Comprehensive Genomic Studies on the Cell Blocks of Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citological Samples
2.2. DNA Quantification and Quality Assessment, and Mutation Testing
2.3. Next-Generation Sequencing and Bioinformatic Analysis
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Specimen Adequacy for Molecular Testing
3.2. PDAC NGS Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Ye, Z.; Qin, Y.; Xu, X.; Yu, X.; Zhuo, Q.; Ji, S. Mutations in Key Driver Genes of Pancreatic Cancer: Molecularly Targeted Therapies and Other Clinical Implications. Acta Pharmacol. Sin. 2021, 42, 1725–1741. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-Y.; Wang, Q.; Wang, S.-M.; Xu, Y.; Pan, Y.-C.; Zhang, X.; Hu, A.-Y.; Zhang, S.-H. Cytomorphological and Immunohistochemical Features of Pancreatic Ductal Adenocarcinoma in Serous Fluids. Diagn. Cytopathol. 2022, 50, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Redegalli, M.; Schiavo Lena, M.; Cangi, M.G.; Smart, C.E.; Mori, M.; Fiorino, C.; Arcidiacono, P.G.; Balzano, G.; Falconi, M.; Reni, M.; et al. Proposal for a New Pathologic Prognostic Index after Neoadjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma (PINC). Ann. Surg. Oncol. 2022, 29, 3492–3502. [Google Scholar] [CrossRef] [PubMed]
- Posta, M.; Győrffy, B. Analysis of a Large Cohort of Pancreatic Cancer Transcriptomic Profiles to Reveal the Strongest Prognostic Factors. Clin. Transl. Sci. 2023, 16, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 1.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2024, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.B.; Centeno, B.A.; Ali, S.Z.; Genevay, M.; Stelow, E.; Mino-Kenudson, M.; Castillo, C.F.-D.; Schmidt, C.M.; Brugge, W.R.; Layfield, L.J. Standardized Terminology and Nomenclature for Pancreatobiliary Cytology: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- IAC-IARC-Who Joint Editorial Board. WHO Reporting System for Pancreaticobiliary Cytopathology; International Agency for Research on Cancer: Lyon, France, 2023; ISBN 978-92-832-4518-6. [Google Scholar]
- Hoda, R.S.; Arpin, R.N.; Rosenbaum, M.W.; Pitman, M.B. Risk of Malignancy Associated with Diagnostic Categories of the Proposed World Health Organization International System for Reporting Pancreaticobiliary Cytopathology. Cancer Cytopathol. 2022, 130, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Proctor, T.; Seide, S.; Chatziioannou, S.S.; Reynolds, J.P.; Ntourakis, D. Diagnostic Performance of Pancreatic Cytology with the Papanicolaou Society of Cytopathology System: A Systematic Review, before Shifting into the Upcoming WHO International System. Int. J. Mol. Sci. 2022, 23, 1650. [Google Scholar] [CrossRef]
- Kuo, K.-K.; Hsiao, P.-J.; Chang, W.-T.; Chuang, S.-C.; Yang, Y.-H.; Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Li, C.-P.; Kato, K.; et al. Therapeutic Strategies Targeting Tumor Suppressor Genes in Pancreatic Cancer. Cancers 2021, 13, 3920. [Google Scholar] [CrossRef]
