Artificial Intelligence in Cutaneous Leishmaniasis Diagnosis: Current Developments and Future Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Literature Search: A systematic search was conducted across five electronic databases including PubMed, Scopus, Web of Science, Science Direct, and Google Scholar.
- Search Terms: We utilized specific keywords related to the following:
- –
- Artificial Intelligence: ‘artificial intelligence’, ‘AI’, ‘AI Algorithm’, ‘Deep Learning’, ‘DL’, ‘Machine Learning’, ‘ML’, ‘Transfer Learning’, ‘Computer Aided Diagnosis’, ‘Convolutional Neural Network’, and ‘CNN’.
- –
- Cutaneous Leishmaniasis: ‘Cutaneous Leishmaniasis’, ‘CL’, and ’Mucocutaneous leishmaniasis’.
- –
- Diagnosis: ‘diagnostic’, ‘diagnosis’, ‘sensitivity’, and ‘specificity’.
- –
- Combined Search Terms: various combinations of search terms were employed, such as ‘Cutaneous leishmaniasis AND artificial intelligence’, ‘CL diagnosis AND machine learning’, ‘CL diagnosis AND deep learning’, and others.
- Search Criteria: Studies published between 2019 and 2024 were considered. The search was conducted in both English and French languages.
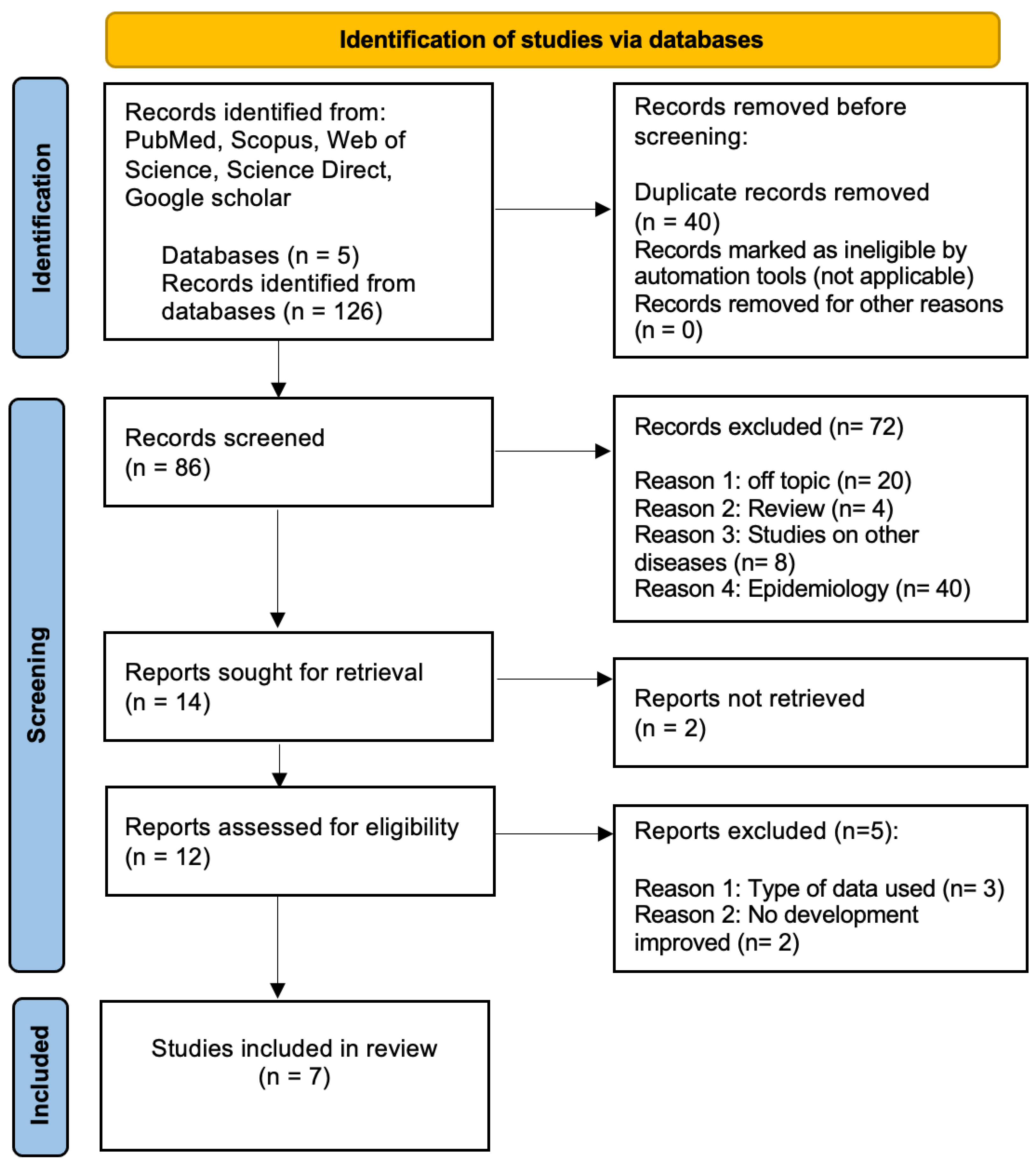
2.2. Study Selection
2.3. Data Extraction
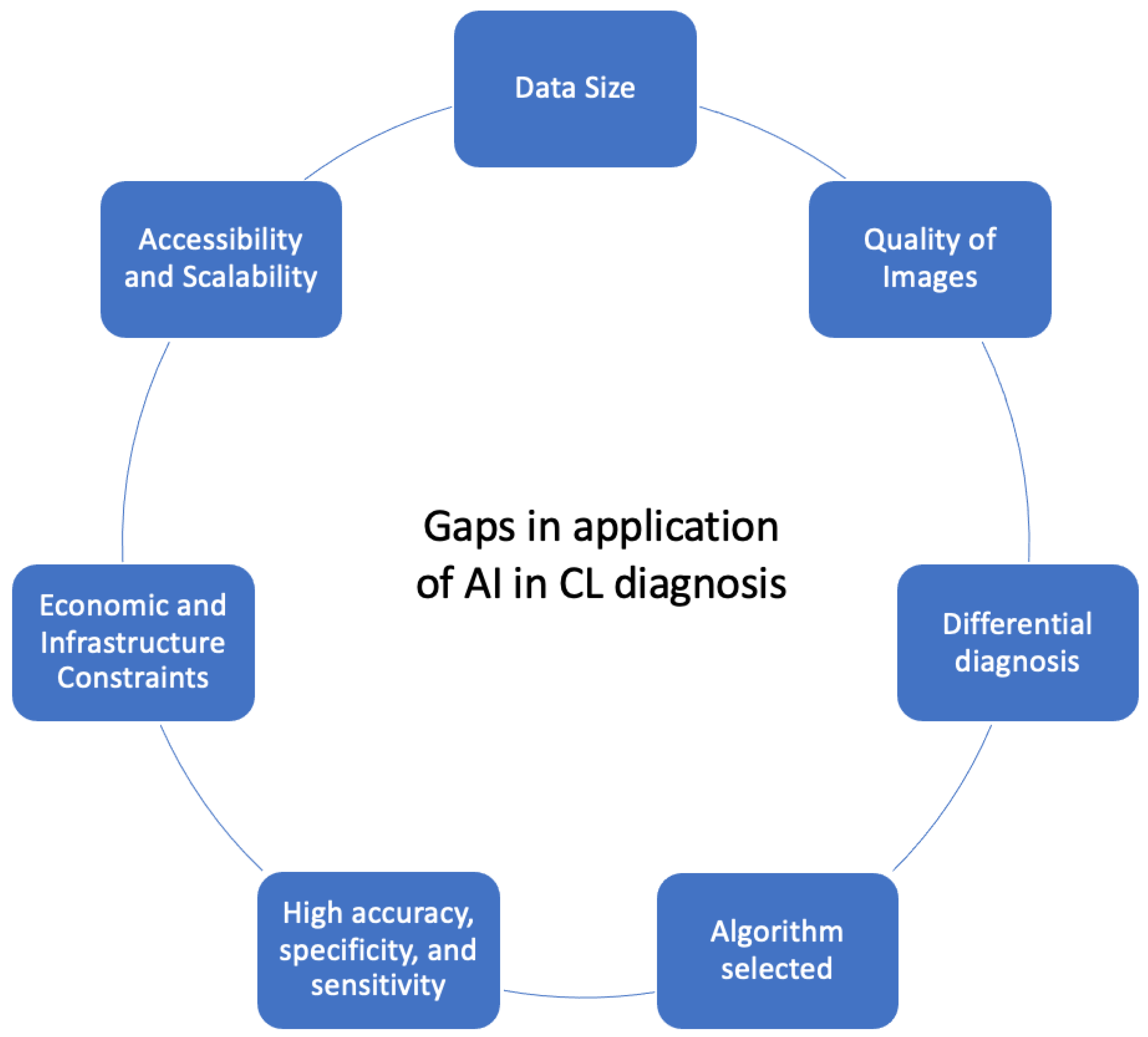
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | Cutaneous Leishmaniasis. |
| WHO | World Health Organization. |
| AI | Artificial Intelligence. |
| NTDs | neglected tropical diseases. |
| NNN | Novy–MacNeal–Nicolle. |
| RPMI | Roswell Park Memorial Institute. |
| RDT | Rapid Diagnostic Test. |
| ML | Machine Learning. |
| DL | Deep Learning. |
| PRISMA | Preferred Reporting Items for Systematic Reviews. |
| BCC | basal cell carcinoma. |
| CNN | Convolutional Neural Network. |
| MLP | Multilayer Perceptron. |
| YOLOv5 | You Only Look Once. |
| BHO-SVM | Black Hole Optimization Support Vector Machine. |
