.; Baselga, J. The epidermal growth factor receptor pathway: A model for targeted therapy. Clin. Cancer Res. 2006, 12, 5268–5272. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Payne, R.E.; Yague, E.; Slade, M.J.; Apostolopoulos, C.; Jiao, L.R.; Ward, B.; Coombes, R.C.; Stebbing, J. Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics 2009, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, E.A.; Atwal, S.K.; Spoerke, J.M.; Savage, H.; Pandita, A.; Yeh, R.F.; Pirzkall, A.; Fine, B.M.; Amler, L.C.; Chen, D.S.; et al. Molecular biomarker analyses using circulating tumor cells. PLoS ONE 2010, 5, e12517. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.R.; Leversha, M.A.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Gu, B.; Anand, A.; Smith, K.; Maslak, P.; Doyle, G.V.; et al. Circulating Tumor Cell Analysis in Patients with Progressive Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2007, 13, 2023–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorges, T.M.; Stein, A.; Quidde, J.; Hauch, S.; Rock, K.; Riethdorf, S.; Joosse, S.A.; Pantel, K. Improved Detection of Circulating Tumor Cells in Metastatic Colorectal Cancer by the Combination of the CellSearch(R) System and the AdnaTest(R). PLoS ONE 2016, 11, e0155126. [Google Scholar] [CrossRef] [PubMed]
- Gasch, C.; Bauernhofer, T.; Pichler, M.; Langer-Freitag, S.; Reeh, M.; Seifert, A.M.; Mauermann, O.; Izbicki, J.R.; Pantel, K.; Riethdorf, S. Heterogeneity of Epidermal Growth Factor Receptor Status and Mutations of KRAS/PIK3CA in Circulating Tumor Cells of Patients with Colorectal Cancer. Clin. Chem. 2012, 59, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musella, V.; Pietrantonio, F.; Di Buduo, E.; Iacovelli, R.; Martinetti, A.; Sottotetti, E.; Bossi, I.; Maggi, C.; Di Bartolomeo, M.; de Braud, F.; et al. Circulating tumor cells as a longitudinal biomarker in patients with advanced chemorefractory, RAS-BRAFwild-type colorectal cancer receiving cetuximab or panitumumab. Int. J. Cancer 2015, 137, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Matsusaka, S.; Minowa, S.; Shibata, H.; Suenaga, M.; Shinozaki, E.; Mizunuma, N.; Ueno, M.; Yamaguchi, T.; Hatake, K. Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res. 2013, 33, 3905–3910. [Google Scholar] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Haley, B.; Perkins, S.; Beitsch, P.; Khan, A.; Euhus, D.; Osborne, C.; et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad. Sci. USA 2004, 101, 9393–9398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehm, T.; Muller, V.; Aktas, B.; Janni, W.; Schneeweiss, A.; Stickeler, E.; Lattrich, C.; Lohberg, C.R.; Solomayer, E.; Rack, B.; et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: A prospective, multicenter trial. Breast Cancer Res. Treat. 2010, 124, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Pestrin, M.; Bessi, S.; Galardi, F.; Truglia, M.; Biggeri, A.; Biagioni, C.; Cappadona, S.; Biganzoli, L.; Giannini, A.; Di Leo, A. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res. Treat. 2009, 118, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzone, E.; Nolé, F.; Goldhirsch, A.; Botteri, E.; Esposito, A.; Zorzino, L.; Curigliano, G.; Minchella, I.; Adamoli, L.; Cassatella, M.C.; et al. Changes of HER2 Status in Circulating Tumor Cells Compared With the Primary Tumor During Treatment for Advanced Breast Cancer. Clin. Breast Cancer 2010, 10, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Rothe, F.; Chaboteaux, C.; Durbecq, V.; Rouas, G.; Criscitiello, C.; Metallo, J.; Kheddoumi, N.; Singhal, S.K.; Michiels, S.; et al. HER2-positive circulating tumor cells in breast cancer. PLoS ONE 2011, 6, e15624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Wang, T.; Bian, L.; Hu, H.; Xu, C.; Liu, B.; Liu, Y.; Cristofanilli, M.; Jiang, Z. Real-time HER2 status detected on circulating tumor cells predicts different outcomes of anti-HER2 therapy in histologically HER2-positive metastatic breast cancer patients. BMC Cancer 2016, 16, 526. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, D.; Schiavon, G.; Vincenzi, B.; Gaeta, L.; Pantano, F.; Russo, A.; Ortega, C.; Porta, C.; Galluzzo, S.; Armento, G.; et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE 2011, 6, e19234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieferdecker, A.; Voigt, M.; Riecken, K.; Braig, F.; Schinke, T.; Loges, S.; Bokemeyer, C.; Fehse, B.; Binder, M. Denosumab mimics the natural decoy receptor osteoprotegerin by interacting with its major binding site on RANKL. Oncotarget 2014, 5, 6647–6653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Bradshaw, J.; Greene, J.; Peach, R.; Bennett, K.L.; Mittler, R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996, 4, 535–543. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinan, E.C.; Gribben, J.G.; Boussiotis, V.A.; Freeman, G.J.; Nadler, L.M. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood 1994, 84, 3261–3282. [Google Scholar] [PubMed]
