ALTEA: A Software Tool for the Evaluation of New Biomarkers for Alzheimer’s Disease by Means of Textures Analysis on Magnetic Resonance Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Data Acquisition
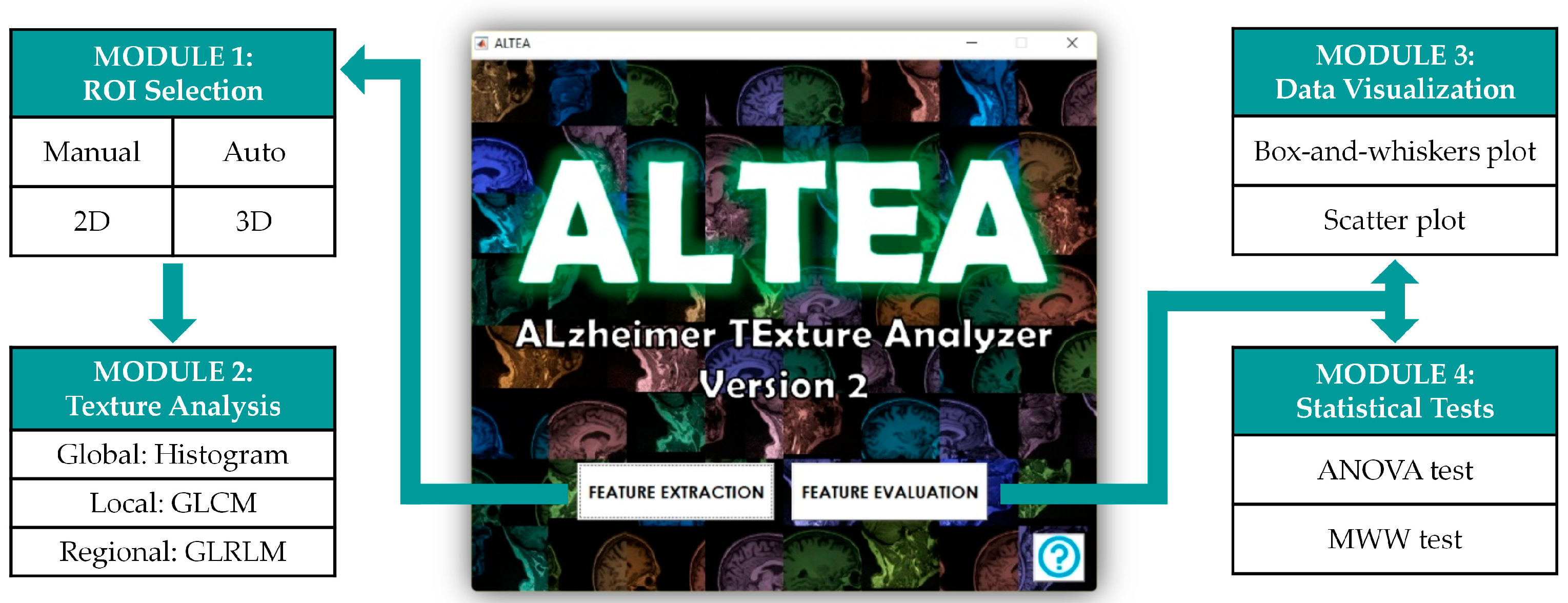
2.2. Software
2.3. Regions of Interest
2.4. Texture Analysis
2.5. Statistical Analysis
3. Results
3.1. ALTEA Software Tool
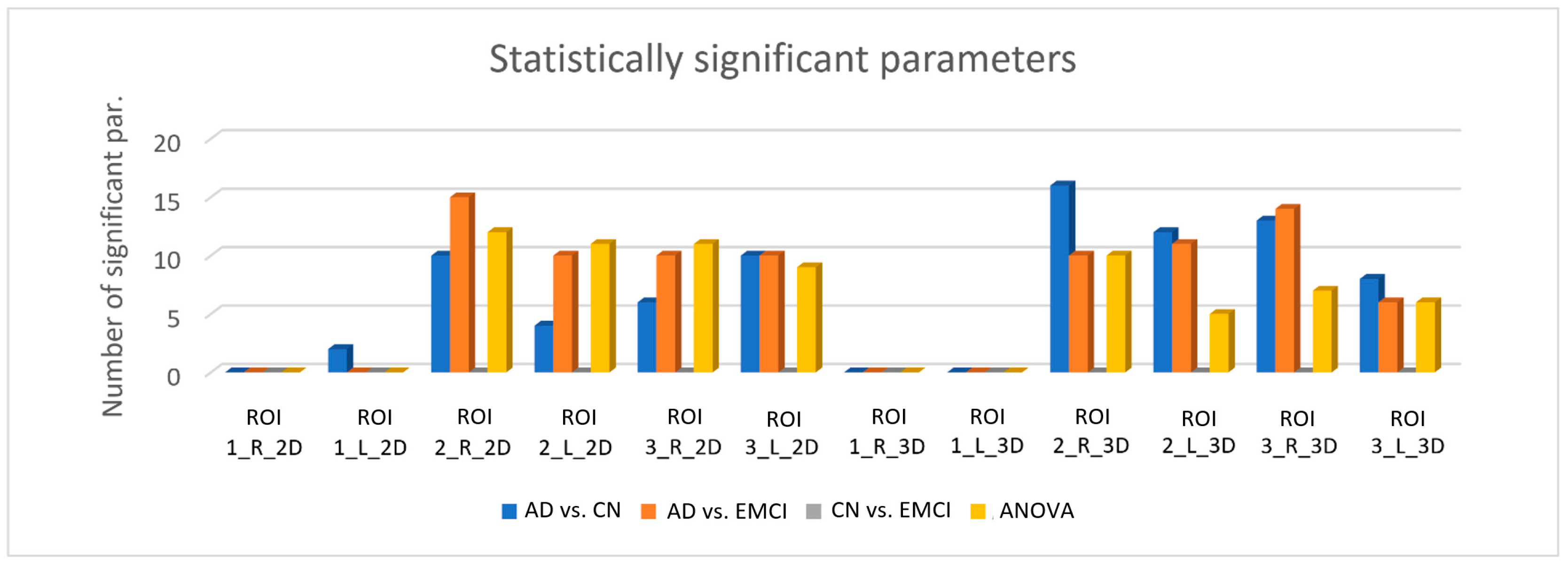
3.2. Results from the Statistical Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ROI | Hippocampus | AD vs. CN | AD vs. EMCI | CN vs. EMCI |
|---|---|---|---|---|
| ROI1 | Right | - | - | - |
| Left | Sum Average, Autocorrelation | - | - | |
| ROI2 | Right | Global Variance, Skewness, Correlation, Sum Average, Autocorrelation, HGRE, SRHGE, LRHGE, GLV, RLV | Global Variance, Kurtosis, Energy, Entropy, Homogeneity, Correlation, Sum Average, Variance, Dissimilarity, Autocorrelation, GLN, HGRE, SRHGE, LRHGE, RLV | - |
| Left | Global Variance, Skewness, Correlation, Autocorrelation | Global Variance, Kurtosis, Correlation, Sum Average, Variance, Autocorrelation, HGRE, SRHGE, LRHGE, RLV | - | |
| ROI3 | Right | Global Variance, Kurtosis, Contrast, Correlation, Dissimilarity, Autocorrelation | Global Variance, Kurtosis, Correlation, Sum Average, Variance, Dissimilarity, Autocorrelation, HGRE, SRHGE, LRHGE | - |
| Left | Global Variance, Kurtosis, Correlation, Sum Average, Variance, Autocorrelation, GLN, HGRE, SRHGE, LRHGE | Global Variance, Kurtosis, Correlation, Variance, Autocorrelation, GLN, HGRE, SRHGE, LRHGE, RLV | - |
| ROI | Hippocampus | AD vs. CN | AD vs. EMCI | CN vs. EMCI |
|---|---|---|---|---|
| ROI1 | Right | - | - | - |
| Left | - | - | - | |
| ROI2 | Right | Global Variance, Kurtosis, Energy, Contrast, Homogeneity, Correlation, Sum Average, Dissimilarity, Autocorrelation, SRE, LRE, RLN, RP, HGRE, SRHGE, LRHGE | Global Variance, Contrast, Homogeneity, Correlation, Dissimilarity, Autocorrelation, SRE, LRE, RLN, RP | - |
| Left | Global Variance, Kurtosis, Contrast, Correlation, Sum Average, Variance, Autocorrelation, Dissimilarity, GLN, HGRE, SRHGE, LRHGE | Global Variance, Skewness, Contrast, Homogeneity, Correlation, Dissimilarity, Autocorrelation, SRE, LRE, HGRE, SRHGE, RLN, RP | - | |
| ROI3 | Right | Global Variance, Kurtosis, Energy, Contrast, Entropy, Homogeneity, Correlation, Sum Average, Variance, Dissimilarity, Autocorrelation, GLN, LRHGE | Global Variance, Skewness, Kurtosis, Contrast, Homogeneity, Correlation, Sum Average, Dissimilarity, Autocorrelation, SRE, LRE, GLN, RLN, RP | - |
| Left | Global Variance, Kurtosis, Contrast, Correlation, Sum Average, Dissimilarity, Autocorrelation, GLN | Global Variance, Kurtosis, Contrast, Correlation, Dissimilarity, Autocorrelation | - |
References