| ACL | Anthroponotic Cutaneous Leishmaniasis. |
References
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 28 January 2023).
- Messaoudene, F.; Boukraa, S.; Boubidi, S.C.; Guerzou, A.; Ouahabi, A. Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria). Microorganisms 2023, 11, 2608. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.S.; Lockwood, D.N.J. Cutaneous Leishmaniasis. Clin. Dermatol. 2007, 25, 203–211. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Lindoso, J.A.L. Current Diagnosis and Treatment of Cutaneous and Mucocutaneous Leishmaniasis. Expert Rev. Anti-Infect. Ther. 2010, 8, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Reimão, J.Q.; Coser, E.M.; Lee, M.R.; Coelho, A.C. Laboratory Diagnosis of Cutaneous and Visceral Leishmaniasis: Current and Future Methods. Microorganisms 2020, 8, 1632. [Google Scholar] [CrossRef]
- Ogado Ceasar Odiwuor, S.; Ageed Saad, A.; De Doncker, S.; Maes, I.; Laurent, T.; El Safi, S.; Mbuchi, M.; Büscher, P.; Dujardin, J.-C.; Van der Auwera, G. Universal PCR Assays for the Differential Detection of All Old World Leishmania Species. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, J.; Niederwieser, I.; Makia, N.D.; Beck, H.-P.; Felger, I. Diagnostic Genotyping of Old and New World Leishmania Species by PCR-RFLP. Diagn. Microbiol. Infect. Dis. 2003, 46, 115–124. [Google Scholar] [CrossRef]
- de Bruijn, M.H.; Barker, D.C. Diagnosis of New World Leishmaniasis: Specific Detection of Species of the Leishmania Braziliensis Complex by Amplification of Kinetoplast DNA. Acta Trop. 2003, 52, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, S.; Amro, A.; Schönian, G.; Lemrani, M. Moroccan Leishmania Infantum: Genetic Diversity and Population Structure as Revealed by Multi-Locus Microsatellite Typing. BMC Proc. 2011, 5, P43. [Google Scholar] [CrossRef]