- Tarhini, A.; Lo, E.; Minor, D.R. Releasing the brake on the immune system: Ipilimumab in melanoma and other tumors. Cancer Biother. Radiopharm. 2010, 25, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Wistuba-Hamprecht, K.; Yuan, J.; Postow, M.A.; Wong, P.; Capone, M.; Madonna, G.; Khammari, A.; Schilling, B.; Sucker, A.; et al. Increases in Absolute Lymphocytes and Circulating CD4+ and CD8+ T Cells Are Associated with Positive Clinical Outcome of Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 4848–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damuzzo, V.; Solito, S.; Pinton, L.; Carrozzo, E.; Valpione, S.; Pigozzo, J.; Arboretti Giancristofaro, R.; Chiarion-Sileni, V.; Mandruzzato, S. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. OncoImmunology 2016, 5, e1249559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, R.; Lapointe, R.; Cocolakis, E.; Thébault, P.; Kazemi, S.; Friedmann, J.E.; Dionne, J.; Cailhier, J.-F.; Bélanger, K.; Ayoub, J.-P.; et al. Peripheral and local predictive immune signatures identified in a phase II trial of ipilimumab with carboplatin/paclitaxel in unresectable stage III or stage IV melanoma. J. ImmunoTher. Cancer 2017, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L.; Tarhini, A.A.; Edington, H.; Butterfield, L.H.; Lin, Y.; Shuai, Y.; Tawbi, H.; Sander, C.; Yin, Y.; Holtzman, M.; et al. Immune Monitoring of the Circulation and the Tumor Microenvironment in Patients with Regionally Advanced Melanoma Receiving Neoadjuvant Ipilimumab. PLoS ONE 2014, 9, e87705. [Google Scholar]
- Khoja, L.; Lorigan, P.; Zhou, C.; Lancashire, M.; Booth, J.; Cummings, J.; Califano, R.; Clack, G.; Hughes, A.; Dive, C. Biomarker Utility of Circulating Tumor Cells in Metastatic Cutaneous Melanoma. J. Investig. Dermatol. 2013, 133, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Klinac, D.; Gray, E.S.; Freeman, J.B.; Reid, A.; Bowyer, S.; Millward, M.; Ziman, M. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 2014, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Sullivan, R.J.; Kalinich, M.; Kwan, T.T.; Giobbie-Hurder, A.; Pan, S.; LiCausi, J.A.; Milner, J.D.; Nieman, L.T.; Wittner, B.S.; et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 2467–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Huang, Z.; Teng, F.; Xing, L.; Yu, J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015, 41, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Callea, M.; Albiges, L.; Gupta, M.; Cheng, S.C.; Genega, E.M.; Fay, A.P.; Song, J.; Carvo, I.; Bhatt, R.S.; Atkins, M.B.; et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol. Res. 2015, 3, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fousek, K.; Ahmed, N. The Evolution of T-cell Therapies for Solid Malignancies. Clin. Cancer Res. 2015, 21, 3384–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Xu, K.; Niu, L.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Lin, M. Comparison of autogeneic and allogeneic natural killer cells immunotherapy on the clinical outcome of recurrent breast cancer. OncoTargets Ther. 2017, 10, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liang, S.-Z.; Shi, J.; Niu, L.-Z.; Chen, J.-B.; Zhang, M.-J.; Xu, K.-C. Circulating tumor cell as a biomarker for evaluating allogenic NK cell immunotherapy on stage IV non-small cell lung cancer. Immunol. Lett. 2017, 191, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chen, J.; Zeng, J.; Niu, L.; Xie, S.; Wang, X.; Liang, Y.; Wu, Z.; Zhang, M. Effect of NK cell immunotherapy on immune function in patients with hepatic carcinoma: A preliminary clinical study. Cancer Biol. Ther. 2017, 18, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [PubMed]
- Romero, P.; Banchereau, J.; Bhardwaj, N.; Cockett, M.; Disis, M.L.; Dranoff, G.; Gilboa, E.; Hammond, S.A.; Hershberg, R.; Korman, A.J.; et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Sci. Transl. Med. 2016, 8, 334ps9. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Mittendorf, E.A.; Holmes, J.P.; Amin, A.; Hueman, M.T.; Ponniah, S.; Peoples, G.E. Quantification and Phenotypic Characterization of Circulating Tumor Cells for Monitoring Response to a Preventive HER2/neu Vaccine-Based Immunotherapy for Breast Cancer: A Pilot Study. Ann. Surg. Oncol. 2007, 14, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Birzele, F.; Voss, E.; Nopora, A.; Honold, K.; Heil, F.; Lohmann, S.; Verheul, H.; Le Tourneau, C.; Delord, J.P.; van Herpen, C.; et al. CD44 Isoform Status Predicts Response to Treatment with Anti-CD44 Antibody in Cancer Patients. Clin. Cancer Res. 2015, 21, 2753–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisel, D.; Birzele, F.; Voss, E.; Nopora, A.; Bader, S.; Friess, T.; Goller, B.; Laifenfeld, D.; Weigand, S.; Runza, V. Targeting Tumor Cells with Anti-CD44 Antibody Triggers Macrophage-Mediated Immune Modulatory Effects in a Cancer Xenograft Model. PLoS ONE 2016, 11, e0159716. [Google Scholar] [CrossRef] [PubMed]