- Wortmann, M. Dementia: A global health priority—Highlights from an ADI and World Health Organization report. Alzheimers. Res. Ther. 2012, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International (ADI): London, UK, 2016. [Google Scholar]
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017, 13, 325–373. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain Imaging in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.E.; Savage, G.; Villemagne, V.L.; Ng, S.; Moss, S.A.; Maruff, P.; Mathis, C.A.; Klunk, W.E.; Masters, C.L.; Rowe, C.C. β-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer’s disease. Brain 2007, 130, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Quiroz, Y.T.; Fleisher, A.S.; Chen, K.; Velez-Pardo, C.; Jimenez-Del-Rio, M.; Fagan, A.M.; Shah, A.R.; Alvarez, S.; Arbelaez, A.; et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. Lancet Neurol. 2012, 11, 1048–1056. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Petrella, J.R.; Coleman, R.E.; Doraiswamy, P.M. Neuroimaging and Early Diagnosis of Alzheimer Disease: A Look to the Future. Radiology 2003, 226, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Shiung, M.M.; Gunter, J.L.; O’Brien, P.C.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Ivnik, R.J.; Smith, G.E.; Cha, R.H.; et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 2004, 62, 591–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scahill, R.I.; Schott, J.M.; Stevens, J.M.; Rossor, M.N.; Fox, N.C. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. USA 2002, 99, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, L.-O.; Almkvist, O.; Blennow, K.; Engedahl, K.; Johansson, A.; Waldemar, G.; Wolf, H. Evidence-based Evaluation of Magnetic Resonance Imaging as a Diagnostic Tool in Dementia Workup. Top. Magn. Reson. Imaging 2005, 16, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Leandrou, S.; Mamais, I.; Petroudi, S.; Kyriacou, P.A.; Reyes-Aldasoro, C.C.; Pattichis, C.S. Hippocampal and entorhinal cortex volume changes in Alzheimer’s disease patients and mild cognitive impairment subjects. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 235–238. [Google Scholar]
- Cuingnet, R.; Gerardin, E.; Tessieras, J.; Auzias, G.; Lehéricy, S.; Habert, M.-O.; Chupin, M.; Benali, H.; Colliot, O. Automatic classification of patients with Alzheimer’s disease from structural MRI: A comparison of ten methods using the ADNI database. Neuroimage 2011, 56, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, C.; Cabral, H.; Killiany, R. Multimodal Discrimination between Normal Aging, Mild Cognitive Impairment and Alzheimer’s Disease and Prediction of Cognitive Decline. Diagnostics 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture analysis of medical images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Larroza, A.; Bodí, V.; Moratal, D. Texture analysis in magnetic resonance imaging: Review and considerations for future applications. In Assessment of Cellular and Organ Function and Dysfunction Using Direct and Derived MRI Methodologies; InTech: Rijeka, Croatia, 2016; pp. 75–106. ISBN 978-953-51-2723-9. [Google Scholar]