- Redegalli, M.; Grassini, G.; Magliacane, G.; Pecciarini, L.; Schiavo Lena, M.; Smart, C.E.; Johnston, R.L.; Waddell, N.; Maestro, R.; Macchini, M.; et al. Routine Molecular Profiling in Both Resectable and Unresectable Pancreatic Adenocarcinoma: Relevance of Cytologic Samples. Clin. Gastroenterol. Hepatol. 2023, 21, 2825–2833. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Jhala, N.; Seth, A.; Krall, K.; Navaneethan, U.; Hawes, R.; Wilcox, C.M.; Varadarajulu, S. Standardisation of EUS-Guided FNB Technique for Molecular Profiling in Pancreatic Cancer: Results of a Randomised Trial. Gut 2023, 72, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Razzano, D.; Bouza, S.J.; Hernandez, P.V.; Wang, M.; Robert, M.E.; Walther, Z.; Cai, G. Comprehensive Molecular Profiling of Pancreatic Ductal Adenocarcinoma in FNA, Biopsy, and Resection Specimens. Cancer Cytopathol. 2022, 130, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Roy-Chowdhuri, S.; Duose, D.Y.; Stewart, J.M.; Coronel, E.; Bhutani, M.S.; Lee, J.H.; Weston, B.; Ge, P.S.; Ross, W.A.; et al. Adequacy Evaluation and Use of Pancreatic Adenocarcinoma Specimens for Next-Generation Sequencing Acquired by Endoscopic Ultrasound-Guided FNA and FNB. Cancer Cytopathol. 2022, 130, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wei, S. Overview of Molecular Testing of Cytology Specimens. Acta Cytol. 2020, 64, 136–146. [Google Scholar] [CrossRef]
- Turner, S.A.; Abou Shaar, R.; Yang, Z. The Basics of Commonly Used Molecular Techniques for Diagnosis, and Application of Molecular Testing in Cytology. Diagn. Cytopathol. 2023, 51, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Kerr, K.M.; Garrido, P.; Thunnissen, E.; Dequeker, E.; Normanno, N.; Patton, S.J.; Fairley, J.; Kapp, J.; de Ridder, D.; et al. Expert Opinion on NSCLC Small Specimen Biomarker Testing—Part 1: Tissue Collection and Management. Virchows Arch. 2022, 481, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Souza Da Silva, R.; Schmitt, F. Optimal Assessment of Metastatic Breast Carcinoma: The Value of Cytopathology Combined with Molecular Analysis. JMP 2022, 3, 329–338. [Google Scholar] [CrossRef]
- Sahu, S.; Gupta, P.; Dey, P. Molecular Testing on Serous Effusion: An Update. Cytojournal 2021, 18, 35. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Chen, H.; Singh, R.R.; Krishnamurthy, S.; Patel, K.P.; Routbort, M.J.; Manekia, J.; Barkoh, B.A.; Yao, H.; Sabir, S.; et al. Concurrent Fine Needle Aspirations and Core Needle Biopsies: A Comparative Study of Substrates for next-Generation Sequencing in Solid Organ Malignancies. Mod. Pathol. 2017, 30, 499–508. [Google Scholar] [CrossRef]
- Achterberg, F.B.; Mulder, B.G.S.; Janssen, Q.P.; Koerkamp, B.G.; Hol, L.; Quispel, R.; Bonsing, B.A.; Vahrmeijer, A.L.; van Eijck, C.H.J.; Roos, D.; et al. Targeted Next-Generation Sequencing Has Incremental Value in the Diagnostic Work-up of Patients with Suspect Pancreatic Masses; a Multi-Center Prospective Cross Sectional Study. PLoS ONE 2023, 18, e0280939. [Google Scholar] [CrossRef] [PubMed]
