On-Site Validation of a Microwave Breast Imaging System, before First Patient Study
Abstract
1. Introduction
2. Materials and Methods
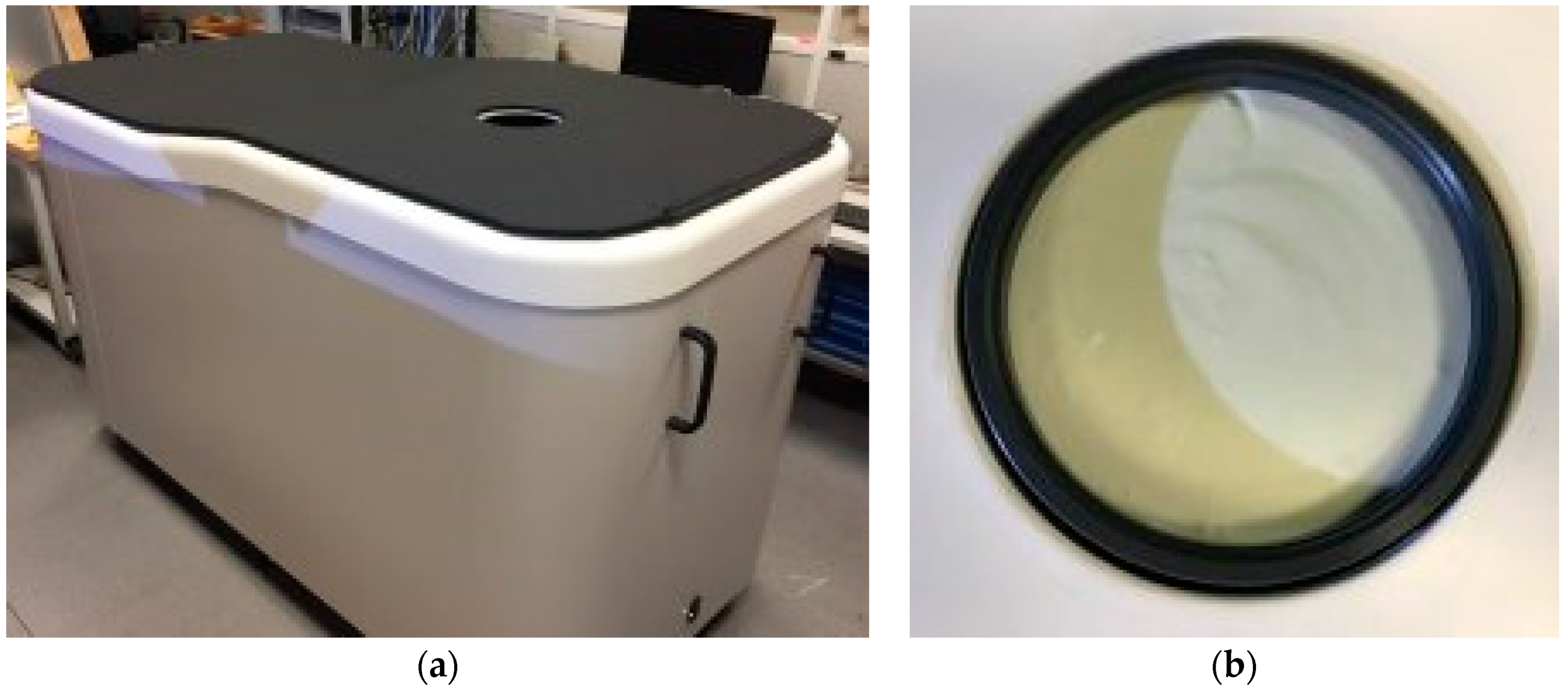
2.1. The Wavelia Microwave Breast Imaging System Prototype
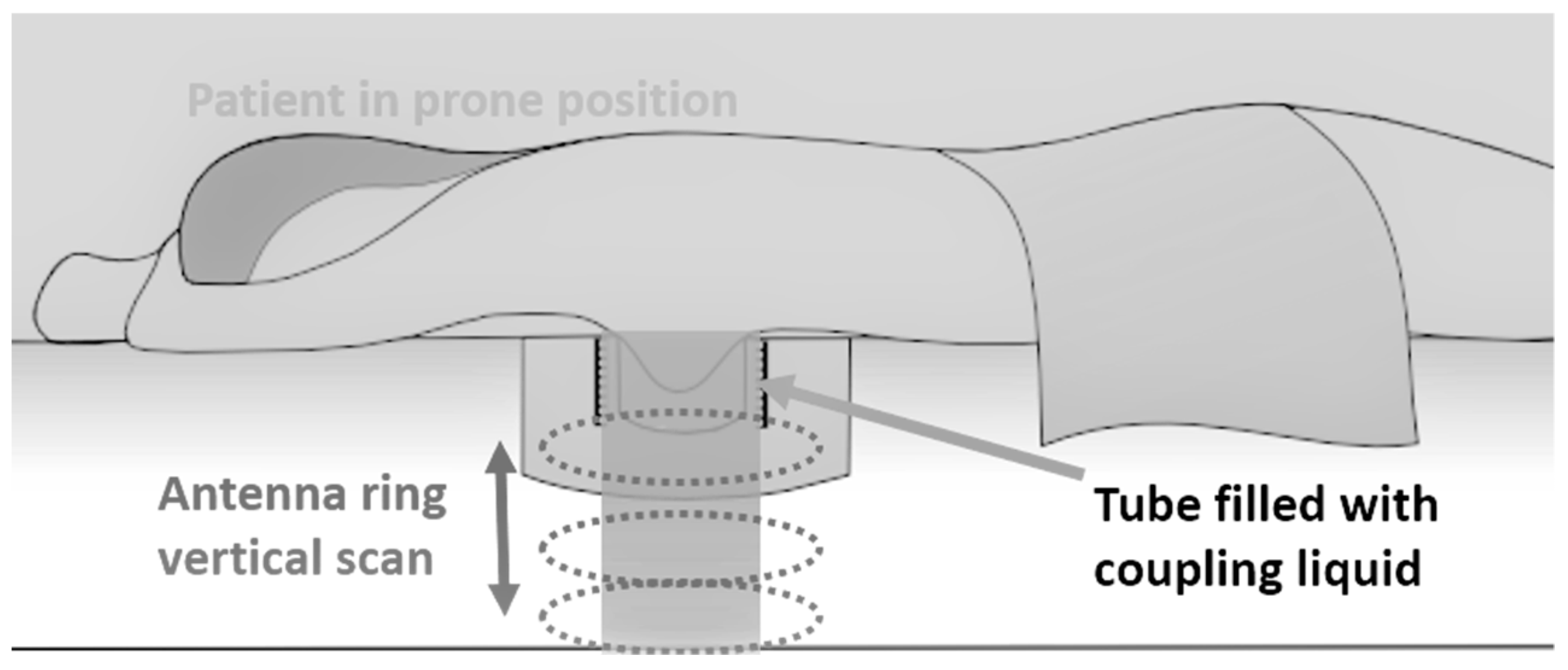
2.2. Microwave Breast Imaging at Prone Position: The Principle
- An adipose layer directly below the skin. This layer consists of vesicular cells filled with fat, which are aggregated into lobules and separated by Cooper’s ligaments;
- The mammary glands: the innermost tissue of the breast consists of about 15–20 sections, termed lobes, with many smaller sections of mammary glands, which are arranged in a circular fashion. These lobes and ducts are also surrounded by Cooper’s ligaments, which have the function of maintaining the inner structure of the breast and supporting the tissue attached to the chest wall;
- Posterior to the breast is the major pectoral muscle, as well as ribs two to six.
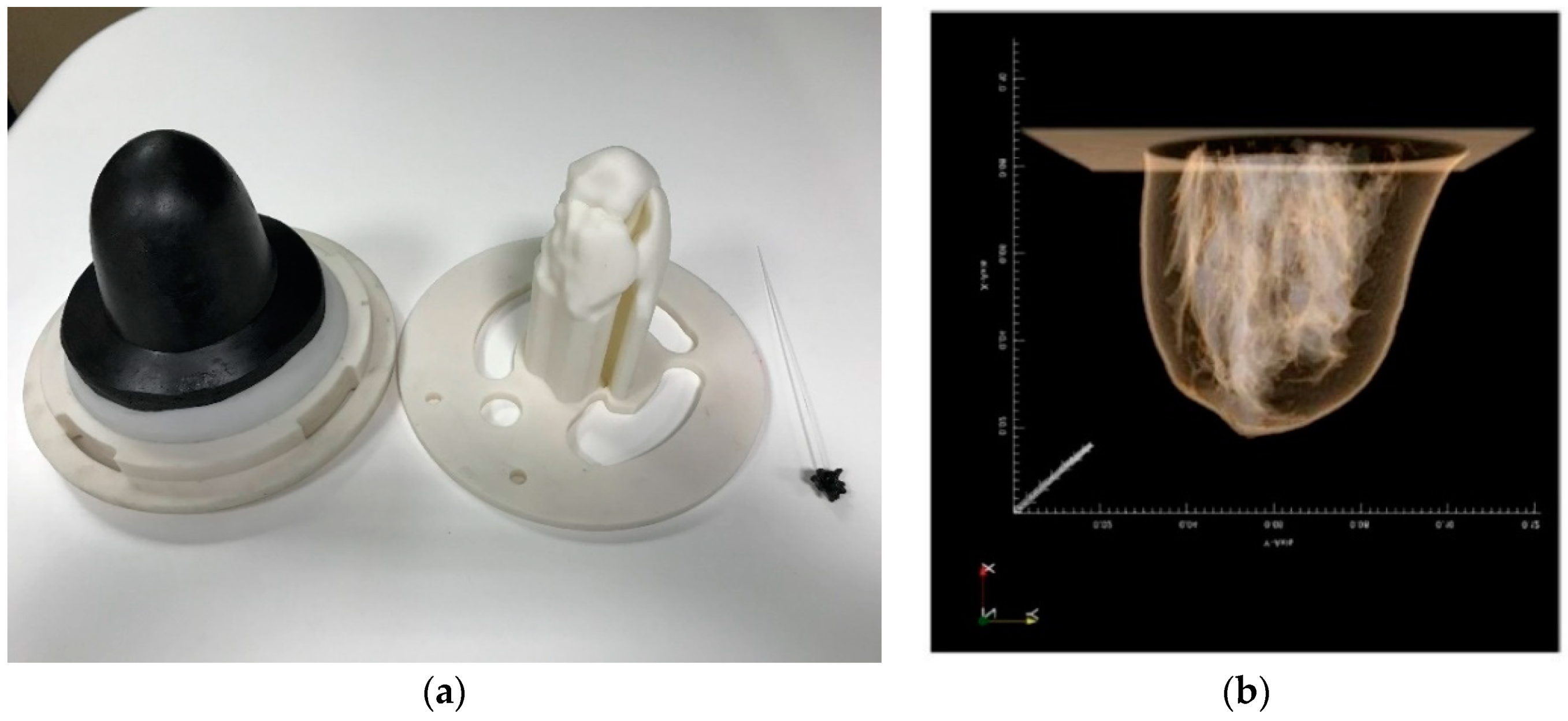
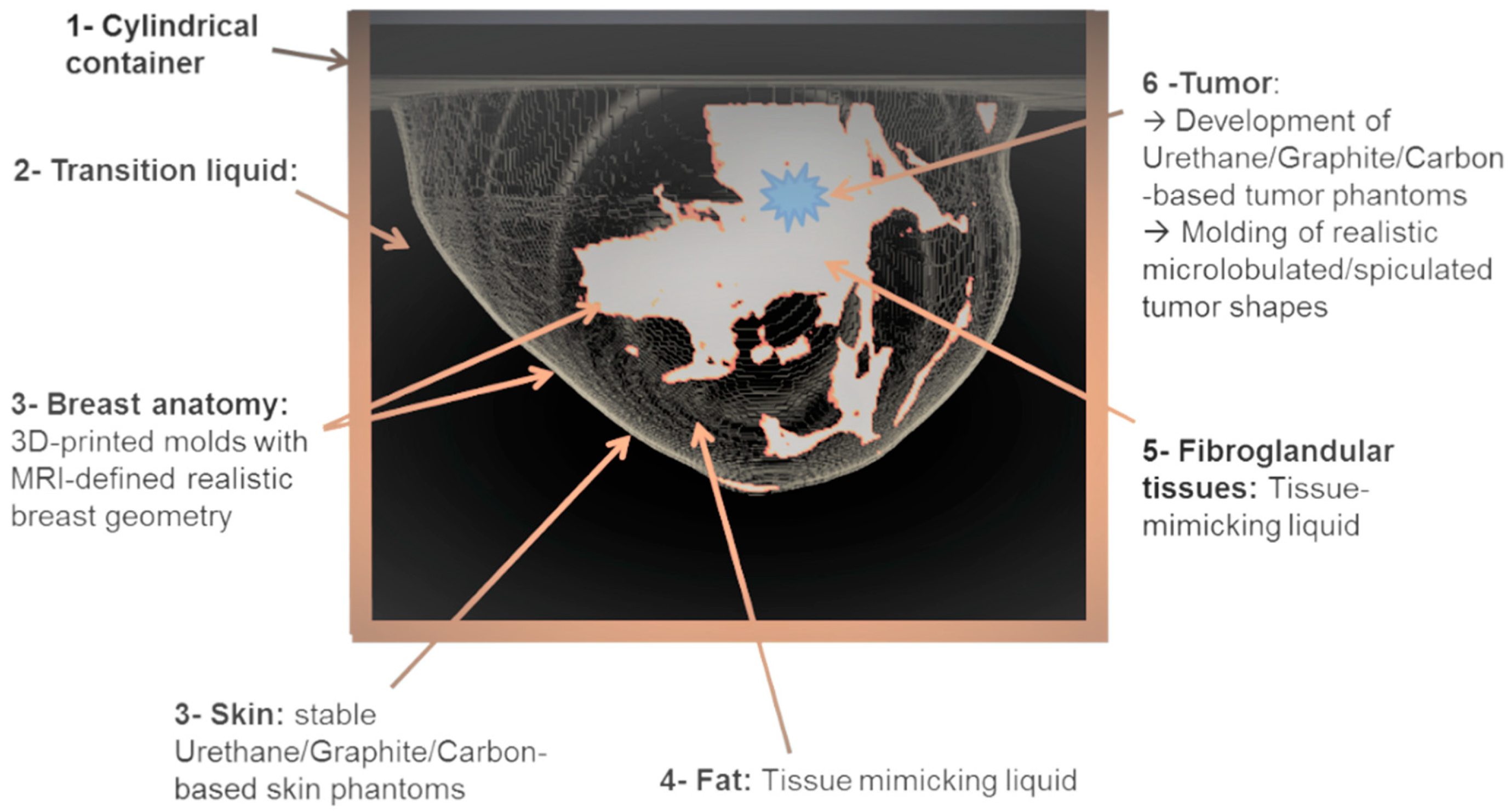
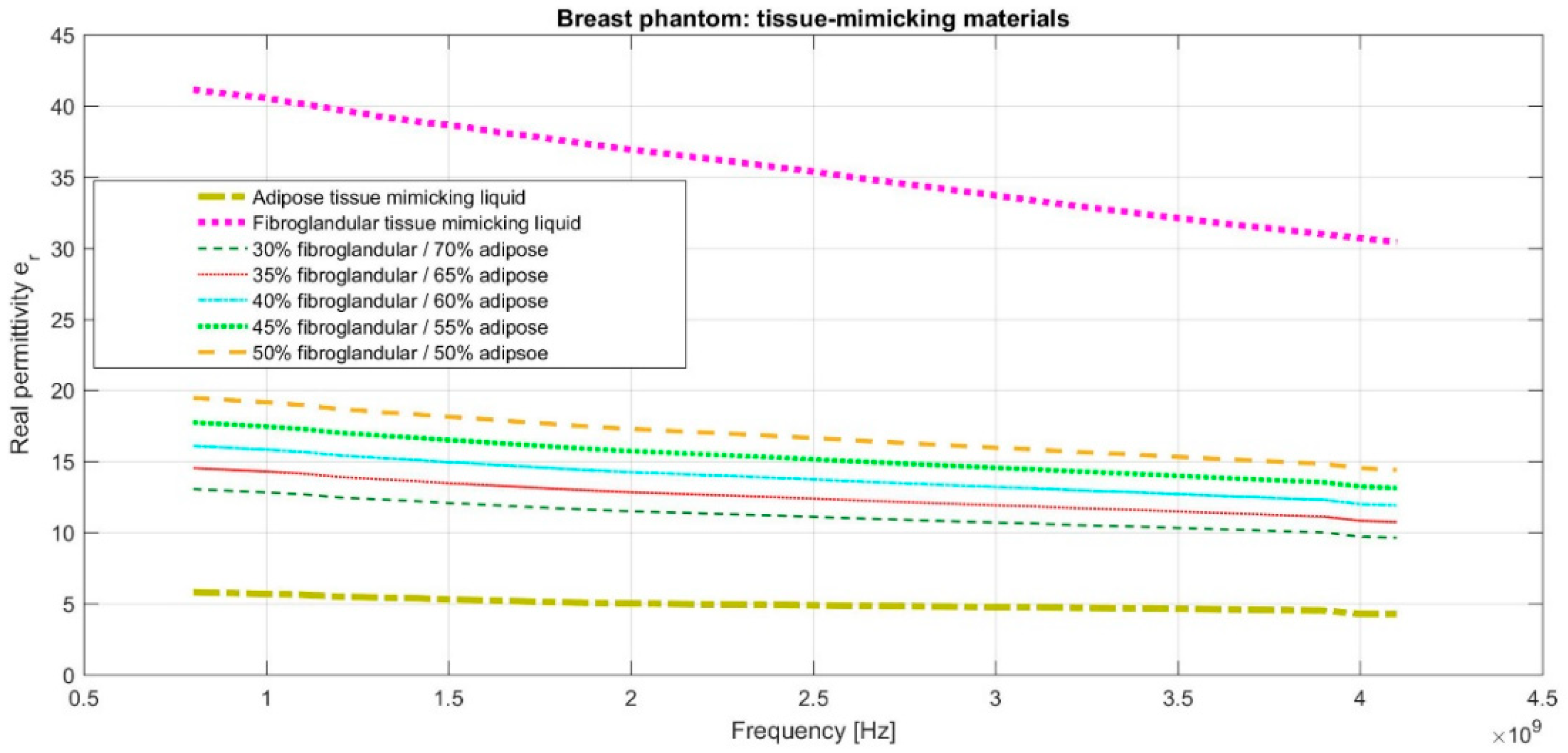
2.3. The Breast Phantoms
- ▪
- Realistic breast shapes extracted from a publicly available database of real MRI breast images [27];
- ▪
- ▪
2.4. The Imaging Algorithm
2.4.1. The Physical Considerations and Modeling
- Transmitting antenna → Cylinder → Transition liquid → Skin → Fat → Glandular tissue → Tumor
- Receiving antenna ← Cylinder ← Transition liquid ← Skin ← Fat ← Glandular tissue ← Tumor
2.4.2. The Data Pre-Processing Steps
- Data calibration at the presence of the breast
- Reconstruction of the breast external envelope
- Independent Component Analysis, in the frequency domain
- Data filtering: IC Selection with Appropriate Spectral and Geometry-based Features
- ▪
- ▪
- The estimated location from which each IC radar echo originates: an inverse fast Fourier transform (IFFT) for transformation of the IC from the frequency domain to the time domain is applied for this purpose; the correspondence between time and distance is established using as input the prior estimate of the breast contour, the known dielectric properties of the transition liquid, and an assumption on the average dielectric properties in the interior of the breast (directly derived from an assumption on the percentage of fibro-glandular versus adipose tissue in the breast).
- ▪
- Filtering-out ICs with spectral profile incompatible with radar target echoes originating from the breast tissues, given the expected level of frequency dispersion; in the future, additional pattern features may be identified and employed at this filtering step, based on measurements with real breast tissues.
- ▪