- Kassner, A.; Thornhill, R.E. Texture analysis: A review of neurologic MR imaging applications. AJNR Am. J. Neuroradiol. 2010, 31, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ramón, R.; Larroza, A.; Ruiz-España, S.; Arana, E.; Moratal, D. Classifying brain metastases by their primary site of origin using a radiomics approach based on texture analysis: A feasibility study. Eur. Radiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Larroza, A.; Moratal, D.; Paredes-Sánchez, A.; Soria-Olivas, E.; Chust, M.L.; Arribas, L.A.; Arana, E. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging 2015, 42, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Valdés Hernández, M.C.; González-Castro, V.; Chappell, F.M.; Sakka, E.; Makin, S.; Armitage, P.A.; Nailon, W.H.; Wardlaw, J.M. Application of Texture Analysis to Study Small Vessel Disease and Blood–Brain Barrier Integrity. Front. Neurol. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Rittner, L.; Appenzeller, S.; Ruocco, H.H.; Lotufo, R. Etiology-based classification of brain white matter hyperintensity on magnetic resonance imaging. J. Med. Imaging 2015, 2, 014002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yu, C.; Jiang, G.; Liu, W.; Tong, L. 3D texture analysis on MRI images of Alzheimer’s disease. Brain Imaging Behav. 2012, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, H.; Zhou, Z.; Tong, L. 3D texture analysis of hippocampus based on MR images in patients with alzheimer disease and mild cognitive impairment. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; pp. 1–4. [Google Scholar]
- Sørensen, L.; Igel, C.; Liv Hansen, N.; Osler, M.; Lauritzen, M.; Rostrup, E.; Nielsen, M. Early detection of Alzheimer’s disease using MRI hippocampal texture. Hum. Brain Mapp. 2016, 37, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.S.; Balthazar, M.L.F.; D’Abreu, A.; Yasuda, C.L.; Damasceno, B.P.; Cendes, F.; Castellano, G. MR Imaging Texture Analysis of the Corpus Callosum and Thalamus in Amnestic Mild Cognitive Impairment and Mild Alzheimer Disease. Am. J. Neuroradiol. 2011, 32, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Oppedal, K.; Eftestøl, T.; Engan, K.; Beyer, M.K.; Aarsland, D. Classifying Dementia Using Local Binary Patterns from Different Regions in Magnetic Resonance Images. Int. J. Biomed. Imaging 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Murcia, F.J.; Górriz, J.M.; Ramírez, J.; Segovia, F.; Salas-Gonzalez, D.; Castillo-Barnes, D.; Illán, I.A.; Ortiz, A. Evaluating Alzheimer’s Disease Diagnosis Using Texture Analysis. In MIUA 2017. Communications in Computer and Information Science, Proceedings of the Medical Image Understanding and Analysis 2017, Edinburgh, UK, 11–13 July 2017; Valdés Hernández, M.C., Gonzalez-Castro, V., Eds.; Springer: Cham, Switzerland, 2017; Volume 723, pp. 470–481. [Google Scholar]