- Boité, M.C.; Mauricio, I.L.; Miles, M.A.; Cupolillo, E. New Insights on Taxonomy, Phylogeny and Population Genetics of Leishmania (Viannia) Parasites Based on Multilocus Sequence Analysis. PLoS Negl. Trop. Dis. 2012, 6, e1888. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Bennis, I.; Verdonck, K.; el Khalfaoui, N.; Riyad, M.; Fellah, H.; Dujardin, J.-C.; Sahibi, H.; Bouhout, S.; Van der Auwera, G.; Boelaert, M. Accuracy of a Rapid Diagnostic Test Based on Antigen Detection for the Diagnosis of Cutaneous Leishmaniasis in Patients with Suggestive Skin Lesions in Morocco. Am. J. Trop. Med. Hyg. 2018, 99, 716–722. [Google Scholar] [CrossRef]
- Retmi, K.; Ouzayd, F.; Ech-Cheikh, H.; Guarret, C.; Taleb, Y. State of Art on Object Detection Solution Applied to COVID 19’s Spreading Prevention. In Proceedings of the 2021 IEEE International Conference on Data Science and Computer Application (ICDSCA), Dalian, China, 29–31 October 2021; pp. 364–368. [Google Scholar]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V.K. Overview of Artificial Intelligence in Medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Patel, S.; Lee, J.B.; Motaparthi, K. Artificial Intelligence in Dermatopathology: Diagnosis, Education, and Research. J. Cutan. Pathol. 2021, 48, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bamorovat, M.; Sharifi, I.; Rashedi, E.; Shafiian, A.; Sharifi, F.; Khosravi, A.; Tahmouresi, A. A Novel Diagnostic and Prognostic Approach for Unresponsive Patients with Anthroponotic Cutaneous Leishmaniasis Using Artificial Neural Networks. PLoS ONE 2021, 16, e0250904. [Google Scholar] [CrossRef]
- Arce-Lopera, C.A.; Diaz-Cely, J.; Quintero, L. Presumptive Diagnosis of Cutaneous Leishmaniasis. Front. Health Inform. 2021, 10, 75. [Google Scholar] [CrossRef]