- Grugan, K.D.; McCabe, F.L.; Kinder, M.; Greenplate, A.R.; Harman, B.C.; Ekert, J.E.; van Rooijen, N.; Anderson, G.M.; Nemeth, J.A.; Strohl, W.R.; et al. Tumor-Associated Macrophages Promote Invasion while Retaining Fc-Dependent Anti-Tumor Function. J. Immunol. 2012, 189, 5457–5466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, S.; Hepp, F.; Kentenich, C.R.; Janni, W.; Pantel, K.; Riethmuller, G.; Willgeroth, F.; Sommer, H.L. Monoclonal antibody therapy with edrecolomab in breast cancer patients: Monitoring of elimination of disseminated cytokeratin-positive tumor cells in bone marrow. Clin. Cancer Res. 1999, 5, 3999–4004. [Google Scholar] [PubMed]
- Schwartzberg, L.S. Clinical experience with edrecolomab: A monoclonal antibody therapy for colorectal carcinoma. Crit. Rev. Oncol. Hematol. 2001, 40, 17–24. [Google Scholar] [CrossRef]
- Richter, C.E.; Cocco, E.; Bellone, S.; Silasi, D.-A.; Rüttinger, D.; Azodi, M.; Schwartz, P.E.; Rutherford, T.J.; Pecorelli, S.; Santin, A.D. High-grade, chemotherapy-resistant ovarian carcinomas overexpress epithelial cell adhesion molecule (EpCAM) and are highly sensitive to immunotherapy with MT201, a fully human monoclonal anti-EpCAM antibody. Am. J. Obstetr. Gynecol. 2010, 203, 582-e1. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, D.; Lindhofer, H.; Bokemeyer, C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM×anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 2010, 36, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Zaloudik, J.; Li, W.; Jacob, L.; Kieny, M.P.; Somasundaram, R.; Acres, B.; Song, H.; Zhang, T.; Li, J.; Herlyn, D. Inhibition of tumor growth by recombinant vaccinia virus expressing GA733/CO17-1A/EpCAM/KSA/KS1-4 antigen in mice. Cancer Gene Ther. 2002, 9, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudry, M.A.; Sales, K.; Ruf, P.; Lindhofer, H.; Winslet, M.C. EpCAM an immunotherapeutic target for gastrointestinal malignancy: Current experience and future challenges. Br. J. Cancer 2007, 96, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosolits, S.; Markovic, K.; Frodin, J.E.; Virving, L.; Magnusson, C.G.; Steinitz, M.; Fagerberg, J.; Mellstedt, H. Vaccination with Ep-CAM protein or anti-idiotypic antibody induces Th1-biased response against MHC class I- and II-restricted Ep-CAM epitopes in colorectal carcinoma patients. Clin. Cancer Res. 2004, 10, 5391–5402. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, S.J.; Park, Y.S.; Park, H.S.; Yang, K.M.; Heo, K. EpCAM peptide-primed dendritic cell vaccination confers significant anti-tumor immunity in hepatocellular carcinoma cells. PLoS ONE 2018, 13, e0190638. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.H.; David, J.M.; Dominguez, C.; Palena, C. Development of Cancer Vaccines Targeting Brachyury, a Transcription Factor Associated with Tumor Epithelial-Mesenchymal Transition. Cells Tissues Organs 2017, 203, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackl, H.; Charoentong, P.; Finotello, F.; Trajanoski, Z. Computational genomics tools for dissecting tumour-immune cell interactions. Nat. Rev. Genet. 2016, 17, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.T.; Wittner, B.S.; Ligorio, M.; Vincent Jordan, N.; Shah, A.M.; Miyamoto, D.T.; Aceto, N.; Bersani, F.; Brannigan, B.W.; Xega, K.; et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014, 8, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andree, K.C.; Mentink, A.; Zeune, L.L.; Terstappen, L.; Stoecklein, N.H.; Neves, R.P.; Driemel, C.; Lampignano, R.; Yang, L.; Neubauer, H.; et al. Towards a real liquid biopsy in metastatic breast and prostate cancer: Diagnostic LeukApheresis increases CTC yields in a European prospective multi-center study (CTCTrap). Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Scherr, D.; Batra, J.; Jhanwar, Y.; Robinson, B.; Nanus, D.; Beltran, H.; Molina, A.; Christos, P.; Bander, N. Anti-prostate-specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 immunotherapy for prostate cancer. Ann. Oncol. 2016, 27, 772TiP. [Google Scholar] [CrossRef]