- Filtering-out ICs that are associated with radar target echoes originating from either very short distances (residual coupling) or very long distances (multipath) with respect to the sensors; the ICs that are filtered out at this step cannot physically correspond to the breast tissues, in terms of geometry.
- Propagation Loss Compensation
2.4.3. The TR-MUSIC (Time-Reversal Multiple Signal Classification) Imaging Algorithm
- ▪
- A limited number of frequency points is selected from the total of measured frequency points in the operating band.
- ▪
- Sectorization is performed, such that multiple images are formed at each frequency and each vertical position of the sensor network, each time using a different sector of the circular network. The selected number of sensors in the sector is further denoted as . The total number of sectors required to scan over the full 360° around the breast is denoted as .
- The multistatic frequency response matrix (MFRM) is formed using the calibrated and filtered data at the specific frequency:where:as defined in Equation (3).
- The time-reversal operator is subsequently formed as:with H denoting the Hermitian transpose.
- Eigenvalue decomposition is performed on , and an appropriate model order selection criterion is used to separate the resulting eigenspace into signal and noise subspaces [59]:where Mord is the selected model order. The separation can be a challenging task, if in the imaging scene there are multiple interacting non-point targets, as is typically the case for breast imaging. The effective separability between the signal and noise subspace has a significant direct impact on the final imaging result, given that the principle for the formation of this type of image is the orthogonality between the two subspaces.
- The image, or the so-called TR-MUSIC pseudospectrum at the pixel p and the frequency f, when using the sector of sensors sect at the vertical scan position hj, is formed as:where:is the illumination vector of the sector sensor array sect at the frequency f and the pixel location p in the imaging zone.
2.4.4. The Composite Image Formation
2.4.5. The Focusing Metrics, as a Means of Adjustment of the Breast Mean Permittivity
- ▪
- is the Hankel function of first kind and zero order: ,
- ▪
- is the wavenumber for propagation in the transition liquid,
- ▪
- is an ‘average’ differential wavenumber for propagation in the breast,
- ▪
- is the speed of light in vacuum,
- ▪
- is the known dielectric constant of the transition liquid,
- ▪
- is an estimate of the average equivalent dielectric constant of the breast, and
- ▪
- is an estimate of the propagation path in the breast, in the case of a wave propagating from the sensor to the pixel , knowing the wavefront corresponding to the external surface of the breast.