- Steyve, N.; Steve, P.; Ghislain, M.; Ndjakomo, S.; Pierre, E. Optimized Real-Time Diagnosis of Neglected Tropical Diseases by Automatic Recognition of Skin Lesions. Inform. Med. Unlocked 2022, 33, 101078. [Google Scholar] [CrossRef]
- Zare, M.; Akbarialiabad, H.; Parsaei, H.; Asgari, Q.; Alinejad, A.; Bahreini, M.S.; Hosseini, S.H.; Ghofrani-Jahromi, M.; Shahriarirad, R.; Amirmoezzi, Y.; et al. A Machine Learning-Based System for Detecting Leishmaniasis in Microscopic Images. BMC Infect. Dis. 2022, 22, 48. [Google Scholar] [CrossRef]
- Noureldeen, A.M.; Masoud, K.S.; Almakhzoom, O.A. Deep Learning Model for Cutaneous Leishmaniasis Detection and Classification Using Yolov5. Afr. J. Adv. Pure Appl. Sci. (AJAPAS) 2023, 270–280. [Google Scholar]
- Leal, J.F.d.C.; Barroso, D.H.; Trindade, N.S.; de Miranda, V.L.; Gurgel-Gonçalves, R. Automated Identification of Cutaneous Leishmaniasis Lesions Using Deep-Learning-Based Artificial Intelligence. Biomedicines 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Abdelmula, A.M.; Mirzaei, O.; Güler, E.; Süer, K. Assessment of Deep Learning Models for Cutaneous Leishmania Parasite Diagnosis Using Microscopic Images. Diagnostics 2024, 14, 12. [Google Scholar] [CrossRef]
- Leha, A.; Hellenkamp, K.; Unsöld, B.; Mushemi-Blake, S.; Shah, A.M.; Hasenfuß, G.; Seidler, T. A Machine Learning Approach for the Prediction of Pulmonary Hypertension. PLoS ONE 2019, 14, e0224453. [Google Scholar] [CrossRef] [PubMed]
- Sippy, R.; Farrell, D.F.; Lichtenstein, D.A.; Nightingale, R.; Harris, M.A.; Toth, J.; Hantztidiamantis, P.; Usher, N.; Cueva Aponte, C.; Barzallo Aguilar, J.; et al. Severity Index for Suspected Arbovirus (SISA): Machine Learning for Accurate Prediction of Hospitalization in Subjects Suspected of Arboviral Infection. PLoS Negl. Trop. Dis. 2020, 14, e0007969. [Google Scholar] [CrossRef] [PubMed]