- Heery, C.R.; Palena, C.; McMahon, S.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Dirmeier, U.; Cordes, L.; Marte, J.; Dahut, W.; et al. Phase I Study of a Poxviral TRICOM-Based Vaccine Directed Against the Transcription Factor Brachyury. Clin. Cancer Res. 2017, 23, 6833–6845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heery, C.R.; Donahue, R.; Lepone, L.; Grenga, I.; Richards, J.; Metenou, S.; Fernando, R.I.; Dirmeier, U.; Singh, H.; Madan, R.; et al. Phase I, dose-escalation, clinical trial of MVA-Brachyury-TRICOM vaccine demonstrating safety and brachyury-specific T cell responses. J. ImmunoTher. Cancer 2015, 3, P132. [Google Scholar] [CrossRef] [Green Version]
- Bidard, F.; cottu, P.; Dubot, C.; Venat-Bouvet, L.; Lortholary, A.; Bourgeois, H.; Bollet, M.; Hanon, V.S.; Luporsi-Gely, E.; Espie, M.; et al. 117P—Anti-HER2 therapy efficacy in HER2-negative metastatic breast cancer with HER2-amplified circulating tumor cells: Results of the CirCe T-DM1 trial. Ann. Oncol. 2017, 28 (Suppl. 5), v22–v42. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Rack, B.; Rothe, F.; Riethdorf, S.; Decraene, C.; Bonnefoi, H.; Dittrich, C.; Messina, C.; Beauvois, M.; Trapp, E.; et al. Liquid biopsy-based clinical research in early breast cancer: The EORTC 90091-10093 Treat CTC trial. Eur. J. Cancer 2016, 63, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Imhof, M.; Lipovac, M.; Angleitner-Boubenizek, L.; Barta, J.; Gomez, I.; Hrdina, A.; Krupa, E.; Lafleur, J.; Lang, I.; Pieta, K.; et al. Double-loaded mature dendritic cell (DC) therapy for non-HLA-restricted patients with advanced ovarian cancer: Final results of a clinical phase I study. J. Clin. Oncol. 2013, 31, 3052. [Google Scholar]
- Van den Heuvel, M.M.; Verheij, M.; Boshuizen, R.; Belderbos, J.; Dingemans, A.M.; De Ruysscher, D.; Laurent, J.; Tighe, R.; Haanen, J.; Quaratino, S. NHS-IL2 combined with radiotherapy: Preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J. Transl. Med. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Bilusic, M.; Hodge, J.W.; Tsang, K.Y.; Arlen, P.M.; Heery, C.R.; Rauckhorst, M.; McMahon, S.; Intrivici, C.; Ferrara, T.A.; et al. A phase I trial of a yeast-based therapeutic cancer vaccine targeting CEA. J Clin. Oncol. 2011, 29, 2604. [Google Scholar] [CrossRef]
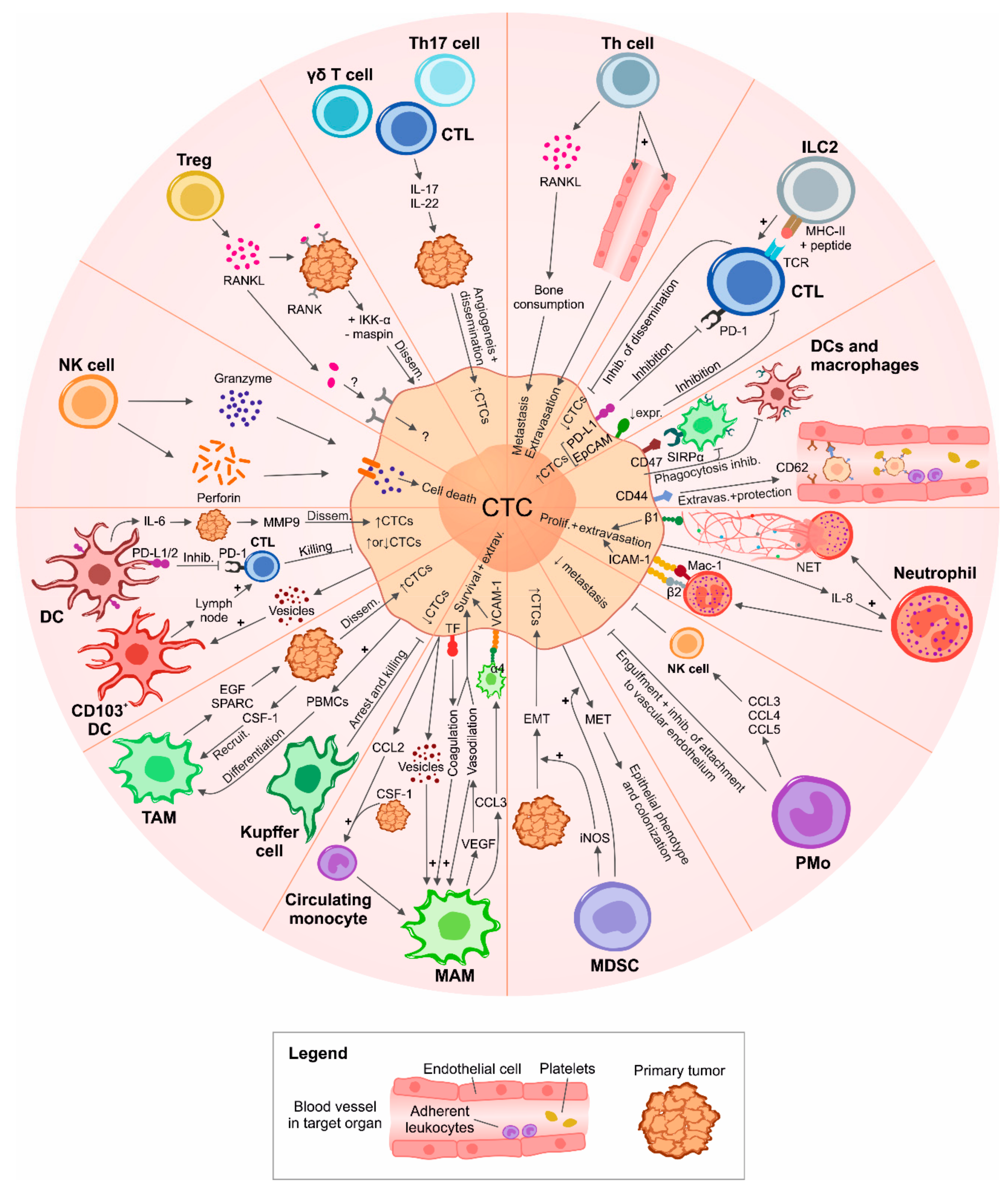
| Trial Number | Official Title | Study Type | Status | Phase | Estimated/Actual Enrollment | Disease | CTC/Other Biomarker Measurem. Method | Outcome Measures/Objectives | Sponsor/ Collaborators | Start/ Completion Date | Publications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03515798 | A Prospective Multicenter Open-label, Randomized Phase II Study of Pembrolizumab in Combination with Neoadjuvant (F)EC–Paclitaxel Regimen in HER2-negative Inflammatory Breast Cancer | Int | Not yet recruiting | Phase 2 | 81 | Inflammatory breast cancer | NP | Evaluation of: pathological CR and DLT rates, IDFS, EFS, and OS; PD-L1 expression in pre-, per-, and post-treatment tissue. Occurrence of adverse events. Measurement of baseline CTCs for prospective validation of their prognostic value in IBC; disease monitoring (ctDNA sequencing). Identification of mechanisms of treatment resistance (immune profiling, NGS, and mouse xenografing) | Institut Paoli-Calmettes; MSD France | June 2018–April 2025 | NP |
| NCT03213041 | I-CURE-1: A Phase II, Single Arm Study of Pembroluzimab Combined with Carboplatin in Patients with Circulating Tumor Cells (CTCs) Positive Her-2 Negative Metastatic Breast Cancer (MBC) | Int | Recruiting | Phase 2 | 100 | Metastatic breast cancer | CellSearch, CellSieve, sequencing | Evaluation of: PFS, OS, ORR, CBR, immune-related response, immune-related clinical benefit, TTNM in CTC-positive mBC patients previously treated with anthracyclines and taxanes undergoing treatment with carboplatin–pembrolizumab; ORR and CBR in relation to PD-L1 expression in tissue and CTCs. Measurement of: PD-L1 in CTCs and immune cells such as CAMLs and their correlation with therapeutic benefit. Measurement of ctDNA and sequencing analysis of TCR and their correlation with CTC enumeration and therapeutic benefit | Northwestern University; Merck Sharp & Dohme Corp.; National Cancer Institute (NCI) | September 2017–July 2022 | NP |
| NCT03434912 | Identification and Evaluation of the Potential Biomarkers on Circulating Tumor Cells and Tumor Related Rare Cells in Cancer Patients Undergoing Immunotherapy | Obs | Recruiting | NP | 80 | NP | NP | Isolation and analysis of CTCs and tumor-related rare cells before and after immunotherapy. Identification of potential biomarkers associated with clinical outcome. Association between changes of rare tumor cells and clinical outcome | MiCareo Taiwan Co., Ltd.; Taipei Veterans General Hospital; Taiwan | May 2017–April 2020 | NP |