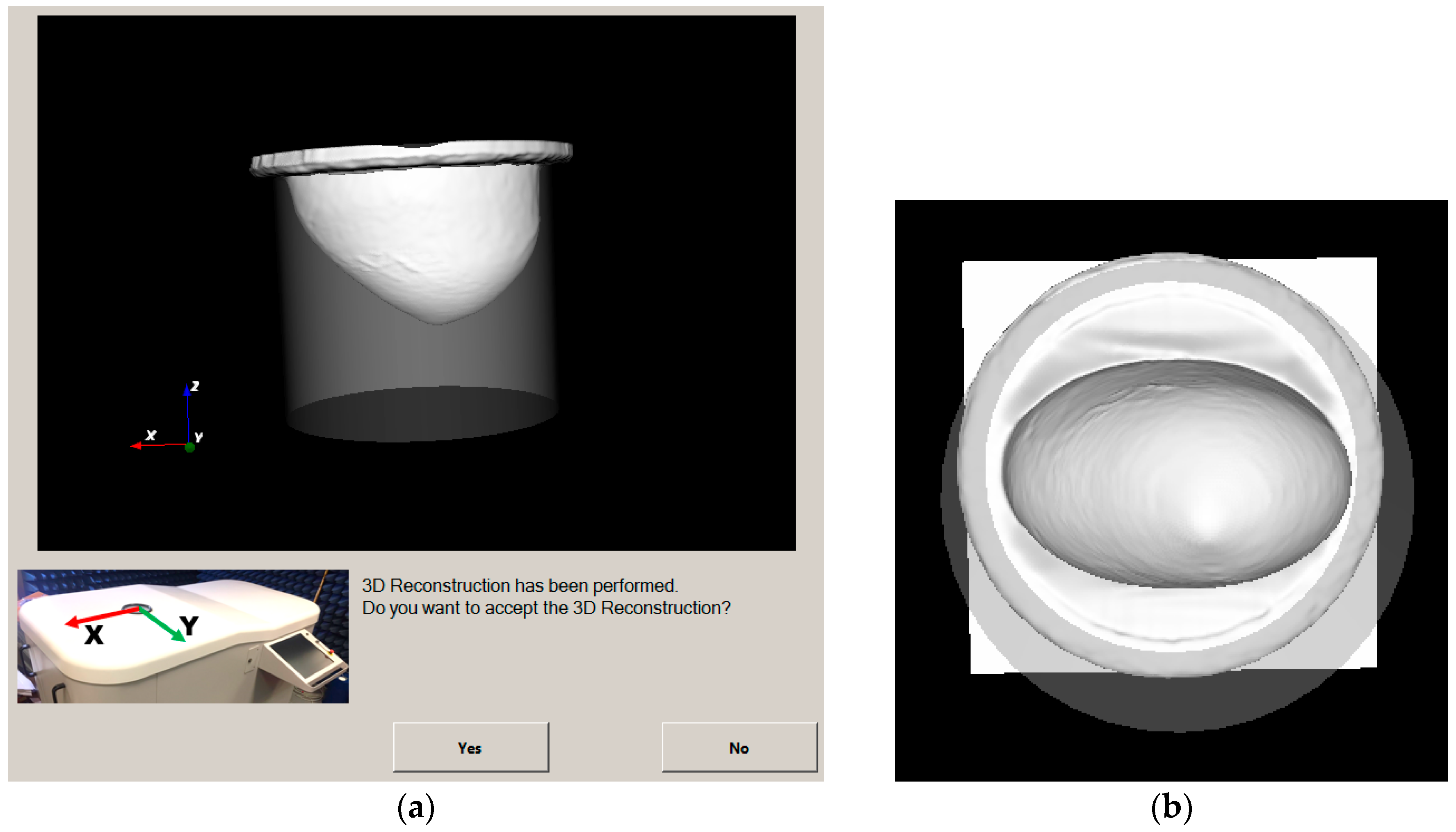
2.5. The Optical Breast Scan and Metrology
- ▪
- Compute the volume of the patient’s breast, thus indirectly deriving the required volume of transition liquid such that the container of the microwave breast imaging subsystem is optimally filled after immersion of the breast;
- ▪
- Compute the vertical extent of the pendulous breast, in order to optimally dimension the vertical scan of the microwave breast imaging system;
- ▪
- Reconstruct fully the external envelope of the breast, with high precision; such information will further serve to control the potential level of deformation of the breast due to immersion in the transition liquid during the microwave imaging scan. It may also serve as an intermediate step when registering the 3D microwave image with reference to the 2D mammographic projections of the patient’s breast, for comparison and validation of the microwave breast imaging modality.
2.6. The On-Site Validation Test Procedure for the Microwave Breast Imaging System
2.6.1. Controlled Environmental Conditions for Nominal Operation of the Imaging System
- ▪
- container filled with transition liquid: measurement at the center and close to the borders of the container
- ▪
- breast mold compartments filled with fibroglandular tissue-mimicking liquid: measurement at three different points, or compartments