- Aguerchi, K.; Jabrane, Y.; Habba, M.; El Hassani, A.H. A CNN Hyperparameters Optimization Based on Particle Swarm Optimization for Mammography Breast Cancer Classification. J. Imaging 2024, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hamet, P.; Tremblay, J. Artificial Intelligence in Medicine. Metabolism 2017, 69S, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Moon, I.J.; Kim, S.H.; Na, J.-I.; Kim, M.S.; Park, G.H.; Park, I.; Kim, K.; Lim, W.; Lee, J.H.; et al. Assessment of Deep Neural Networks for the Diagnosis of Benign and Malignant Skin Neoplasms in Comparison with Dermatologists: A Retrospective Validation Study. PLoS Med. 2020, 17, e1003381. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.P.; Kim, B.J.; Paolino, A.; Tan, W.R.; Lim, S.M.L.; Seo, J.; Tan, S.P.; Francis, L.; Tsakok, T.; Simpson, M.; et al. Systematic Review of Deep Learning Image Analyses for the Diagnosis and Monitoring of Skin Disease. NPJ Digit. Med. 2023, 6, 180. [Google Scholar] [CrossRef]
- Srinivasu, P.N.; SivaSai, J.G.; Ijaz, M.F.; Bhoi, A.K.; Kim, W.; Kang, J.J. Classification of Skin Disease Using Deep Learning Neural Networks with MobileNet V2 and LSTM. Sensors 2021, 21, 2852. [Google Scholar] [CrossRef]
- Goceri, E. Diagnosis of Skin Diseases in the Era of Deep Learning and Mobile Technology. Comput. Biol. Med. 2021, 134, 104458. [Google Scholar] [CrossRef]
- AlSuwaidan, L. Deep Learning Based Classification of Dermatological Disorders. Biomed Eng. Comput. Biol. 2023, 14, 11795972221138470. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.L.; Tada, M.; So, A.; Torres, R. Artificial Intelligence and Skin Cancer. Front. Med. 2024, 11, 1331895. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Skin NTDs App—Apps on Google Play. Available online: https://play.google.com/store/apps/details?id=com.universaldoctor.skin_ntds&hl=en_US (accessed on 3 February 2024).