| NCT03070002 | A Phase II, Open Label Study to Evaluate Denosumab in Patients with ER and/or PR-Positive, HER2-Negative Metastatic Breast Cancer (MBC) With Bone Metastases and Detectable Circulating Tumor Cells (CTCs) | Int | Recruiting | Phase 2 | 42 | Metastatic breast cancer | NP | Assessment of: the effect of denosumab in patients with bone metastases and ≥5 CTCs who are in PR or SD after starting systemic therapy by measuring the fraction of patients with reduction in CTCs; the effect of denosumab on CTC enumeration considered as a continuous variable (percent change from baseline); median-PFS through statistical analysis evaluating the relationship between longitudinal CTC counts and PFS; the effect on CTC profiling and characterization of stem cell phenotype (CTC-EMT); the type of PD. Analysis of RANKL expression | Northwestern University; Amgen; National Cancer Institute (NCI) | March 2017–March 2022 | NP |
| NCT02978118 | Exploring Relevant Immune-based Biomarkers and Circulating Tumor Cells During Treatment with Immune Checkpoint Inhibitors in Genitourinary Malignancies | Obs | Recruiting | NP | 10 | Metastatic renal cell carcinoma and metastatic urothelial carcinoma | NP | Evaluation of: change in the number of T cells, B cells, myeloid cells before and after treatment with immune therapies; number of patients with detectable CTCs; prevalence of TILs and TAMs at baseline; change in CTCs over time; distribution of CTC difference scores across tumor response categories of CR, PR, SD, and PD | Duke University; University of Wisconsin, Madison | March 2017–March 2019 | NP |
| NCT02948985 | Evaluation of Individual Peripheral Blood Circulating Tumor Cells Combined with Tumor Marker Detection of Efficacy of Chemotherapy in Patients with Advanced Colorectal Cancer: An Observational Clinical Trial | Obs | Not yet recruiting | NP | 100 | Metastatic colorectal cancer | SE-iFISH, BEAMing | Correlation of: RAS status on CTCs with clinical outcome of patients with histologically-confirmed RAS and B-Raf wild-type mCRC treated with FOLFIRI ± cetuximab; mutations in ctDNA with cetuximab resistance | Shanghai General Hospital; Shanghai Jiao Tong University School of Medicine | January 2017–December 2019 | NP |
| NCT03114631 | Single-center Trial Evaluating the Safety and Efficacy of MUC-1/WT-1 Peptide or Tumor Lysate-pulsed Dendritic Cell Immunotherapy for the Patients with Pancreatic Cancer | Int | Recruiting | Phase 1 and phase 2 | 30 | Pancreatic cancer | NP | Assessment of: number of participants with PR or CR at 1 year; number of participants who survived at 1, 2, 3 years or more; immune response (increase of antigen-specific T cells, decrease of Tregs and other cell subsets associated with tumor progression); CTC count (decrease of EpCAM+ CD45- CTCs) | The Republican Research and Practical Center for Epidemiology and Microbiology; Belarusian state medical university | January 2017–December 2019 | NP |
| NCT02456571 | Defining the Relevant Immune Checkpoints Expressed on Metastatic Prostate Cancer Circulating Tumor Cells | Obs | Recruiting | NP | 40 | Metastatic prostate cancer | CellSearch | Evaluation of: change in expression of PD-L1, PD-L2, B7-H3, and CTLA-4 on CTCs of four groups of patients undergoing different treatments; change over time in mutational profiles, AR-variant expression and immune/tumor-related RNA signatures in CTC-enriched blood; expression (prevalence) of PD-L1, PD-L2, B7-H3, and CTLA-4 in metastatic tumor tissue obtained by elective CT or US-guided research biopsies in up to 10 patients and comparison of this expression percentage with CTC immune checkpoint expression | Duke University; Janssen Research & Development, LLC | November 2016–June 2019 | NP |
| NCT02933255 | Phase I/II Study of PROSTVAC in Combination with Nivolumab in Men With Prostate Cancer | Int | Recruiting | Phase 1 and phase 2 | 29 | Prostate cancer | NP | Evaluation of: safety and changes in T cell infiltration in the tumor after neoadjuvant treatment; change in peripheral PSA-specific T cells in patients treated with PROSTVAC and nivolumab; intraprostatic Treg infiltration with CD4 and FOX-P3 stainings; PSA changes secondary to vaccination, including rate of biochemical recurrence after prostatectomy; MRI changes secondary to treatment; changes in PD-L1 expression; pathologic responses (including pathologic CR); changes in immune cell subsets in the periphery; changes in soluble immune-mediating factors (such as cytokines, etc.) in sera; changes in CTC levels in mCRPC cohort | National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC) | October 2016–August 2021 | NP |
| NCT02915445 | T Cells Armed with Chimeric Antigen Receptor Recognizing EpCAM for Patients With Nasopharyngeal Carcinoma and Breast Cancer | Int | Recruiting | Phase 1 | 30 | Nasopharyngeal carcinoma or breast cancer | NP | Evaluation of: number of participants with treatment-related adverse events/dose-limiting toxicity as assessed by CTCAE v4.0; RR of participants treated with EpCAM+ CAR-T cells assessed by RECIST v1.1; persistence of EpCAM+ CAR-T cells and correlation with the RR; persistence of EpCAM+ CTCs | Sichuan University | July 2016–July 2019 | NP |