2.6.2. System Stability Verification
- Verify that the amplitude envelope of the raw measured data keeps consistent with the lower and upper-level masks, as predefined at factory;
- Perform first and second-order statistics on raw measured data after drift correction: evaluate the stability, both in amplitude and phase, of the reference channel
- Perform first and second-order statistics on calibrated data: evaluate the multi-run stability, both in amplitude and phase, on a limited set of Tx/Rx couples.
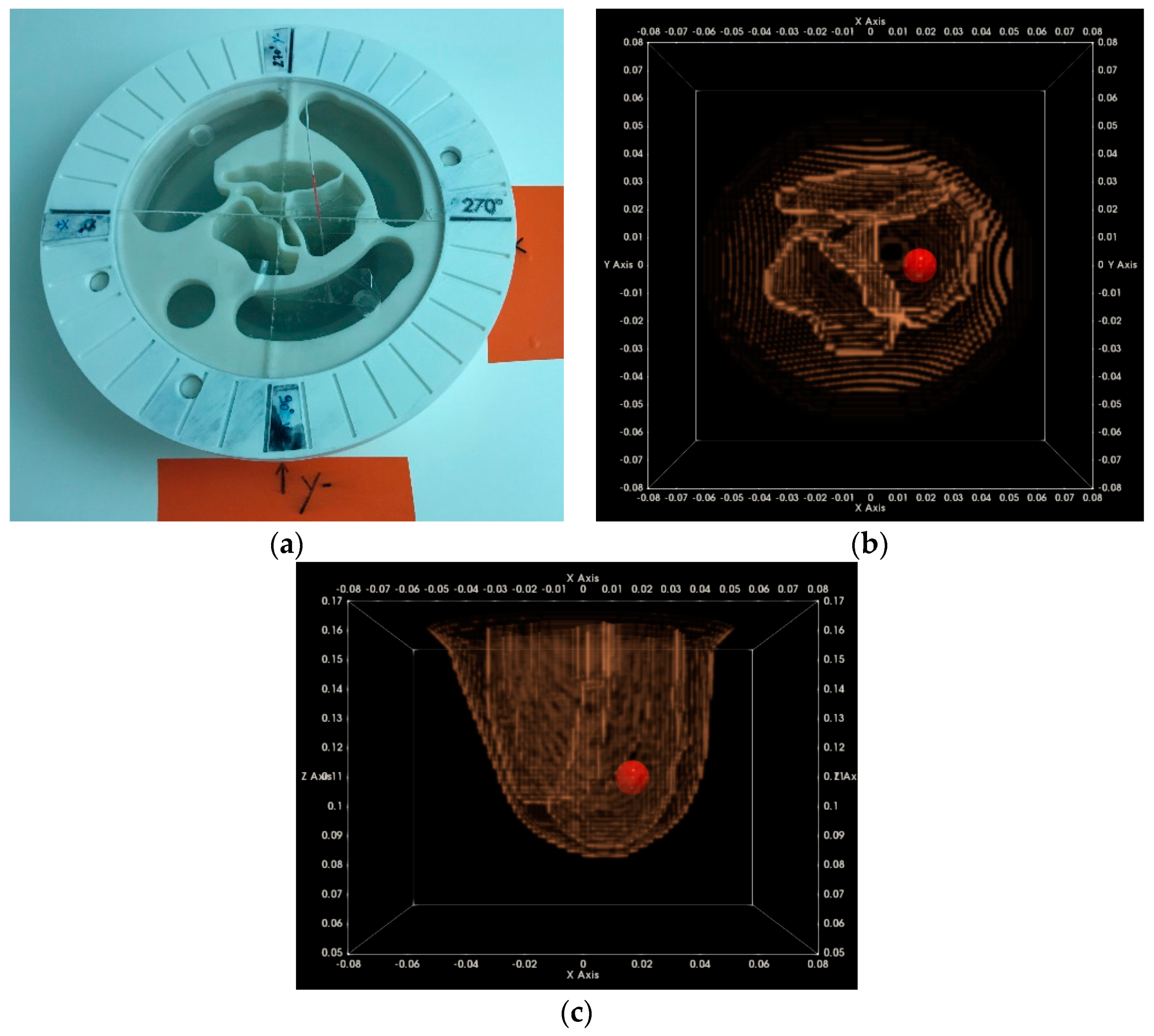
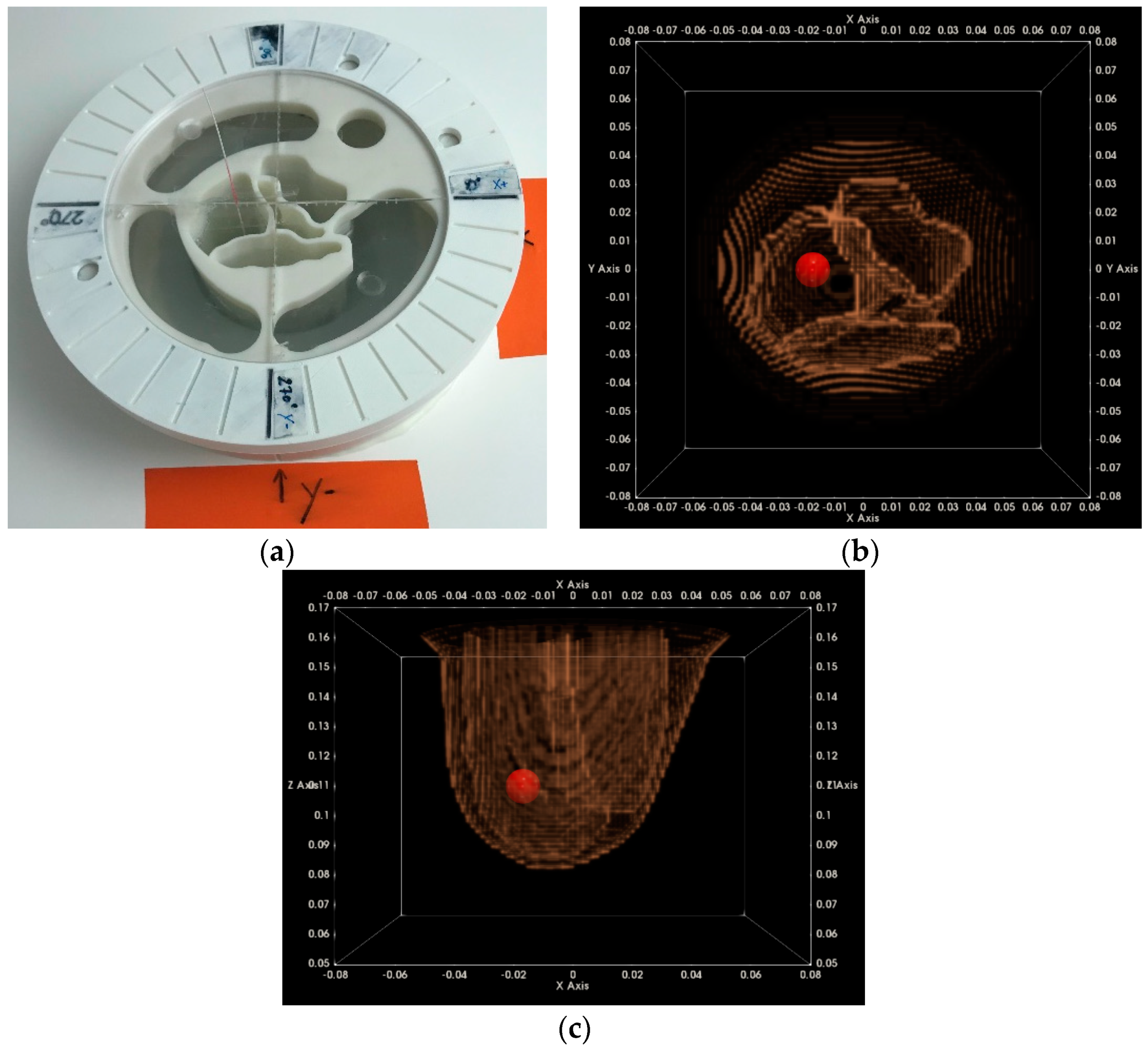
2.6.3. Imaging Test with Complex Breast Phantom at Two Azimuthal Rotational Positions
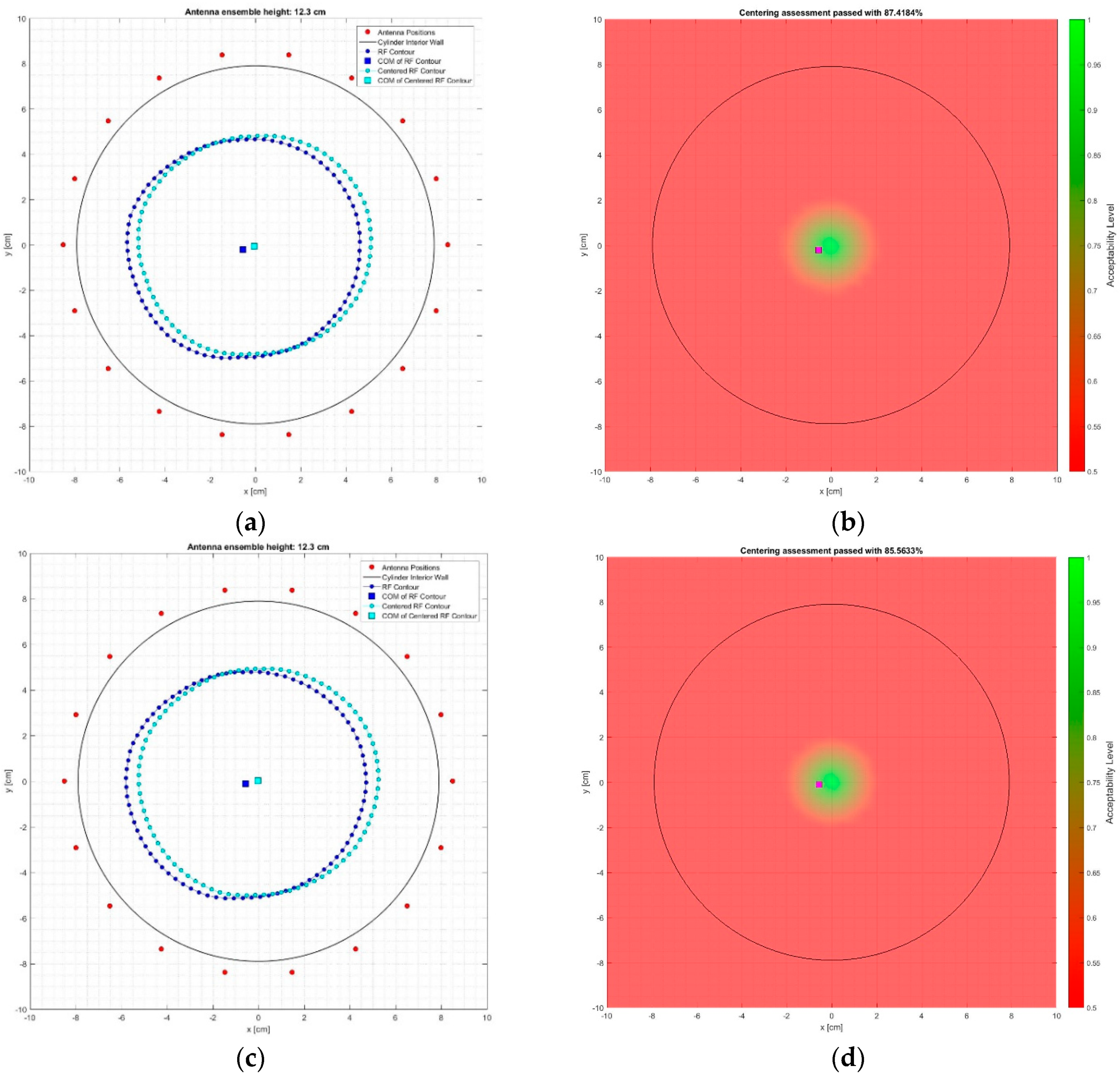
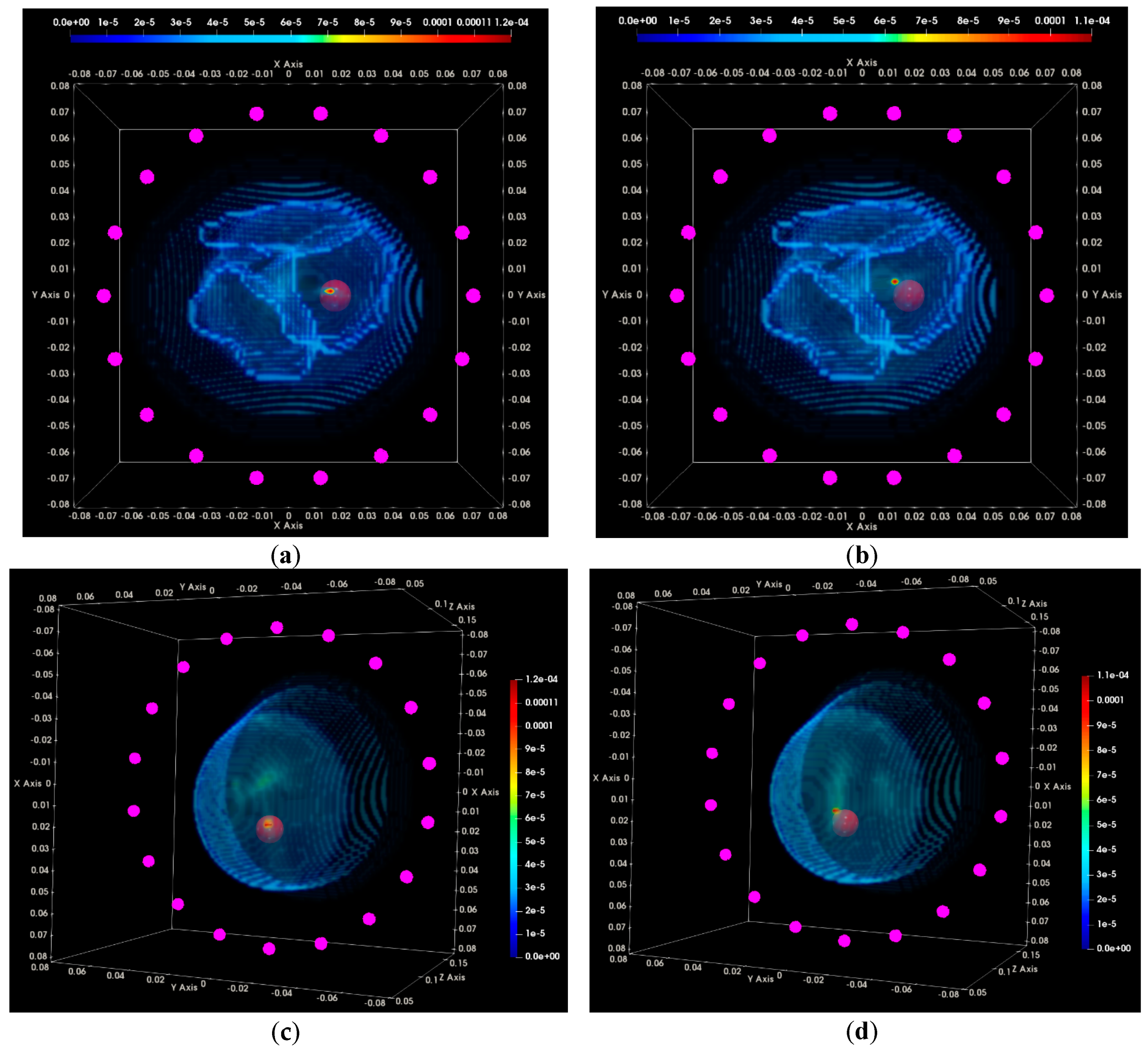
2.6.4. Centering Assessment of the Reconstructed Breast Outer Surface
2.6.5. Image Quality Assessment (QA) Metrics for System Performance Acceptance
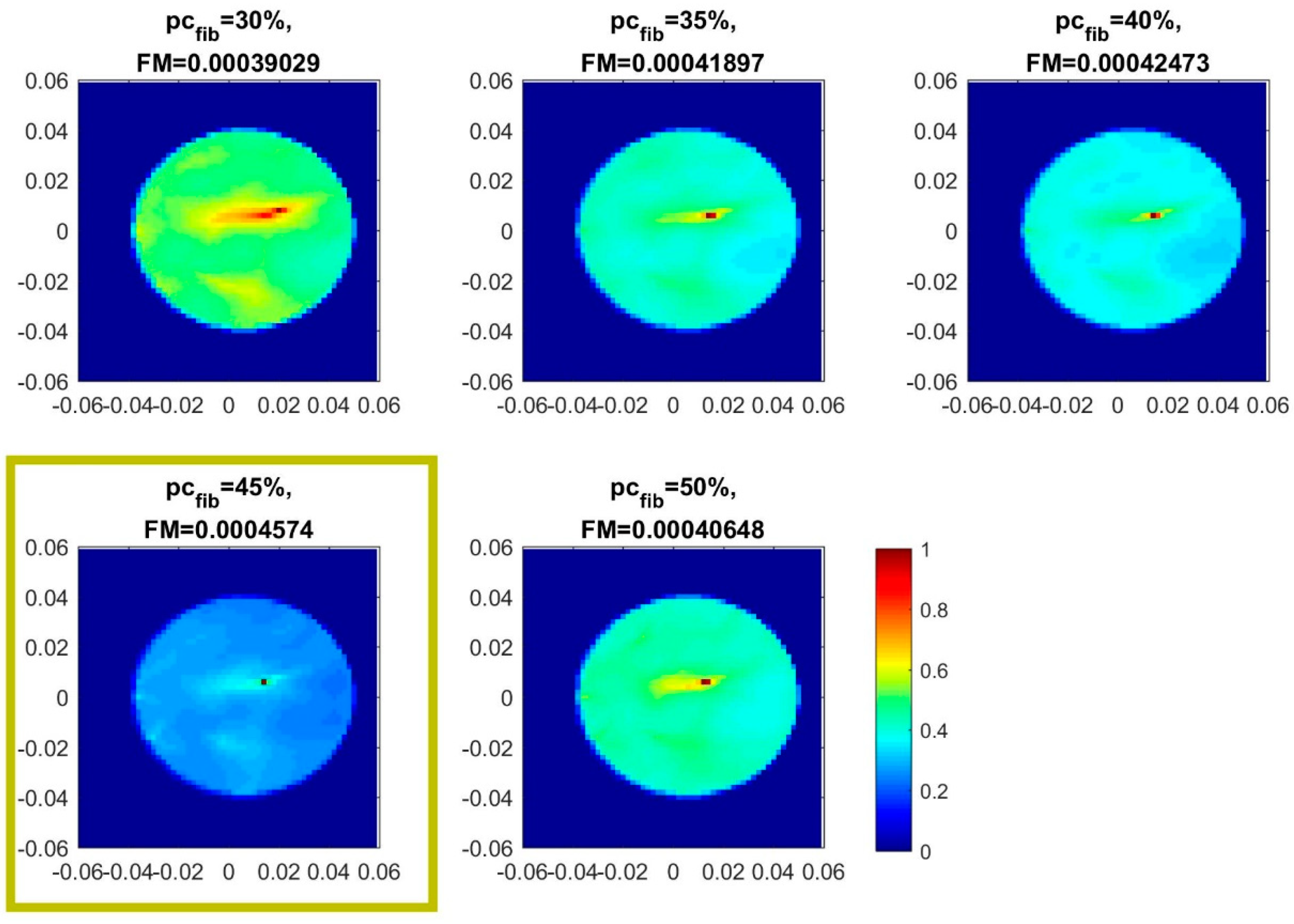
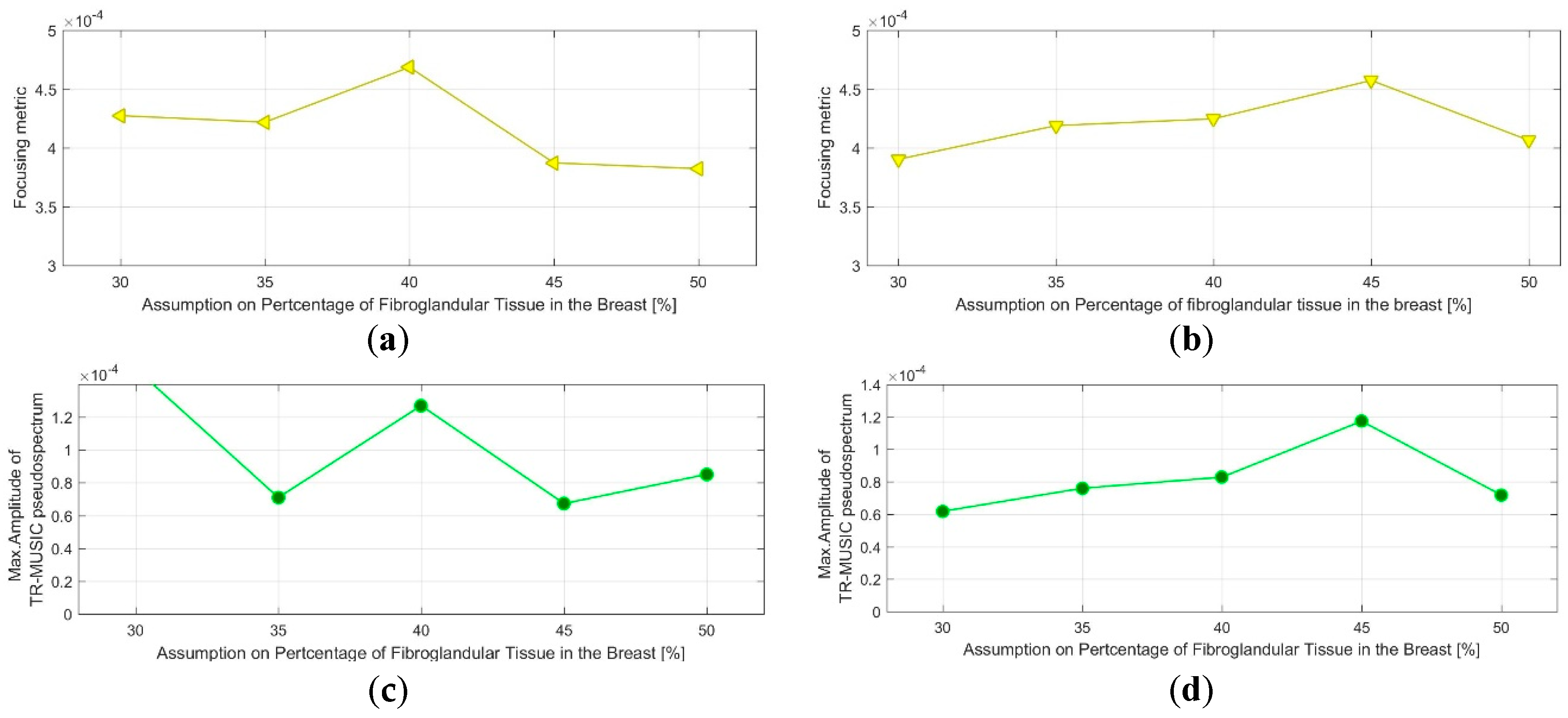
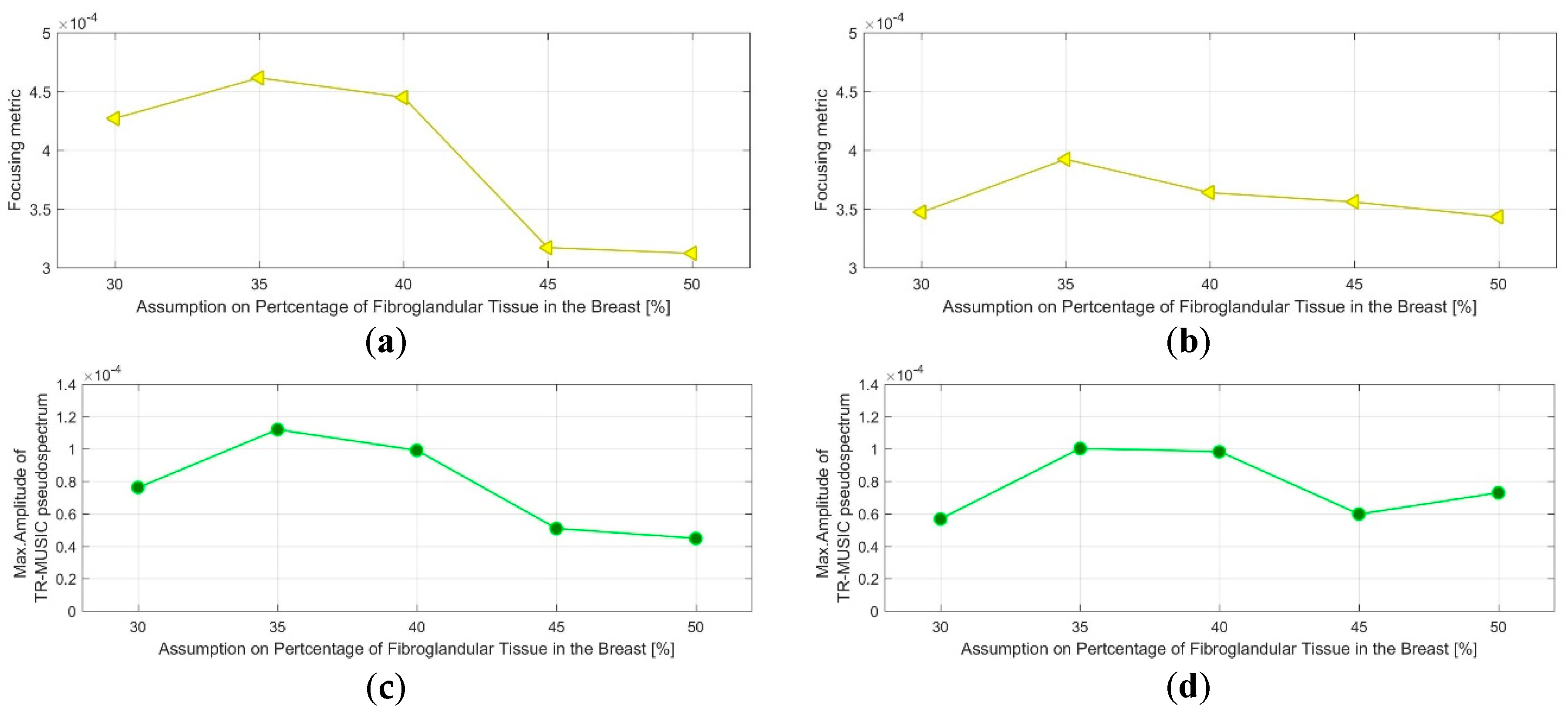
- QA Metric 1: Focusing Metric (FM) evaluated on the composite image formed at single vertical position of the sensor network (as per Equation (13)), in front of the tumor:
- ◌
- Acceptance Criterion (AC) #1: The optimal value, for which the focusing measure is maximized, should remain constant at every repetition of the controlled imaging test, and for every rotational position of the breast phantom (testing with intervals equal to 5%).