- Valero, V.; Saunders, T.J.; He, J.; Weiss, M.J.; Cameron, J.L.; Dholakia, A.; Wild, A.T.; Shin, E.J.; Khashab, M.A.; O’Broin-Lennon, A.M.; et al. Reliable Detection of Somatic Mutations in Fine Needle Aspirates of Pancreatic Cancer with Next-Generation Sequencing: Implications for Surgical Management. Ann. Surg. 2016, 263, 153–161. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Layfield, L.J.; Zhang, T.; Esebua, M. Molecular Features of Pancreaticobiliary Neoplasms: Implications for Diagnosis, Prognostication, and Therapy Selection. Diagn. Cytopathol. 2023, 51, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, W.; Ren, L.; Yang, Y.; Zhang, Y.; Ge, B.; Li, S.; Zheng, X.; Liu, J.; Zhang, S.; et al. Progress on Diagnostic and Prognostic Markers of Pancreatic Cancer. Oncol. Res. 2023, 31, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.R.; Rubinson, D.A.; Nowak, J.A.; Morales-Oyarvide, V.; Dunne, R.F.; Kozak, M.M.; Welch, M.W.; Brais, L.K.; Da Silva, A.; Li, T.; et al. Association of Alterations in Main Driver Genes with Outcomes of Patients with Resected Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2018, 4, e173420. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.R.; Zhu, Y.; Yin, L.; Javed, A.A.; Ding, D.; Tenior, J.; Wright, M.; Ali, S.Z.; Burkhart, R.A.; Burns, W.; et al. Reliable Detection of Somatic Mutations for Pancreatic Cancer in Endoscopic Ultrasonography-Guided Fine Needle Aspirates with Next-Generation Sequencing: Implications from a Prospective Cohort Study. J. Gastrointest. Surg. 2021, 25, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Doi, T.; Morita, R.; Kataoka, S.; Miyake, H.; Yamaguchi, K.; Moriguchi, M.; Sogame, Y.; Yasuda, H.; et al. Genomic Landscape and Clinical Features of Rare Subtypes of Pancreatic Cancer: Analysis with the National Database of Japan. J. Gastroenterol. 2023, 58, 575–585. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef]
- Hong, J.Y.; Cho, H.J.; Kim, S.T.; Park, Y.S.; Shin, S.H.; Han, I.W.; Lee, J.; Heo, J.S.; Park, J.O. Comprehensive Molecular Profiling to Predict Clinical Outcomes in Pancreatic Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211038478. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, V.H.F.; Mathias-Machado, M.C.; de Farias, J.P.F.; Aruquipa, M.P.S.; Jácome, A.A.; Peixoto, R.D. Targeting KRAS in Pancreatic Ductal Adenocarcinoma: The Long Road to Cure. Cancers 2023, 15, 5015. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A.; Digklia, A. Pancreatic Adenocarcinomas without KRAS, TP53, CDKN2A and SMAD4 Mutations and CDKN2A/CDKN2B Copy Number Alterations: A Review of the Genomic Landscape to Unveil Therapeutic Avenues. Chin. Clin. Oncol. 2023, 12, 1–14. [Google Scholar] [CrossRef]
- Philip, P.A.; Azar, I.; Xiu, J.; Hall, M.J.; Hendifar, A.E.; Lou, E.; Hwang, J.J.; Gong, J.; Feldman, R.; Ellis, M.; et al. Molecular Characterization of KRAS Wild-Type Tumors in Patients with Pancreatic Adenocarcinoma. Clin. Cancer Res. 2022, 28, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Dorman, K.; Zhang, D.; Heinrich, K.; Reeh, L.; Weiss, L.; Haas, M.; Beyer, G.; Rössler, D.; Goni, E.; Renz, B.W.; et al. Precision Oncology in Pancreatic Cancer: Experiences and Challenges of the CCCMunichLMU Molecular Tumor Board. Target. Oncol. 2023, 18, 257–267. [Google Scholar] [CrossRef]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Albanese, L.; Signoriello, G.; Napoli, C.; Molinari, A.M. Pancreatic Cancer with Mutation in BRCA1/2, MLH1, and APC Genes: Phenotype Correlation and Detection of a Novel Germline BRCA2 Mutation. Genes 2022, 13, 321. [Google Scholar] [CrossRef]