- Freeborough, P.A.; Fox, N.C. MR image texture analysis applied to the diagnosis and tracking of Alzheimer’s disease. IEEE Trans. Med. Imaging 1998, 17, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; van Cappellen van Walsum, A.-M.; Slump, C.H. Classification and localization of early-stage Alzheimer’s disease in magnetic resonance images using a patch-based classifier ensemble. Neuroradiology 2014, 56, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; Slump, C.; van Cappellen van Walsum, A.M. Using local texture maps of brain MR images to detect Mild Cognitive Impairment. In Proceedings of the 21st International Conference on Pattern Recognition (ICPR2012), Tsukuba, Japan, 11–15 November 2012; pp. 153–156. [Google Scholar]
- Hwang, E.-J.; Kim, H.-G.; Kim, D.; Rhee, H.Y.; Ryu, C.-W.; Liu, T.; Wang, Y.; Jahng, G.-H. Texture analyses of quantitative susceptibility maps to differentiate Alzheimer’s disease from cognitive normal and mild cognitive impairment. Med. Phys. 2016, 43, 4718–4728. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Petersen, R.C.; Donohue, M.C.; Gamst, A.; Raman, R.; Thomas, R.G.; Walter, S.; Trojanowski, J.Q.; Shaw, L.M.; Beckett, L.A.; et al. Clinical core of the Alzheimer’s disease neuroimaging initiative: Progress and plans. Alzheimer’s Dement. 2010, 6, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Wolfsgruber, S.; Wiese, B.; Bickel, H.; Mösch, E.; Kaduszkiewicz, H.; Pentzek, M.; Riedel-Heller, S.G.; Luck, T.; Fuchs, A.; et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014, 10, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Konrad, C.; Ukas, T.; Nebel, C.; Arolt, V.; Toga, A.W.; Narr, K.L. Defining the human hippocampus in cerebral magnetic resonance images—An overview of current segmentation protocols. Neuroimage 2009, 47, 1185–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collewet, G.; Strzelecki, M.; Mariette, F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn. Reson. Imaging 2004, 22, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.; Turnbull, L.W. Textural analysis of contrast-enhanced MR images of the breast. Magn. Reson. Med. 2003, 50, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourtsoyianni, S.; Doumou, G.; Prezzi, D.; Taylor, B.; Stirling, J.J.; Taylor, N.J.; Siddique, M.; Cook, G.J.R.; Glynne-Jones, R.; Goh, V. Primary Rectal Cancer: Repeatability of Global and Local-Regional MR Imaging Texture