- ◌
- AC#2: the value of the focusing measure, for the optimal , should exceed a preset threshold value .
- QA Metric 2: Intensity of the TR-MUSIC pseudospectrum at the tumor location (Immax), evaluated on the composite image formed at a single vertical position of the sensor network (as per Equation (13)), in front of the tumor:
- ◌
- AC#3: The optimal value for which Immax is maximized should remain constant at every repetition of the controlled imaging test, and for every rotational position of the breast phantom (testing with intervals equal to 5%).
- ◌
- AC#4: The value of Immax, for the optimal value, should exceed a preset threshold value .
- ◌
- AC#5: The two patterns FM() and Immax() should be consistent with each other, meaning that maximization and identical slope(s) are observed for the same values on both patterns.
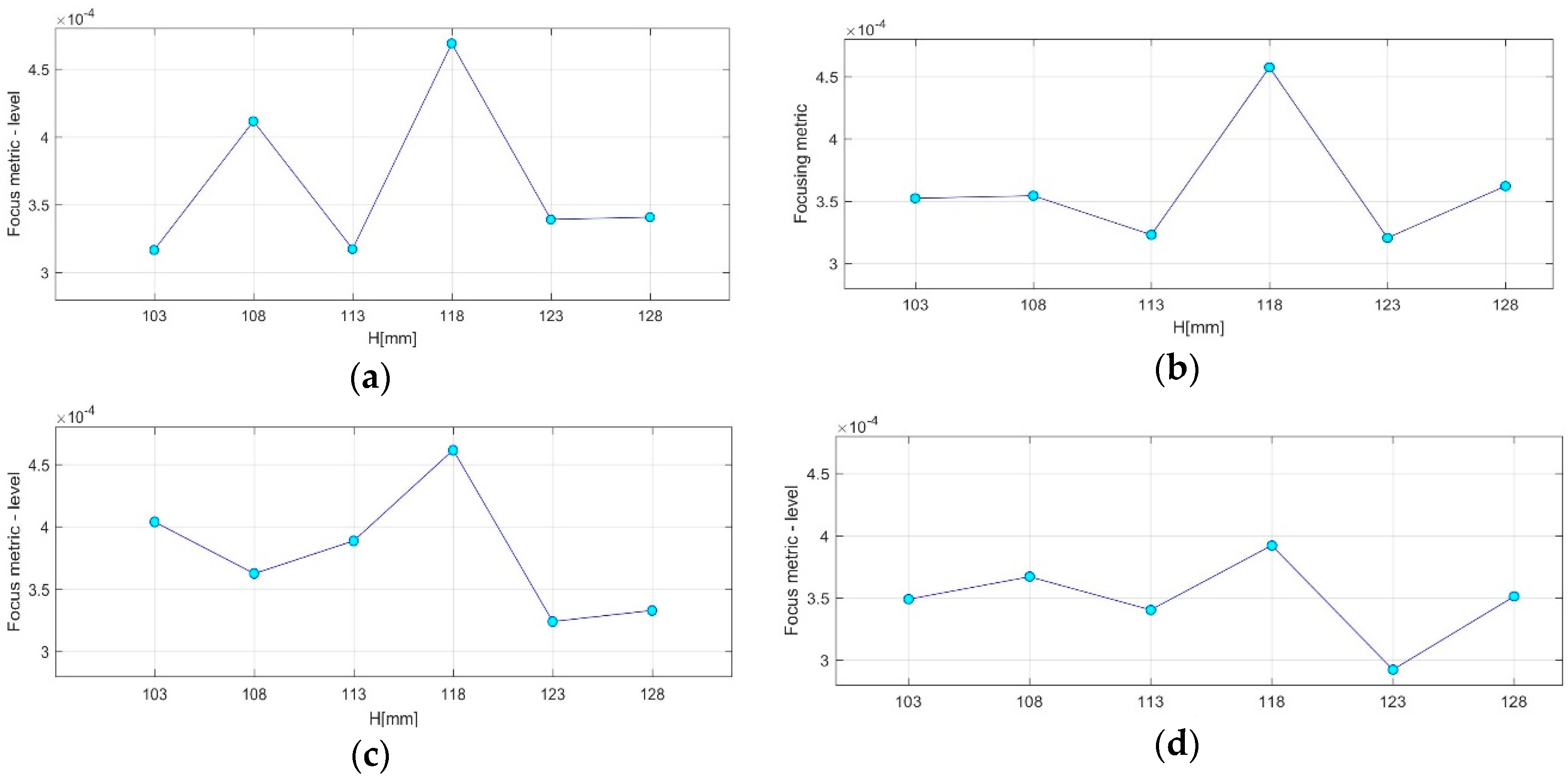
- QA Metric 3: Variation of the maximal achievable focusing FM over the height, evaluated for images formed using various vertical scan positions of the sensor network (as per Equation (14)):
- ◌
- AC#6: The maximal FM should be observed at the same height: the one closer to the tumor, at every repetition of the controlled imaging test, and for every rotational position of the breast phantom.
