Scintigraphic Small Intestinal Transit Time and Defaecography in Patients with J-Pouch
Abstract
:1. Introduction
2. Experimental Section
2.1. Questionnaires
2.2. Inflammation
2.3. Scintigraphy
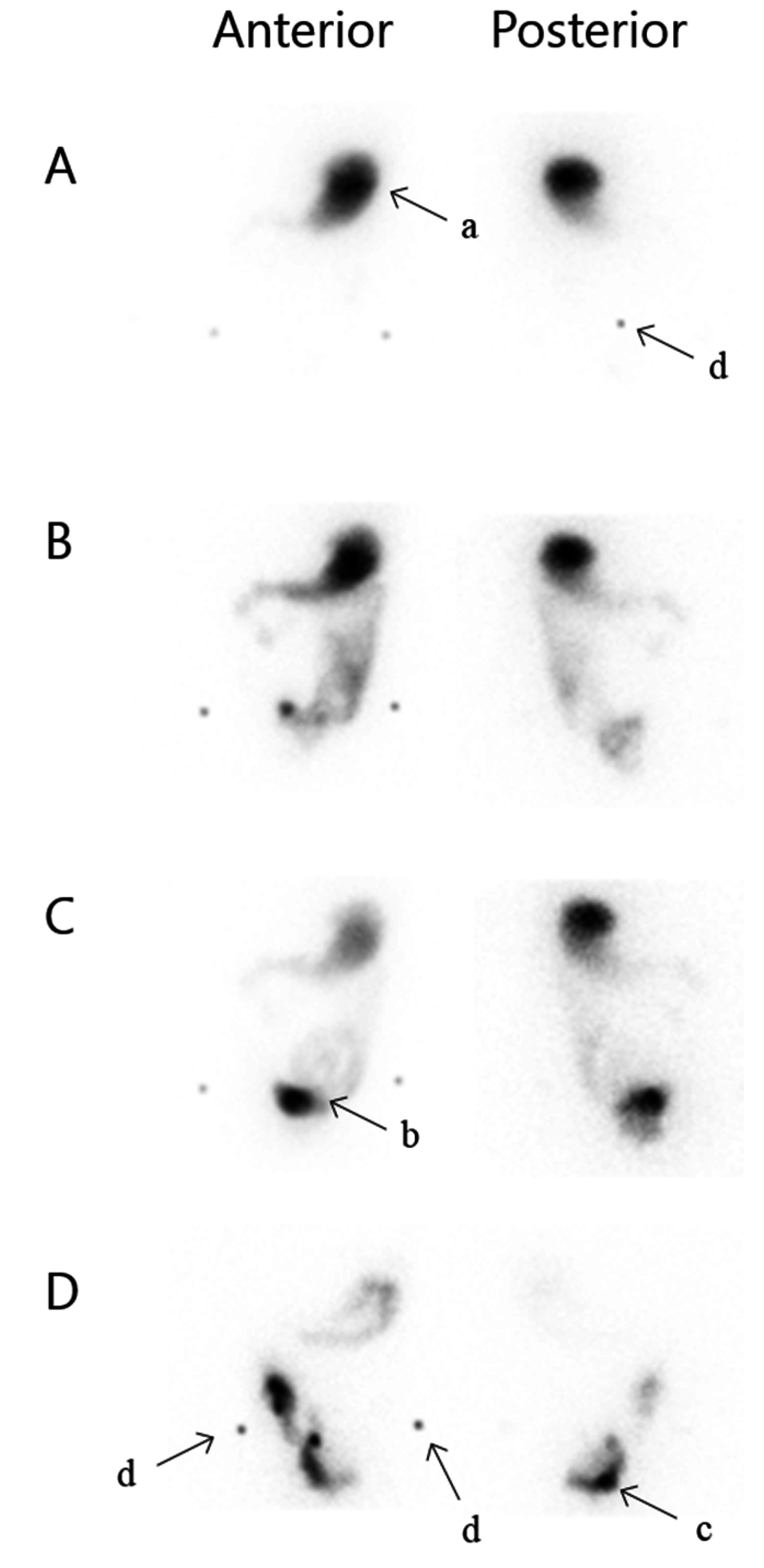
2.4. Defaecography
2.5. Statistics
3. Results
3.1. Patients and Baseline Characteristics
| Variable | Descriptive Statistics n =21 |
|---|---|
| Sex (M/F) | 9/12 |
| Age (years) | 41.3 (25.8–67.9) |
| BMI | 23.8 (18.7–36.1) 1 |
| Top-study (days) | 932 (481–3491) |
| Smoking (%) | |
| Yes | 1 (5) |
| No | 11 (52) |
| Earlier | 9 (43) |
| Extra intestinal symptoms (%) | 10 (48) |
| Diverting stoma after IPAA operation (%) | 13 (62) |
| Laparoscopically assisted/conventional open approach (%) | 8/13 (38/62) |
| OP stages (%) | |
| 1 | 3 (14) |
| 2 | 6 (29) |
| 3 | 12 2 (57) |
| Postoperative complications (%) | |
| Pouchitis | 10 (50) |
| Ileus requiring surgery | 3 (15) |
| Leakage | 1 (5) |
| Fistula requiring surgery | 1 (5) |
| Perforated ulcer | 1 (5) |
| SIBDQ | 55 (22–67) |
| Stool frequency, total | 9 (4–25) |
| Incontinence, minor and major (%) | 6 (29) |
| Daytime | 3 (14) |
| Night time | 6 (29) |
| Pouch volume (mL) | 222.5 (100–360) |
| Histology (%) | |
| No inflammation | 2 (10) |
| Light inflammation | 12 (57) |
| Moderate-severe inflammation | 7 (33) |
| Faecal calprotectin (mg·kg−1) | 200.5 (19–3750) |
3.2. Pouch Function, SIBDQ, and Inflammation
3.3. Scintigraphic Assessments
| Median Small Intestinal Transit Time (min) | 188.5 (105–365) |
|---|---|
| Evacuation fraction A (%) | 49 (3–77) |
| Evacuation fraction B (%) | 62 (17–98) |
| Median gastric transit time, (min) | 78 (40–115) |
| Interval from mouth to 10% in pouch (min) | 172.5 (90–270) |
| Median spectrum of transit times, β (min) | 45.5 (16.7–166.7) |
| Variable | Univariate (n = 20) | Multivariate (n = 20) | |||||
|---|---|---|---|---|---|---|---|
| Association with transit time | R2 | Coefficient | 95% CI | Ptransit | Coefficient | R2 = 0.70 95% CI | Ptransit |
| Sex | 0.06 | 35.5 | −31 to 102 | ns | 67.5 | −5.8 to 140 | 0.067 |
| Age | 0.16 | 2.6 | −0.4 to 5.6 | 0.081 | 2.8 | −0.6 to 6.2 | 0.097 |
| BMI | 0.18 | 6.4 | −0.2 to 13 | 0.057 | 10.7 | 3.6 to 17.7 | 0.007 |
| Stool frequency | 0.01 | 1.4 | −5.0 to 7.7 | ns | −10.2 | −18.3 to −2.0 | 0.02 |
| Pouch volume | 0.004 | 0.01 | −0.6 to 0.5 | ns | −0.4 | −1.0 to 0.2 | ns |
| Histological inflammation—severe | 0.08 | 8.2 | −116 to 132 | ns | −26.0 | −140 to 88 | ns |
| Evacuation fraction A | 0.01 | −0.4 | −2.0 to 1.2 | ns | −0.8 | −4.7 to 3.2 | ns |