| NCT02827344 | Feasibility Study of PD-L1 Expression Analysis on Circulating Tumor Cells by Immunocytochemistry and MDSCs Level Evolution Analysis in Non-small Cell Lung Cancer Treated With PD-L1 or PD1 Inhibitor | Obs | Unknown status | NP | 51 | Stage IV non-small cell lung cancer | ISET, immunocytochemistry | Assessment of: feasibility of analysis of PD-L1 expression on CTCs; percentage of CTCs expressing PD-L1 after 4 cycles of immunotherapy; evolution of MDSC counts in response to treatment | University Hospital, Toulouse | October 2015 –October 2016 | [168] |
| NCT02552394 | Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 in Patients with Advanced Prostate Cancer and Unfavorable Circulating Tumor Cell Counts | Int | Recruiting | Phase 1 | 24 | Metastatic prostate cancer | CellSearch | Assessment of: decrease of CTC count in mPCa patients with elevated baseline CTC counts undergoing mAb Hu-J591 treatment with different dose levels; PSA decline rate across all dose levels; 89Zr-DFO-huJ591 PET/CT imaging both pre- and post-mAb hu-J591 infusion to describe objective imaging responses; duration of biochemical and/or measurable disease response through PSA dosage and/or CT scans; number of participants with treatment-related adverse events as assessed by CTCAE v4.0; overall and prostate cancer specific survival rate of subjects following treatment; pain change from baseline as reported on the brief pain inventory questionnaire | Weill Medical College of Cornell University | July 2015–July 2019 | [251] |
| NCT02510781 | A Study on Neoadjuvant Therapy for Her-2 Positive Breast Cancer and the Prognosis by Detecting Circulating Tumor Cells | Int | Unknown status | Phase 2 | 200 | Stage II–III breast cancer | NP | Assessment of: pCR rate; clinical response rate; number of adverse events | Hospital Affiliated to Military Medical Science, Beijing | January 2015–December 2017 | NP |
| NCT02179515 | An Open Label Phase I Study to Evaluate the Safety and Tolerability of a Modified Vaccinia Ankara (MVA) Based Vaccine Modified to Express Brachyury and T-Cell Costimulatory Molecules (MVA Brachyury-TRICOM) | Int | Completed | Phase 1 | 38 | Lung, breast, prostate, ovarian tumors (others) | NP | Evaluation of: safety and tolerability of escalating doses of MVA-brachyury-TRICOM vaccine; CD8+ and CD4+ cell immunologic response as measured by an increase in brachyury-specific T cells; evidence of clinical benefit (such as PFS), RECIST criteria, reduction in serum markers, and/or reduction in CTCs; frequency of immune cell subsets in peripheral blood and changes in serum levels of cytokines and Abs to brachyury; correlation of brachyury expression in tissue samples with clinical outcomes | National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC) | June 2014–February 2018 | [252,253] |
| NCT01804465 | A Randomized Phase 2 Trial of Immediate vs. Delayed Anti-CTLA4 Blockade Following Sipuleucel-T Treatment for Prostate Cancer Immunotherapy | Int | Recruiting | Phase 2 | 54 | Prostate cancer | NP | Evaluation of: impact of the timing of ipilimumab administration on PAP/PA2024-specific immune responses by SipT; PSA measurements; radiographic clinical responses; modulation of effector and regulatory T cells; safety of combining ipilimumab with SipT; CTC count; T cell gene and microRNA signatures | University of California, San Francisco; M.D. Anderson Cancer Center; Bristol-Myers Squibb; Dendreon | January 2014–December 2019 | NP |
| NCT02412462 | Phase I Dose Escalation Study of AB-16B5 in Subjects with an Advanced Solid Malignancy | Int | Completed | Phase 1 | 15 | Advanced solid malignancies | NP | Assessment of: number of participants with an adverse event; plasma concentrations of AB-16B5; objective tumor responses in subjects with measurable disease according to RECIST. Monitoring of EMT and stem cell biomarkers in CTCs and paired tumor biopsies | Alethia Biotherapeutics | April 2015–January 2017 | NP |
| NCT01975142 | Validity of HER2-amplified Circulating Tumor Cells to Select Metastatic Breast Cancer Considered HER2-negative for Trastuzumab-emtansine (T-DM1) Treatment | Int | Active, not recruiting | Phase 2 | 155 | Metastatic breast cancer considered HER2 negative on primary tumor | FISH, IF | Assessment of: tumor response rate to T-DM1 in patients with HER2-amplified CTCs; detection rate of HER2-amplified CTCs; heterogeneity rate between CTCs and correlations with patient characteristics; technical failure rate and reproducibility of HER2 FISH on CTCs; correlation between HER2 FISH and IF on CTCs; PFS; disease control rate (responsive and stable cases); correlation between treatment efficacy and HER2 FISH results; changes in CTC numbers during treatment; ctDNA before and during treatment; toxicity | Institut Curie | October 2013–November 2017 | [254] |