- ◌
- AC#7: The contrast between the maximal FM and the FM achievable at all of the other heights should exceed a given threshold .
- QA Metric 4: Ratio between the average image intensity at the exterior of the breast and the Immax in the interior of the breast: Evaluation on the composite image formed using data from multiple vertical scan positions of the sensor network (as per Equation (14)):
- ◌
- AC#8: The ratio should not exceed a preset upper-limit value .
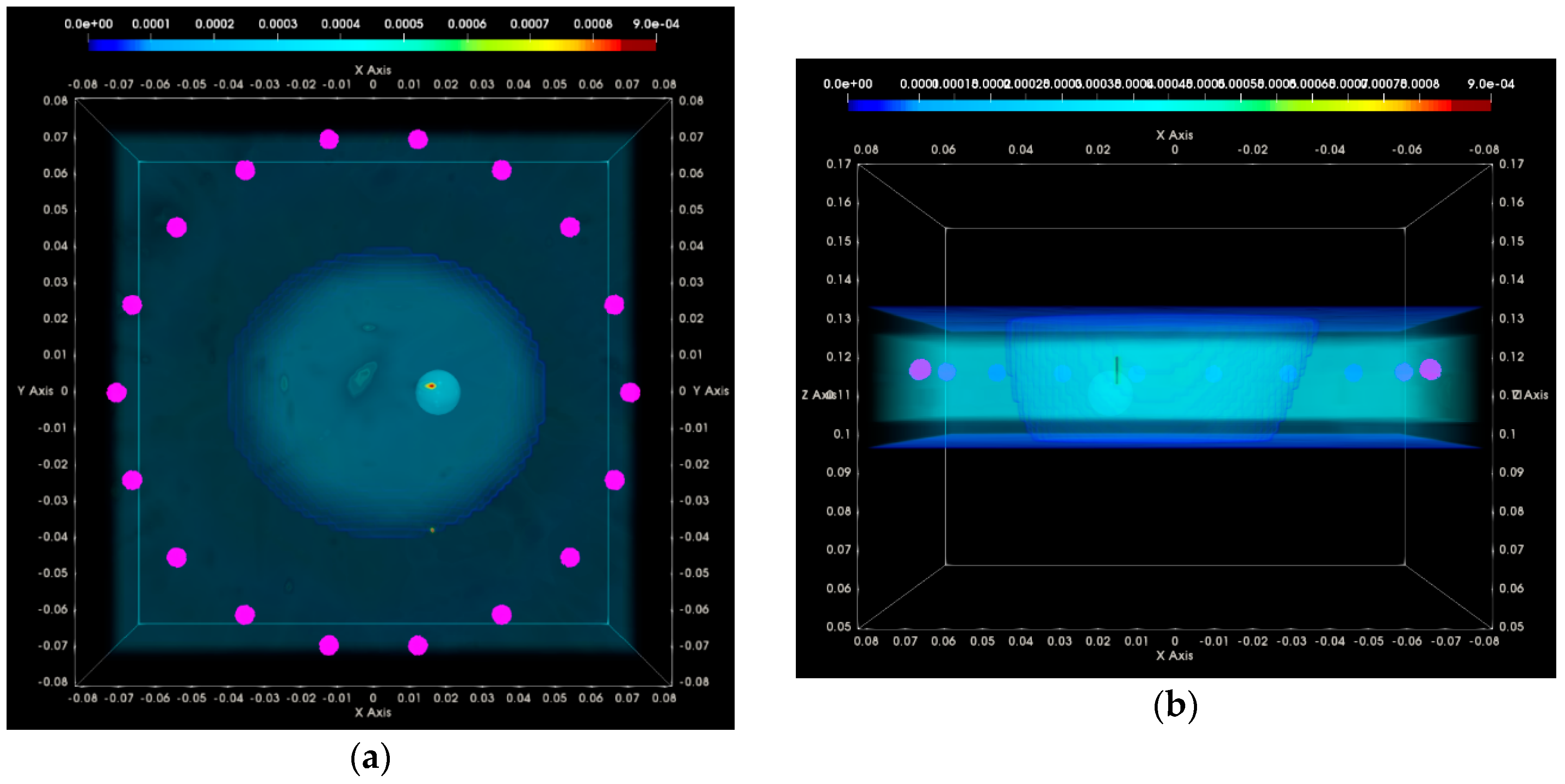
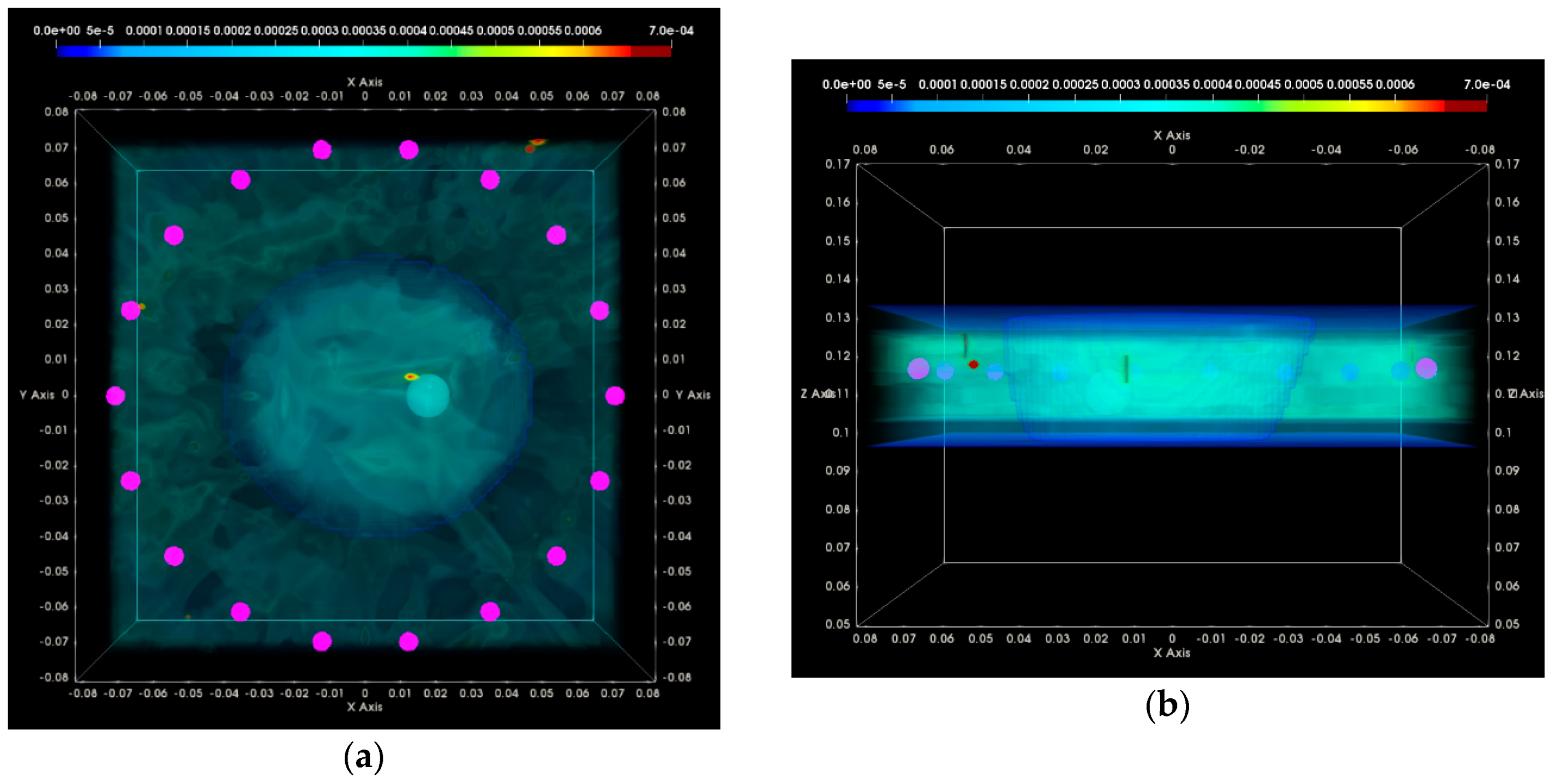
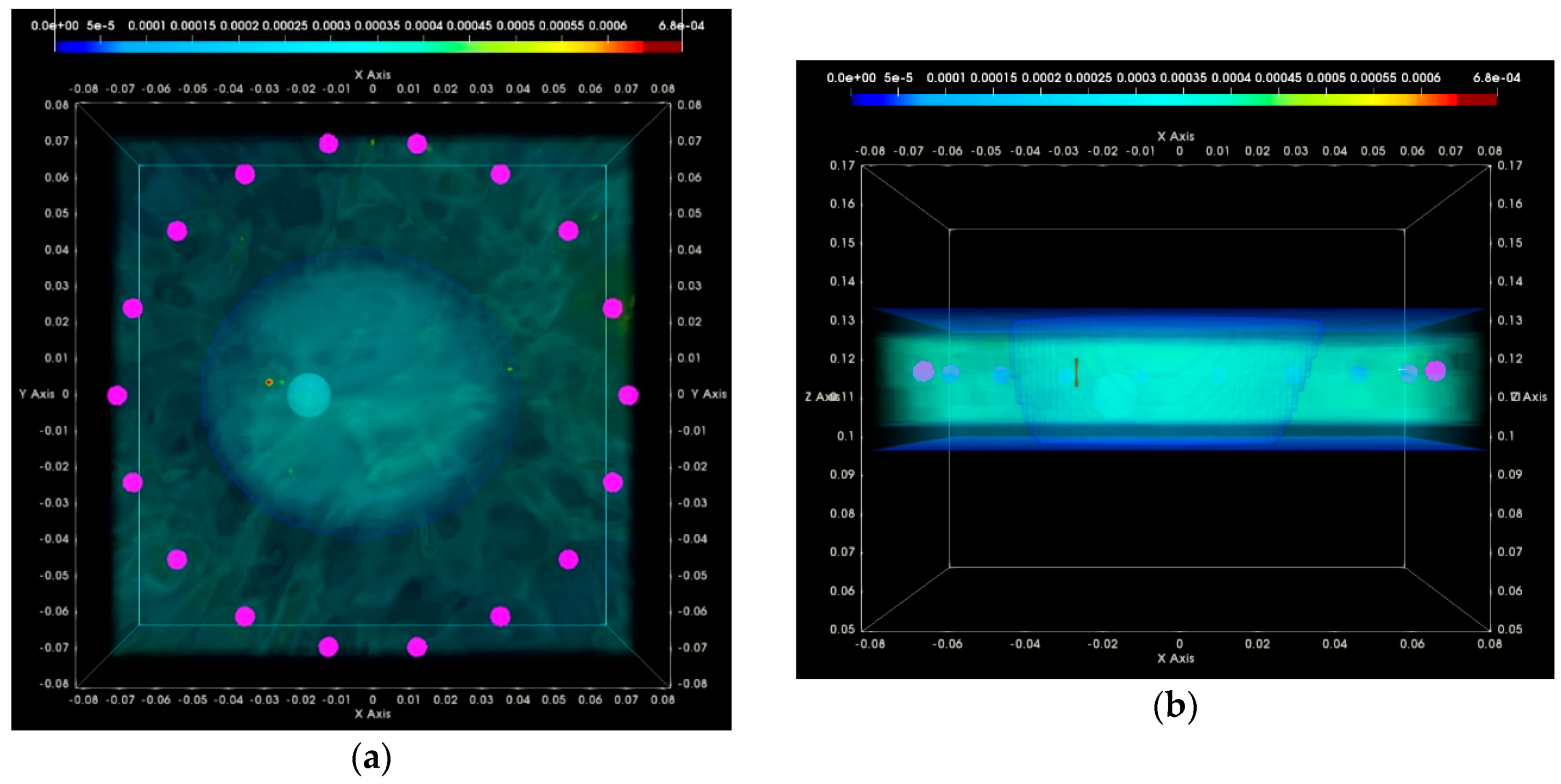
3. Results
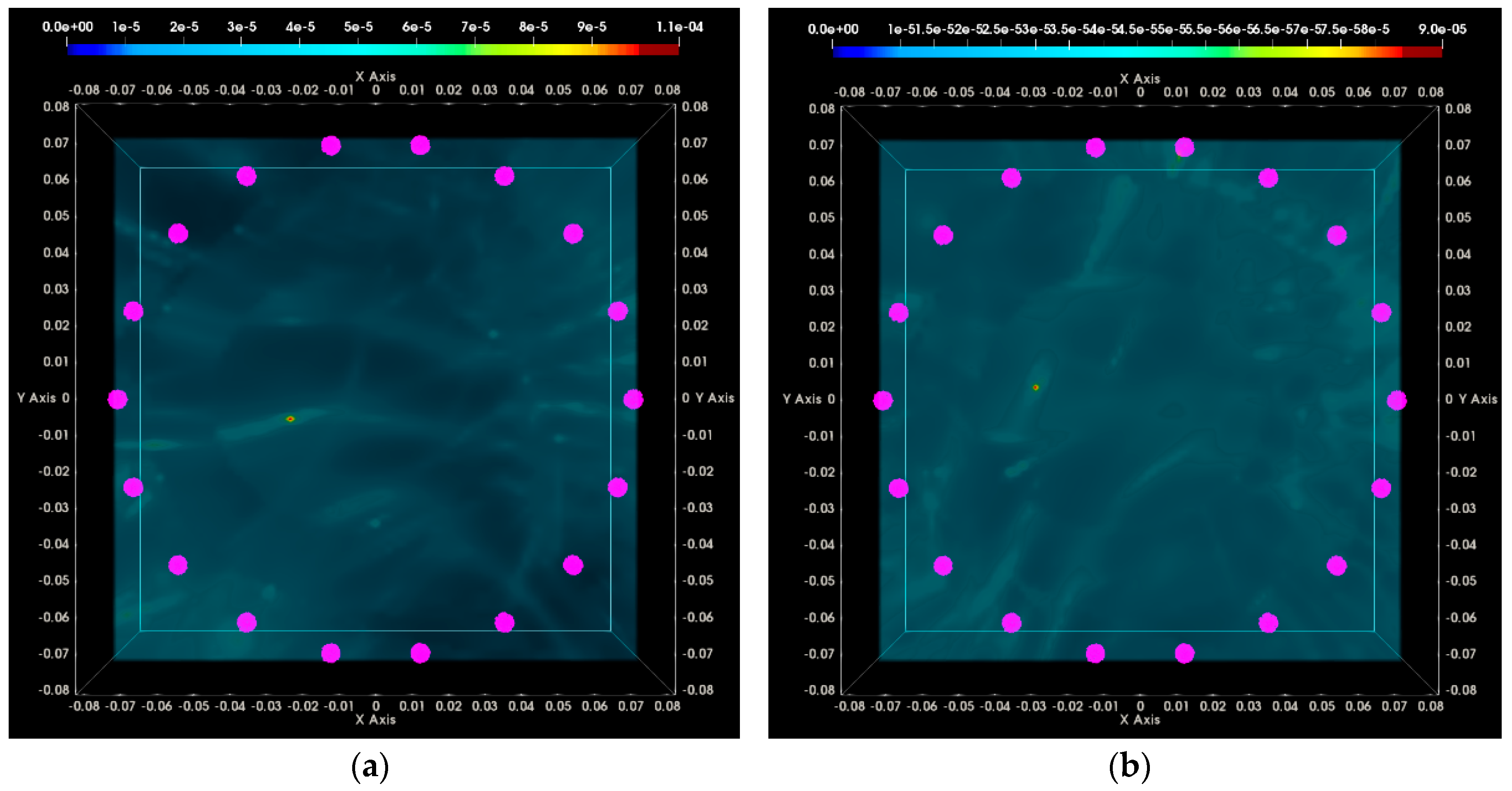
3.1. QA Metrics 1 and 2: Images Formed at Single Vertical Position of the Sensor Network
3.1.1. Breast Rotational Position #1
- ▪
- a given number Nfreq of frequency points, uniformly spanning the working frequency band,
- ▪
- a given number Nsec of sectors of antenna sub-arrays spanning the full 360° azimuth domain around the breast.
3.1.2. Breast Rotational Position #2
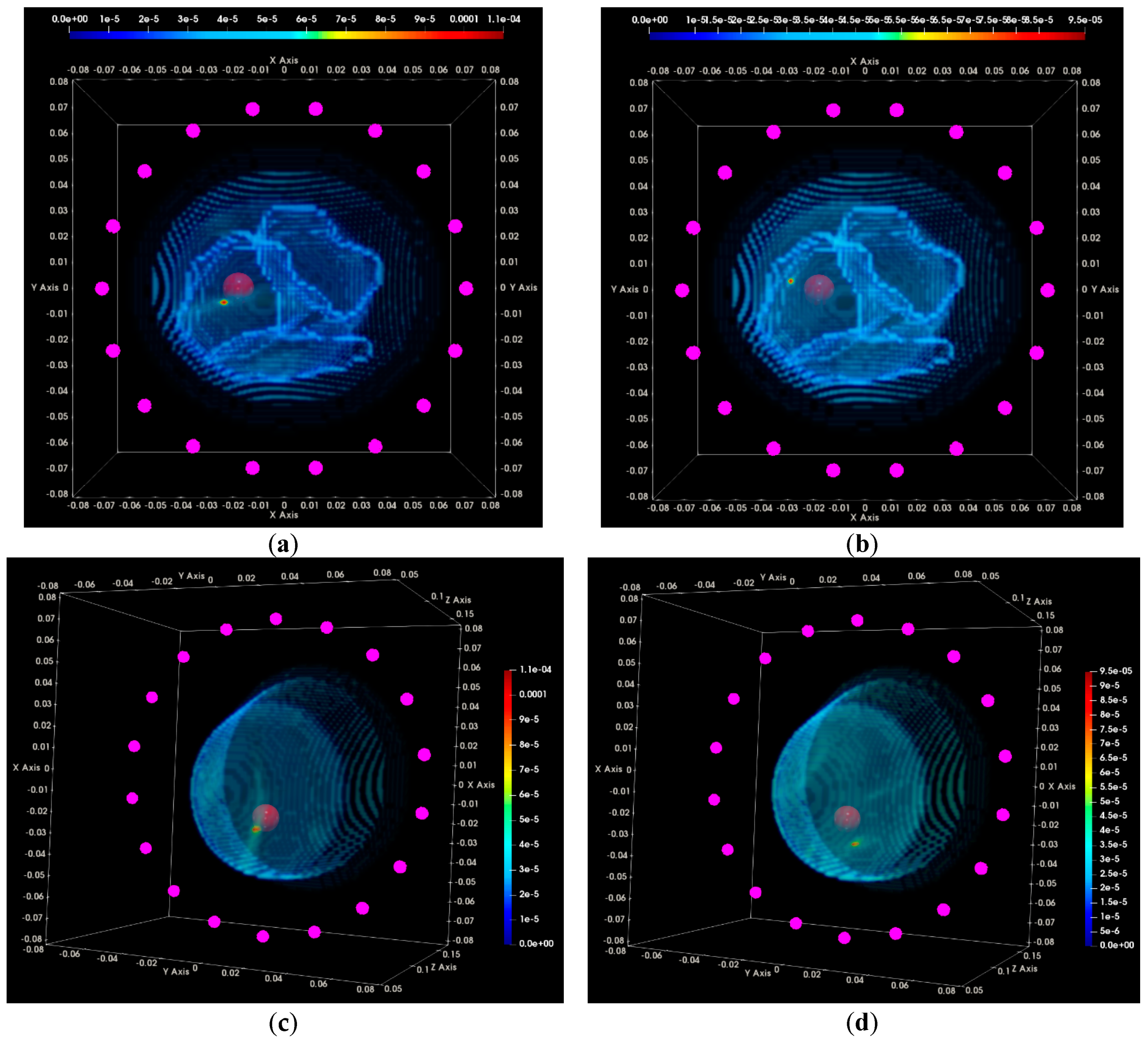
3.2. QA Metrics 3 and 4: Images Formed at Multiple Vertical Positions of the Sensor Network
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fear, E.C.; Stuchly, M.A. Microwave detection of breast cancer. IEEE Trans. Microw. Theory Tech. 2000, 48, 1854–1863. [Google Scholar] [CrossRef]
- Fear, E.C.; Hagness, S.C.; Meaney, P.M.; Okoniewski, M.; Stuchly, M.A. Enhancing breast tumor detection with near-field imaging. IEEE Microw. Mag. 2002, 3, 48–56. [Google Scholar] [CrossRef]
- Fear, E.C.; Meaney, P.M.; Stuchly, M.A. Microwaves for Breast Cancer Detection? IEEE Potentials 2003, 22, 12–18. [Google Scholar] [CrossRef]
- Klemm, M.; Craddock, I.; Leendertz, J.; Preece, A.; Benjamin, R. Experimental and clinical results of breast cancer detection using UWB microwave radar. In Proceedings of the 2008 IEEE Antennas and Propagation Society International Symposium, San Diego, CA, USA, 5–11 July 2008. [Google Scholar] [CrossRef]
- Porter, E.; Santorelli, A.; Coates, M.; Popovic, M. An experimental system for time-domain microwave breast imaging. In Proceedings of the 5th European Conference on Antennas and Propagation (EUCAP), Rome, Italy, 11–15 April 2011. [Google Scholar]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fanning, M.W.; Zhou, T.; Golnabi, A.; Geimer, S.D.; Paulsen, K.D. Clinical microwave breast imaging—2D results and the evolution to 3D. In Proceedings of the 2009 International Conference on Electromagnetics in Advanced Applications, Torino, Italy, 14–18 September 2009. [Google Scholar] [CrossRef]
- Meaney, P.M.; Kaufman, P.A.; Muffly, L.S.; Click, M.; Poplack, S.P.; Wells, W.A.; Schwartz, G.N.; di Florio-Alexander, R.M.; Tosteson, T.D.; Li, Z.; et al. Microwave imaging for neoadjuvant chemotherapy monitoring: Initial clinical experience. Breast Cancer Res. 2013, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Shere, M.; Preece, A.; Craddock, I.; Leendertz, J.; Klemm, M. Multistatic radar: First trials of a new breast imaging modality. Breast Cancer Res. 2009, 11 (Suppl. S2), O5. [Google Scholar] [CrossRef]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Porter, E.; Coates, M.; Popovic, M. An Early Clinical Study of Time-Domain Microwave Radar for Breast Health Monitoring. IEEE Trans. Biomed. Eng. 2016, 63, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; O’Halloran, M.J.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Conceição, R.; Mohr, J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Hadjiiski, L.; Sahiner, B.; Chan, H.-P. Advances in computer-aided diagnosis for breast cancer. Curr. Opin. Obstet. Gynecol. 2006, 18, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Rangayyan, R.M.; Xu, J.; el Naqa, I.; Yang, Y. Computer-aided detection and diagnosis of breast cancer with mammography: Recent advances. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nishikawa, R.M. Computer-Aided Detection and Diagnosis in Medical Imaging; Taylor & Francis: Thames, UK, 2010. [Google Scholar]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; Report N.AL/OE-TR-1996-0037; King’s College London: London, UK, 1996. [Google Scholar]
- Campbell, A.M.; Land, D.V. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gunawan, E.; Low, K.S.; Wang, S.C.; Soh, C.B.; Putti, T.C. Effect of lesion morphology on microwave signature in 2-D ultra-wideband breast imaging. IEEE Trans. Biomed. Eng. 2008, 55, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.K.; van Veen, B.D.; Hagness, S.C.; Kelcz, F. Breast tumor characterization based on ultrawideband microwave backscatter. IEEE Trans. Biomed. Eng. 2008, 55, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, R.C.; O’Halloran, M.; Capote, R.M.; Ferreira, C.S.; Matela, N.; Ferreira, H.A.; Glavin, M.; Jones, E.; Almeida, P. Development of breast and tumour models for simulation of novel multimodal PEM-UWB technique for detection and classification of breast tumours. In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), Anaheim, CA, USA, 27 October–3 November 2012. [Google Scholar] [CrossRef]