- Mitropoulos, P.; Konidas, P.; Durkin-Konidas, M. New World Cutaneous Leishmaniasis: Updated Review of Current and Future Diagnosis and Treatment. J. Am. Acad. Dermatol. 2010, 63, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hajiarbabi, M. Skin Cancer Detection Using Multi-Scale Deep Learning and Transfer Learning. J. Med Artif. Intell. 2023, 6. [Google Scholar] [CrossRef]
- Baweja, A.K.; Aditya, S.; Kanchana, M. Leprosy Diagnosis Using Explainable Artificial Intelligence Techniques. In Proceedings of the 2023 International Conference on Sustainable Computing and Data Communication Systems (ICSCDS), Erode, India, 23–25 March 2023; pp. 551–556. [Google Scholar]
- Han, S.S.; Moon, I.J.; Lim, W.; Suh, I.S.; Lee, S.Y.; Na, J.-I.; Kim, S.H.; Chang, S.E. Keratinocytic Skin Cancer Detection on the Face Using Region-Based Convolutional Neural Network. JAMA Dermatol. 2020, 156, 29–37. [Google Scholar] [CrossRef]
- Salim, F.; Saeed, F.; Basurra, S.; Qasem, S.N.; Al-Hadhrami, T. DenseNet-201 and Xception Pre-Trained Deep Learning Models for Fruit Recognition. Electronics 2023, 12, 3132. [Google Scholar] [CrossRef]
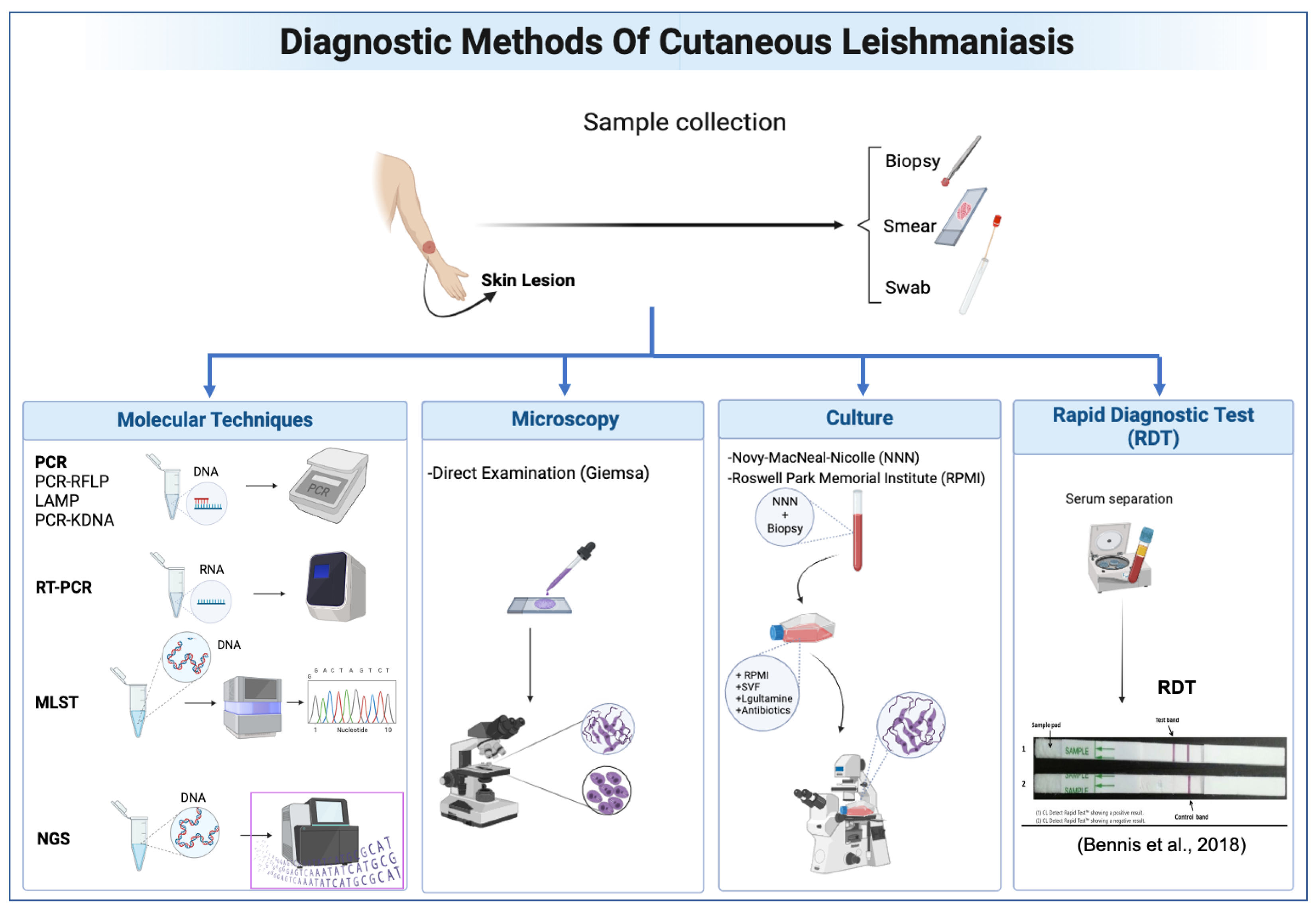