| NCT02450448 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Renal Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 60 | Stage II-IV renal cancer | Multiparameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT02450435 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Prostatic Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 60 | Stage II-IV prostatic cancer | Multiparameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT02450422 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Colorectal Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 60 | Stage II-IV colorectal cancer | Multipa-rameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT02450357 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Breast Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 60 | Stage II–IV breast cancer | Multipa-rameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT02416635 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Liver Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 60 | Stage II–IV liver cancer | Multipa-rameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT02412384 | The Detection of Circulating Tumor Cells (CTCs) in Patients with Lung Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 120 | Stage II–IV lung cancer | Multipa-rameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–February 2016 | NP |
| NCT02406846 | The Detection of CTCs in Patients with Pancreatic Cancer Undergoing Cryosurgery Combined With DC-CIK Treatment | Obs | Completed | NP | 80 | Stage II–IV pancreatic cancer | Multipa-rameter FCM and RT-PCR | Enumeration of CTCs after cryosurgery in patients receiving or not receiving DC-CIK treatment | Fuda Cancer Hospital, Guangzhou | June 2013–December 2015 | NP |
| NCT01548677 | TRastuzumab in HER2-negative Early Breast Cancer as Adjuvant Treatment for Circulating Tumor Cells (CTC) (“TREAT CTC” Trial) | Int | Completed | Phase 2 | 1317 | HER2 negative primary breast cancer | NP | Detection of CTCs. Comparison of CTC detection rate at week 18 between the trastuzumab treatment arm and the observational arm. Evaluation of: RFI, IDFS, DFS, and OS; feasibility, reliability, within-patient reproducibility, and variability of CTC assays; correlation of CTC detection rate with RFI, IDFS, DFS, and OS; cardiac safety of trastuzumab treatment | European Organisation for Research and Treatment of Cancer—EORTC; Hoffmann-La Roche; Janssen Diagnostics, LLC; SUCCESS; UNICANCER | April 2013–March 2017 | [255] |
| NCT01640444 | Randomized Phase II Study to Explore the Influence of BRAF and PIK3K Status on the Efficacy of FOLFIRI Plus Bevacizumab or Cetuximab, as First Line Therapy of Patients with RAS Wild-type Metastatic Colorectal Carcinoma and <3 Circulating Tumor Cells | Int | Active, not recruiting | Phase 2 | 240 | Metastatic colorectal cancer | NP | Evaluation of: PFS, OS, RR, and R0 surgery rate; baseline CTC count and its correlation to PFS, OS, and RR; adverse events; correlation of molecular status of biomarkers related to cellular and tumoral reproduction and/or mode of action and PFS, OS, and RR | Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD); Roche Pharma AG | July 2012–November 2018 | NP |
| NCT01640405 | Phase III, Randomized Clinical Trial to Evaluate FOLFOX + Bevacizumab Versus FOLFOXIRI + Bevacizumab as First Line Treatment of Patients with Metastatic Colorectal Cancer Not Previously Treated and With Three or More Circulating Tumoral Cells | Int | Active, not recruiting | Phase 3 | 350 | Metastatic colorectal cancer | NP | Evaluation of: PFS, OS, RR, and R0 surgery rate; baseline CTC count and its correlation to PFS, OS, and RR; correlation of RAS, BRAF, and PI3K mutations and PFS, OS, and RR; adverse events; correlation of molecular status of biomarkers related to cellular and tumoral reproduction and/or mode of action and PFS, OS, and RR | Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD); Roche Phara AG | July 2012–November 2018 | NP |
| NCT01185509 | A Phase II, Single Arm, Open Label Study to Evaluate the Efficacy and Safety of Trastuzumab and Vinorelbine in Advanced Breast Cancer Patients with HER2 Negative Primary Tumors and HER2 Positive Circulating Tumor Cells | Int | Terminated | Phase 2 | 31 | HER2 negative primary breast cancer | NP | Evaluation of: ORR, CBR, PFS, and CTC levels. Description of CTC number and CTC characteristics before and after therapy, and correlation of these findings with response; safety and tolerability of trastuzumab and vinorelbine treatment | Dana-Farber Cancer Institute; Brigham and Women’s Hospital; Beth Israel Deaconess Medical Center; Massachusetts General Hospital; Genentech, Inc. | November 2010–September 2017 | NP |