- Cherniakov, M. Bistatic Radar: Emerging Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Skolnik, M.I. Radar Handbook; McGraw-Hill Education–Europe: London, UK, 2008. [Google Scholar]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; van Veen, B.D.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Biomed. Eng. 2008, 55, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, T.; Kubota, S.I.; Kuroki, S.I.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex permittivities of breast tumor tissues obtained from cancer surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric Properties Characterization From 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2016, 65, 998–1011. [Google Scholar] [CrossRef]
- Conceição, R.C.; O’Halloran, M.; Glavin, M.; Jones, E. Numerical modelling for ultra wideband radar breast cancer detection and classification. Prog. Electromagn. Res. B 2011, 34, 145–171. [Google Scholar] [CrossRef]
- Fasoula, A.; Bernard, J.; Robin, G.; Duchesne, L. Elaborated breast phantoms and experimental benchmarking of a microwave breast imaging system before first clinical studyTitle. In Proceedings of the 12th European Conference on Antennas and Propagation 2018, London, UK, 9–13 April 2018. [Google Scholar]
- Fasoula, A.; Anwar, S.; Toutain, Y.; Duchesne, L. Microwave vision: From RF safety to medical imaging. In Proceedings of the 11th European Conference on Antennas and Propagation, Paris, France, 19–24 March 2017. [Google Scholar] [CrossRef]
- Joachimowicz, N.; Conessa, C.; Henriksson, T.; Duchêne, B. Breast phantoms for microwave imaging. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 1333–1336. [Google Scholar] [CrossRef]
- Garrett, J.; Fear, E. A New Breast Phantom with a Durable Skin Layer for Microwave Breast Imaging. IEEE Trans. Antennas Propag. 2015, 63, 1693–1700. [Google Scholar] [CrossRef]
- Meaney, P.M.; Shubitidze, F.; Fanning, M.W.; Kmiec, M.; Epstein, N.R.; Paulsen, K.D. Surface wave multipath signals in near-field microwave imaging. Int. J. Biomed. Imaging 2012, 2012, 697253. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.; Fasoula, A.; Duchesne, L. RF-based Breast Surface Estimation—Registration with Reference Imaging Modality. In Proceedings of the IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Boston, MA, USA, 8–13 July 2018. [Google Scholar]
- Hyvärinen, A.; Oja, E. Independent Component Analysis: Algorithms and Applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Karhunen, J.; Oja, E. Applications of ICA. Feature Extraction by ICA. Indep. Compon. Anal. 2001, 1, 391–405. [Google Scholar]
- Eguizabal, A.; Laughney, A.M.; García-Allende, P.B.; Krishnaswamy, V.; Wells, W.A.; Paulsen, K.D.; Pogue, B.W.; Lopez-Higuera, J.M.; Conde, O.M. Direct identification of breast cancer pathologies using blind separation of label-free localized reflectance measurements. Biomed. Opt. Express 2013, 4, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Laughney, A.M.; Krishnaswamy, V.; Rizzo, E.J.; Schwab, M.C.; Barth, R.J.; Cuccia, D.J.; Tromberg, B.J.; Paulsen, K.D.; Pogue, B.W.; Wells, W.A. Spectral discrimination of breast pathologies in situ using spatial frequency domain imaging. Breast Cancer Res. 2013, 15, R61. [Google Scholar] [CrossRef] [PubMed]
- Chaumette, E.; Comon, P.; Muller, D. ICA-based technique for radiating sources estimation: Application to airport surveillance. IEE Proc. Part F Radar Signal Process. 1993, 140, 395–401. [Google Scholar] [CrossRef]
- Gaikwad, A.N.; Singh, D.; Nigam, M.J. Application of clutter reduction techniques for detection of metallic and low dielectric target behind the brick wall by stepped frequency continuous wave radar in ultra-wideband range. IET Radar Sonar Navig. 2011, 5, 416–425. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Vanhala, J. Separating respiration artifact in microwave Doppler radar heart monitoring by independent component analysis. In Proceedings of the 2010 IEEE Sensors, Kona, HI, USA, 1–4 November 2010. [Google Scholar] [CrossRef]
- Zarzoso, V.; Comon, P. Automated Extraction of Atrial Fibrillation Activity from the Surface ECG Using Independent Component Analysis in the Frequency Domain. In Proceedings of the Medical Physics and Biomedical Engineering World Congress, Munchen, Germany, 7–12 September 2009. [Google Scholar]
- Stoica, P.; Moses, R.L.; Hall, P. Introduction to Spectral Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar] [CrossRef]
- DeCarlo, L.T. On the Meaning and Use of Kurtosis. Psychol. Methods 1997, 2, 292–307. [Google Scholar] [CrossRef]
- Aly, O.A.M.; Omar, A.S. Detection and localization of RF radar pulses in noise environments using wavelet packet transforn and higher order statistics. Prog. Electromagn. Res. 2006, 58, 301–317. [Google Scholar] [CrossRef]