| Evacuation fraction B | 0.02 | −0.4 | −2.1 to 1.2 | ns | −0.9 | −4.9 to 3.1 | ns |
| Association with evacuation fraction A | R2 | Coefficient | 95% CI | PEF | Coefficient | R2 = 0.92 95% CI | PEF |
| Sex | 0.05 | 9.5 | −10.1 to 29.1 | ns | 9.4 | −21.0 to 39.8 | ns |
| Age | 0.06 | 0.5 | −0.4 to 1.4 | ns | 0.5 | −0.9 to 1.9 | ns |
| BMI | <0.01 | −0.1 | 2.4 to 2.2 | ns | 0.7 | −2.3 to 3.6 | ns |
| Stool frequency | 0.01 | −0.3 | −2.2 to 1.5 | ns | −2.2 | −5.2 to 0.9 | ns |
| Pouch volume | 0.04 | −0.1 | −0.2 to 0.1 | ns | −0.1 | −0.4 to 0.1 | ns |
| Histological inflammation—severe | 0.06 | 16.4 | −20.2 to 52.9 | ns | 3.2 | −44.1 to 50.5 | ns |
4. Discussion
Scintigraphy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Zeeuw, S.; Ahmed Ali, U.; Donders, R.A.; Hueting, W.E.; Keus, F.; van Laarhoven, C.J. Update of complications and functional outcome of the ileo-pouch anal anastomosis: Overview of evidence and meta-analysis of 96 observational studies. Int. J. Colorectal Dis. 2012, 27, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Block, M.; Borjesson, L.; Lindholm, E.; Oresland, T. Pouch design and long-term functional outcome after ileal pouch-anal anastomosis. Br. J. Surg. 2009, 96, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Oresland, T.; Fasth, S.; Nordgren, S.; Hulten, L. The clinical and functional outcome after restorative proctocolectomy. A prospective study in 100 patients. Int. J Colorectal Dis. 1989, 4, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, R.E.; Fazio, V.W.; Remzi, F.H.; Tilney, H.S.; Nicholls, R.J.; Tekkis, P.P. Development of a pouch functional score following restorative proctocolectomy. Br. J. Surg. 2010, 97, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Brandsborg, S.; Nicholls, R.J.; Mortensen, L.S.; Laurberg, S. Restorative proctocolectomy for ulcerative colitis: Development and validation of a new scoring system for pouch dysfunction and quality of life. Colorectal Dis. 2013, 15, e719–e725. [Google Scholar] [CrossRef] [PubMed]
- Klas, J.; Myers, G.A.; Starling, J.R.; Harms, B.A. Physiologic evaluation and surgical management of failed ileoanal pouch. Dis. Colon Rectum 1998, 41, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Oresland, T.; Fasth, S.; Nordgren, S.; Akervall, S.; Hulten, L. Pouch size: The important functional determinant after restorative proctocolectomy. Br. J. Surg. 1990, 77, 265–269. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.R.; Kelly, K.A.; Brown, M.L. Scintigraphic assessment of neorectal motor function. J. Nucl. Med. 1986, 27, 460–464. [Google Scholar] [PubMed]