| Study | Country | Dataset Size | Type of Data | AI Model | Purpose of Model | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| Bamorovat et al., 2021 [19] | Iran | 172 | Clinical and demographic data from patients with Anthroponotic CL (ACL). In total, 72 unresponsive and 100 responsive. | ML (MLP) | Classification of patients with ACL as either responsive or unresponsive to treatment | 87.8% | 90.3% | 86% |
| Arce-Lopera et al., 2021 [20] | Colombia | 2022 | Images of CL and other dermatoses. | DL (VGG19) | Classification by CL and non-CL lesions + Mobile App | 93% | 80% | 96% |
| Steyve et al., 2022 [21] | Cameroon | 1054 | A total of 262 images of CL, 372 of leprosy, 420 of Buruli ulcers. | ML (BHO-SVM) | Classification by identifying skin lesions | 96% | 92% | 94% |
| Zare et al., 2022 [22] | Iran | 300 | Microscopic images: 150 positives and 150 negatives. | ML (Viola–Jones) | L. parasite detection | IM:60% IP:70% | IM:50% IP:71% | IM:65% IP:52% |
| Noureldeen et al., 2023 [23] | Libya | 160 | Images taken by mobile phone camera. | DL (YOLOv5) | Detection and classification of CL lesions | 70% | 99% | 98% |
| Leal et al., 2023 [24] | Brazil | 2458 | Images taken by mobile phone camera. In total, 1787 of CL and 671 of other dermatoses. | DL (AlexNet) | Identification of CL lesions | 95.04% | 93.81% | 96.04% |