| NCT01456065 | A Phase I, Open, Randomized, Study to Investigate the Safety of Active Immunotherapy with Fully Mature, TERT-mRNA and Survivin—Peptide Double Loaded Dendritic Cells (DCs) in Subjects With Advanced Epithelial Ovarian Cancer, Enrolled in the Study Within Twelve Weeks After Completing Primary Therapy | Int | Unknown status | Phase 1 | 15 | Ovarian epithelial cancer | NP | Assessment of: incidence of adverse events and clinical relevant deviations from laboratory parameters; number of CTCs prior to vaccination and at follow up; number of autologous DCs loaded with tumor-specific antigens; time to progression (CA-125 and CT); OS; immune monitoring prior to vaccination and during treatment; | Life Research Technologies GmbH | September 2010–April 2013 | [256] |
| NCT00879866 | An Open-label, Phase Ib, Dose-escalation Trial on the Safety, Tolerability, Pharmacokinetics, Immunogenicity, Biological Effects and Antitumor Activity of EMD 521873 in Combination with Local Irradiation (20 Gy) of Primary Tumors or Metastases in Subjects With Non-small Cell Lung Cancer Stage IIIb With Malignant Pleural Effusion or Stage IV With Disease Control (Partial Response or Stable Disease) After Application of 4 Cycles of Platinum-based, First-line Chemotherapy | Int | Completed | Phase 1 | 15 | Non-small cell lung cancer | NP | Assessment of: safety, tolerability, and MTD (if reached) with EMD 521873 doses of up to 0.45 mg/kg; PK of EMD 521873 in combination with local tumor irradiation; immunogenicity of EMD 521873 in combination with local tumor irradiation by measuring the induction of anti-EMD 521873 Abs; best overall response; changes in tumor marker levels and CTC numbers after treatment; best overall response after second-line therapy and duration of the response; PFS and OS; biological/immune responses following treatment by assessing changes in relevant parameters including leukocyte subset analysis and molecular markers of immune activation (e.g., cytokines/chemokines, IL-2 receptor, and neopterin) | Merck KGaA | April 2009–September 2012 | [257] |
| NCT00924092 | Open Label Phase I Study to Evaluate the Safety and Tolerability of Vaccine (GI-6207) Consisting of Whole, Heat-Killed Recombinant Saccharomyces Cerevisiae Genetically Modified to Express CEA Protein in Adults with Metastatic CEA-Expressing Carcinoma | Int | Completed | Phase 1 | 25 | Prostate, breast, lung, colorectal, head and neck cancer | NP | Determination of: safety and tolerability of escalating doses of a heated-killed yeast-based vaccine targeting tumors that express CEA; evaluation of: CD4+ and CD8+ cell immunologic response; humoral immune response to yeast antigen; evidence of clinical benefit such as PFS, OR; decreases in CTCs; tumor markers | National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC) | March 2009–August 2012 | [258] |
| NCT02048540 | Phase 2 Study of Neoadjuvant Bevacizumab Plus DOF Versus DOF in Local Advanced Gastric Carcinoma and Its Association with Circulating Tumor Cell | Int | Completed | Phase 1 and phase 2 | 86 | Stage IIIb-IIIc gastric carcinoma | NP | Assessment of: R0 resection rate; pCR rate; OS; DFS; ORR; safety of perioperative treatment and surgery; CTC number change before and after therapy | Chinese PLA General Hospital | February 2009–December 2013 | NP |
| NCT00429247 | A Pilot Randomized Phase II Study of Adjuvant Administration of Trastuzumab (HERCEPTIN) Versus Observation After the Completion of Adjuvant Chemotherapy and Radiotherapy in Patients with Stage I-III Breast Cancer Who Have Detectable Disseminated and/or Circulating Tumor Cells (DTCs and/or CTCs) in the Bone Marrow or/and the Peripheral Blood Before or/and After the Completion of Adjuvant Treatment | Int | Completed | Phase 2 | 75 | Stage I-III operable breast cancer | NP | Comparison of disease-free interval of patients with early-stage breast cancer. Evaluation of CK19 mRNA-positive CTC elimination | University Hospital of Crete | February 2003–December 2007 | NP |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, K.; Poggiana, C.; Zamarchi, R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics 2018, 8, 59. https://doi.org/10.3390/diagnostics8030059
Leone K, Poggiana C, Zamarchi R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics. 2018; 8(3):59. https://doi.org/10.3390/diagnostics8030059
Chicago/Turabian StyleLeone, Kevin, Cristina Poggiana, and Rita Zamarchi. 2018. "The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy" Diagnostics 8, no. 3: 59. https://doi.org/10.3390/diagnostics8030059