- Selvaggi, F.; Cuocolo, A.; Giuliani, A.; Sciaudone, G.; Riegler, G.; Mainolfi, C.; Caprio, M.G.; Panico, M.R.; Fiume, I. The role of scintigraphic defecography in the assessment of bowel function after restorative proctocolectomy for ulcerative colitis. Int. J Colorectal Dis. 2006, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Sugamata, Y.; Takase, Y.; Oya, M. Scintigraphic comparison of neorectal emptying between colonic J-pouch anastomosis and straight anastomosis after stapled low anterior resection. Int. J. Colorectal Dis. 2003, 18, 355–360. [Google Scholar] [PubMed]
- Takesue, Y.; Sakashita, Y.; Akagi, S.; Murakami, Y.; Ohge, H.; Imamura, Y.; Horikawa, Y.; Yokoyama, T. Gut transit time after ileal pouch-anal anastomosis using a radiopaque marker. Dis. Colon Rectum 2001, 44, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Tomita, R.; Fujisaki, S.; Tanjoh, K. Relationship between gastrointestinal transit time and daily stool frequency in patients after ileal J pouch-anal anastomosis for ulcerative colitis. Am. J. Surg. 2004, 187, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kmiot, W.A.; O’Brien, J.D.; Awad, R.; Keighley, M.R. Estimation of small bowel transit time following colectomy and ileal reservoir construction. Br. J. Surg. 1992, 79, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, P.A.; Kamm, M.A.; Nicholls, R.J.; Morris, G.; Britton, K.E. Contribution of gastrointestinal transit and pouch characteristics in determining pouch function. Gut 1997, 40, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Soper, N.J.; Orkin, B.A.; Kelly, K.A.; Phillips, S.F.; Brown, M.L. Gastrointestinal transit after proctocolectomy with ileal pouch-anal anastomosis or ileostomy. J. Surg. Res. 1989, 46, 300–305. [Google Scholar] [CrossRef]
- Jowett, S.L.; Seal, C.J.; Barton, J.R.; Welfare, M.R. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am. J. Gastroenterol. 2001, 96, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Irvine, E.J.; Zhou, Q.; Thompson, A.K. The short inflammatory bowel disease questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s relapse prevention trial. Am. J. Gastroenterol. 1996, 91, 1571–1578. [Google Scholar] [PubMed]