- Devaney, A.J. Super-Resolution Processing of Multi-Static Data Using Time Reversal and MUSIC, Northeastern University Report. Available online: http://www.ece.neu.edu/faculty/ devaney/ajd/preprints.htm (accessed on 23 March 2018).
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Elsevier: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Gruber, F.K.; Marengo, E.A.; Devaney, A.J. Time-reversal imaging with multiple signal classification considering multiple scattering between the targets. J. Acoust. Soc. Am. 2004, 115, 3042–3047. [Google Scholar] [CrossRef]
- Marengo, E.A.; Gruber, F.K.; Simonetti, F. Time-reversal MUSIC imaging of extended targets. IEEE Trans. Image Process. 2007, 16, 1967–1984. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, P.; Rappaport, C.M. Time reversal with the FDTD method for microwave breast cancer detection. IEEE Trans. Microw. Theory Tech. 2005, 53, 2317–2323. [Google Scholar] [CrossRef]
- Kosmas, P.; Rappaport, C.M. A matched-filter FDTD-based time reversal approach for microwave breast cancer detection. IEEE Trans. Antennas Propag. 2006, 54, 1257–1264. [Google Scholar] [CrossRef]
- Kosmas, P. Application of the DORT technique to FDTD-Based time reversal for microwave breast cancer detection. In Proceedings of the 37th European Microwave Conference, Munich, Germany, 9–12 October 2007. [Google Scholar] [CrossRef]
- Kosmas, P.; Laranjeira, S.; Dixon, J.H.; Li, X.; Chen, Y. Time reversal microwave breast imaging for contrast-enhanced tumor classification. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar] [CrossRef]
- Hossain, M.D.; Mohan, A.S.; Abedin, M.J. Beamspace Time-Reversal Microwave Imaging for Breast Cancer Detection. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 241–244. [Google Scholar] [CrossRef]
- Hossain, M.D.; Mohan, A.S. Cancer Detection in Highly Dense Breasts Using Coherently Focused Time-Reversal Microwave Imaging. IEEE Trans. Comput. Imaging 2017, 3, 928–939. [Google Scholar] [CrossRef]
- Stoica, P.; Selen, Y. Model-order selection. IEEE Signal Process. Mag. 2004, 21, 36–47. [Google Scholar] [CrossRef]
- Pertuz, S.; Puig, D.; Garcia, M.A. Analysis of focus measure operators for shape-from-focus. Pattern Recognit. 2013, 46, 1415–1432. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Krewer, F.; Glavin, M.; Jones, E.; O’Halloran, M. Estimating average dielectric properties for microwave breast imaging using focal quality metrics. In Proceedings of the 2016 10th European Conference on Antennas and Propagation, Davos, Switzerland, 10–15 April 2016. [Google Scholar] [CrossRef]
- O’loughlin, D.; Krewer, F.; Glavin, M.; Jones, E.; O’halloran, M. Focal quality metrics for the objective evaluation of confocal microwave images. Int. J. Microw. Wirel. Technol. 2017, 9, 1365–1372. [Google Scholar] [CrossRef]
- Registered Cinical Trial Protocol. Available online: https://clinicaltrials.gov/ct2/show/NCT03475992 (accessed on 23 March 2018).








| Measures | Breast Phantom #1 | Breast Phantom #2 | ||
|---|---|---|---|---|
| Site | Factory | Site | Factory | |
| Breast Volume (mL) | 698 | 696 | 1099 | 1097 |
| Breast Vertical Extent (mm) | 85 | 84 | 108 | 108 |
| Transition Liquid | Fibroglandular Tissue | ||
|---|---|---|---|
| Before the Scan | After the Scan | Before the Scan | After the Scan |
| 22.7 °C–23.3 °C | 23.0 °C–24.1 °C | 20.6 °C | 21.2 °C |
| Breast | Test Date 1 | Test Date 2 |
|---|---|---|
| Rotational position #1 | 82.97% | 83.40% |
| Rotational position #2 | 87.42% | 85.86% |
| Breast | Test Date 1 | Test Date 2 |
|---|---|---|
| Rotational position #1 | 7.41% | 10.38% |
| Rotational position #2 | 4.68% | 11.42% |
| Acceptance Test (AC) | Rotational Position #1 | Rotational Position #2 | ||
|---|---|---|---|---|
| Test Date 1 | Test Date 2 | Test Date 1 | Test Date 2 | |
| #1 | - | - | - | - |
| #2 | + | + | + | × |
| #3 | - | - | - | - |
| #4 | + | + | + | - |
| #5 | - | + | + | + |
| #6 | + | + | + | + |
| #7 | - | + | - | × |
| #8 | + | - | + | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Lawrence, P.; Robin, G.; Bernard, J.-G. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics 2018, 8, 53. https://doi.org/10.3390/diagnostics8030053
Fasoula A, Duchesne L, Gil Cano JD, Lawrence P, Robin G, Bernard J-G. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics. 2018; 8(3):53. https://doi.org/10.3390/diagnostics8030053
Chicago/Turabian StyleFasoula, Angie, Luc Duchesne, Julio Daniel Gil Cano, Peter Lawrence, Guillaume Robin, and Jean-Gael Bernard. 2018. "On-Site Validation of a Microwave Breast Imaging System, before First Patient Study" Diagnostics 8, no. 3: 53. https://doi.org/10.3390/diagnostics8030053
APA StyleFasoula, A., Duchesne, L., Gil Cano, J. D., Lawrence, P., Robin, G., & Bernard, J.-G. (2018). On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics, 8(3), 53. https://doi.org/10.3390/diagnostics8030053