Features. Radiology 2017, 284, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Fox, N.C.; Warrington, E.K.; Freeborough, P.A.; Hartikainen, P.; Kennedy, A.M.; Stevens, J.M.; Rossor, M.N. Presymptomatic hippocampal atrophy in Alzheimer’s disease. Brain 1996, 119, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Grunwald, M.; Kruggel, F.; Riedel-Heller, S.G.; Angerhöfer, S.; Hojjatoleslami, A.; Hensel, A.; Arendt, T.; Gertz, H.-J. Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol. Aging 2001, 22, 177–186. [Google Scholar] [CrossRef]
- Kovalev, V.A.; Kruggel, F.; Gertz, H.J.; Von Cramon, D.Y. Three-dimensional texture analysis of MRI brain datasets. IEEE Trans. Med. Imaging 2001, 20, 424–433. [Google Scholar] [CrossRef] [PubMed]

| Texture Analysis Method | Scale | Number of Features | Feature Names |
|---|---|---|---|
| Intensity Histogram | Global | 3 | Global Variance |
| Skewness | |||
| Kurtosis | |||
| Gray-Level Co-occurrence Matrix (GLCM) | Local | 9 | Energy |
| Contrast | |||
| Entropy | |||
| Homogeneity | |||
| Correlation | |||
| Sum Average | |||
| Variance | |||
| Dissimilarity | |||
| Autocorrelation | |||
| Gray-Level Run-Length Matrix (GLRLM) | Regional | 13 | Short Run Emphasis (SRE) |
| Long Run Emphasis (LRE) | |||
| Gray-level Non-uniformity (GLN) | |||
| Run-Length Non-uniformity (RLN) | |||
| Run Percentage (RP) | |||
| Low Gray-level Run Emphasis (LGRE) | |||
| High Gray-level Run Emphasis (HGRE) | |||
| Short Run Low Gray-level Emphasis (SRLGE) | |||
| Short Run High Gray-level Emphasis (SRHGE) | |||
| Long Run Low Gray-level Emphasis (LRLGE) | |||
| Long Run High Gray-level Emphasis (LRHGE) | |||
| Gray-level Variance (GLV) | |||
| Run-Length Variance (RLV) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, C.; Ortiz-Ramón, R.; Mollá-Olmos, E.; Moratal, D.; For the Alzheimer’s Disease Neuroimaging Initiative. ALTEA: A Software Tool for the Evaluation of New Biomarkers for Alzheimer’s Disease by Means of Textures Analysis on Magnetic Resonance Images. Diagnostics 2018, 8, 47. https://doi.org/10.3390/diagnostics8030047
López-Gómez C, Ortiz-Ramón R, Mollá-Olmos E, Moratal D, For the Alzheimer’s Disease Neuroimaging Initiative. ALTEA: A Software Tool for the Evaluation of New Biomarkers for Alzheimer’s Disease by Means of Textures Analysis on Magnetic Resonance Images. Diagnostics. 2018; 8(3):47. https://doi.org/10.3390/diagnostics8030047
Chicago/Turabian StyleLópez-Gómez, Carlos, Rafael Ortiz-Ramón, Enrique Mollá-Olmos, David Moratal, and For the Alzheimer’s Disease Neuroimaging Initiative. 2018. "ALTEA: A Software Tool for the Evaluation of New Biomarkers for Alzheimer’s Disease by Means of Textures Analysis on Magnetic Resonance Images" Diagnostics 8, no. 3: 47. https://doi.org/10.3390/diagnostics8030047