- Brinch, K.; Larsson, H.B.; Madsen, J.L. A deconvolution technique for processing small intestinal transit data. Eur. J. Nucl. Med. 1999, 26, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.H.; Camilleri, M.; Donohoe, K.; Knight, L.C.; Madsen, J.L.; Mariani, G.; Parkman, H.P.; van Dolsen, J. The SNMMI and EANM practice guideline for small-bowel and colon transit 1.0. J. Nucl. Med. 2013, 54, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- De Buck van Overstraeten, A.; Wolthuis, A.M.; Vermeire, S.; Van Assche, G.; Laenen, A.; Ferrante, M.; Rutgeerts, P.; D’Hoore, A. Long-term functional outcome after ileal pouch anal anastomosis in 191 patients with ulcerative colitis. J. Crohns Colitis 2014, 8, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, R.; Senninger, N.; Bruewer, M.; Rijcken, E. Pouch function and quality of life after successful management of pouch-related septic complications in patients with ulcerative colitis. Langenbecks Arch. Surg. 2012, 397, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.D.; Laursen, S.B.; Qvist, N.; Kjeldsen, J.; Poornoroozy, P.H. Sexual function and body image are similar after laparoscopy-assisted and open ileal pouch-anal anastomosis. World J. Surg. 2014, 38, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.H. 2013 SNMMI highlights lecture: General clinical nuclear medicine: Clinical SPECT/CT—Time for a new standard of care. J. Nucl. Med. 2013, 54, 19N–27N. [Google Scholar] [PubMed]
- Mariani, G.; Pauwels, E.K.; AlSharif, A.; Marchi, S.; Boni, G.; Barreca, M.; Bellini, M.; Grosso, M.; de Bortoli, N.; Mumolo, G.; et al. Radionuclide evaluation of the lower gastrointestinal tract. J. Nucl. Med. 2008, 49, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, K.; McLeod, R.S.; Walfisch, S.; Yip, K.; Cohen, Z. Pelvic pouch emptying scan: An evaluation of scintigraphic assessment of the neorectum. Int. J. Colorectal Dis. 1991, 6, 29–32. [Google Scholar] [CrossRef] [PubMed]
- De Silva, H.J.; de Angelis, C.P.; Soper, N.; Kettlewell, M.G.; Mortensen, N.J.; Jewell, D.P. Clinical and functional outcome after restorative proctocolectomy. Br. J. Surg. 1991, 78, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjaer, M.D.; Simonsen, J.A.; Hvidsten, S.; Kjeldsen, J.; Gerke, O.; Qvist, N. Scintigraphic Small Intestinal Transit Time and Defaecography in Patients with J-Pouch. Diagnostics 2015, 5, 399-412. https://doi.org/10.3390/diagnostics5040399
Kjaer MD, Simonsen JA, Hvidsten S, Kjeldsen J, Gerke O, Qvist N. Scintigraphic Small Intestinal Transit Time and Defaecography in Patients with J-Pouch. Diagnostics. 2015; 5(4):399-412. https://doi.org/10.3390/diagnostics5040399
Chicago/Turabian StyleKjaer, Mie Dilling, Jane Angel Simonsen, Svend Hvidsten, Jens Kjeldsen, Oke Gerke, and Niels Qvist. 2015. "Scintigraphic Small Intestinal Transit Time and Defaecography in Patients with J-Pouch" Diagnostics 5, no. 4: 399-412. https://doi.org/10.3390/diagnostics5040399