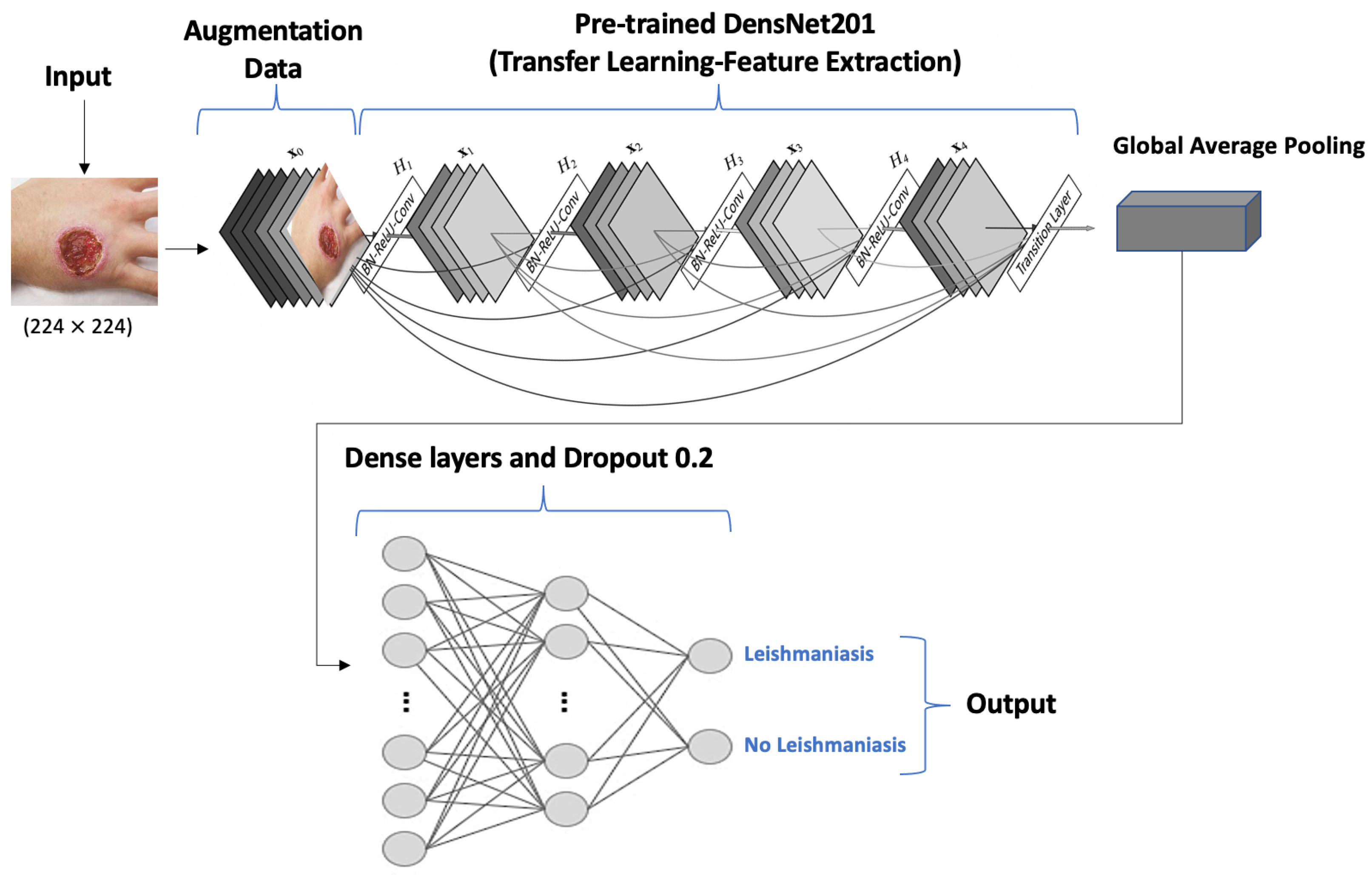
| Abdelmula et al., 2024 [25] | Turkey | - | Microscopic images. | DL (DenseNet201, EfficientNetB0, MobileNetv2, ResNet101, and Xception) | Amastigotes detection | 99.15%, 99.07%, 98.74%, 98.52%, 98.78% | 99.53%, 99.03%, 98.65%, 98.49%, 98.43% | 98.80%, 99.07%, 98.80%, 98.53%, 99.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talimi, H.; Retmi, K.; Fissoune, R.; Lemrani, M. Artificial Intelligence in Cutaneous Leishmaniasis Diagnosis: Current Developments and Future Perspectives. Diagnostics 2024, 14, 963. https://doi.org/10.3390/diagnostics14090963
Talimi H, Retmi K, Fissoune R, Lemrani M. Artificial Intelligence in Cutaneous Leishmaniasis Diagnosis: Current Developments and Future Perspectives. Diagnostics. 2024; 14(9):963. https://doi.org/10.3390/diagnostics14090963
Chicago/Turabian StyleTalimi, Hasnaa, Kawtar Retmi, Rachida Fissoune, and Meryem Lemrani. 2024. "Artificial Intelligence in Cutaneous Leishmaniasis Diagnosis: Current Developments and Future Perspectives" Diagnostics 14, no. 9: 963. https://doi.org/10.3390/diagnostics14090963