- Dal Buono, A.; Poliani, L.; Greco, L.; Bianchi, P.; Barile, M.; Giatti, V.; Bonifacio, C.; Carrara, S.; Malesci, A.; Laghi, L. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients with Pancreatic Cancer or Suspected Related Hereditary Syndromes: Historical Prospective Analysis. Cancers 2023, 15, 1852. [Google Scholar] [CrossRef]
- Lincoln, S.E.; Nussbaum, R.L.; Kurian, A.W.; Nielsen, S.M.; Das, K.; Michalski, S.; Yang, S.; Ngo, N.; Blanco, A.; Esplin, E.D. Yield and Utility of Germline Testing Following Tumor Sequencing in Patients with Cancer. JAMA Netw. Open 2020, 3, e2019452. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Müllauer, L.; Mader, R.M.; Schindl, M.; Prager, G.W. Applied Precision Medicine in Metastatic Pancreatic Ductal Adenocarcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920938611. [Google Scholar] [CrossRef] [PubMed]
- Abdilleh, K.; Khalid, O.; Ladnier, D.; Wan, W.; Seepo, S.; Rupp, G.; Corelj, V.; Worman, Z.F.; Sain, D.; DiGiovanna, J.; et al. Pancreatic Cancer Action Network’s SPARK: A Cloud-Based Patient Health Data and Analytics Platform for Pancreatic Cancer. JCO Clin. Cancer Inform. 2024, 8, e2300119. [Google Scholar] [CrossRef] [PubMed]

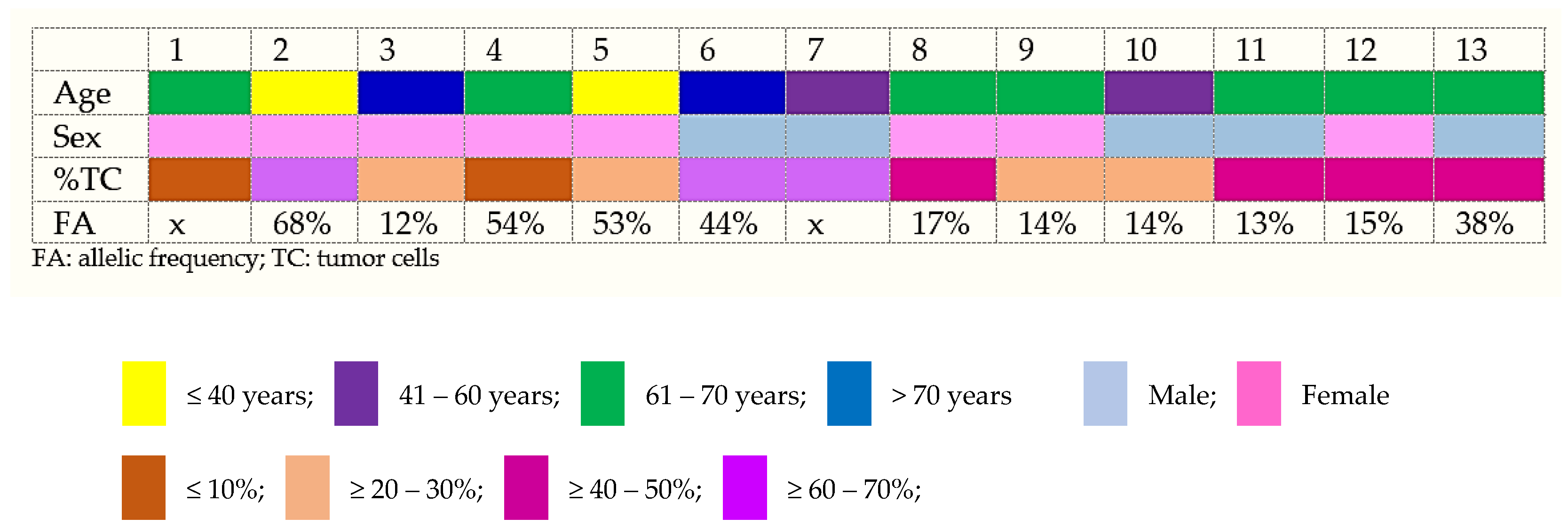
| Cases | %TC | DNA Yield, ng/μL | Input DNA, ng | NGS Panel | Genes Alteration | Variant Name | Chromos. no. | Position (hg 19) | Type | Variant Annotation/Variant Effect | Variant Allele Fraction | Amino Acid Change | Coverage | Exon | DNA | RNA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mapped Reads | On Target | Mean Depth | Uniformity | Mapped Reads | On Target | |||||||||||||||
| 1 | 5% | 0.142 | 2.24 | Oncomine™ BRCA RA | X | X | X | X | X | X | X | X | X | X | 603,223 | 99.26% | 2.63 | 99.58% | NA | NA |
| 2 | 60% | TOO LOW * | Oncomine™ CA v3 | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 39.36 | p.Gly12Val | 1992 | 2 | 3,283,028 | 97.02% | 892.7 | 76.90% | NA | NA | |
| TP53 | c.388C>T p.(Leu130Phe) | 17 | chr17:7578542 | SNV | missense | 99.56 | p.Leu130Phe | 229 | 5 | |||||||||||
| RAD51C | c.709C>T p.(Arg237Ter) | 17 | chr17:56787223 | SNV | nonsense | 68.92 | p.Arg237Ter | 637 | 5 | |||||||||||
| 3 | 20% | TOO LOW * | Oncomine™ CA v3 | KRAS | c.34G>C p.(Gly12Arg) | 12 | chr12:25398284 | SNV | missense | 12.57 | p.Gly12Arg | 1520 | 2 | 3,503,790 | 89.13% | 843.9 | 86.25% | NA | NA | |
| 4 | 10% | 0.216 | 1.62 | Oncomine™ CA v3 | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 33.05 | p.Gly12Val | 1652 | 2 | 2,354,334 | 95.62% | 655.3 | 92.11% | NA | NA |
| FANCA | c.923G>A p.(Gly308Asp) | 16 | chr16:89862397 | SNV | missense | 54.17 | p.Gly308Asp | 216 | 11 | |||||||||||
| TP53 | c.234dup p.(Ala79Serfs * 70) | 17 | chr17:7579452 | INDEL | Frameshift Insertion | 33.56 | p.Ala79fs | 298 | 4 | |||||||||||
| ATM | c.2804C>T p.(Thr935Met) | 11 | chr11:108139302 | SNV | missense | 49.28 | p.Thr935Met | 828 | 18 | |||||||||||
| 5 | 20% | 0.346 | 2.60 | Oncomine™ CA v3 | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 21.64 | p.Gly12Val | 1996 | 2 | 9,660,836 | 95.25% | 2.613 | 87.27% | NA | NA |
| FANCA | c.1844C>G p.(Pro615Arg) | 16 | chr16:89842206 | SNV | missense | 54.63 | p.Pro615Arg | 1966 | 21 | |||||||||||
| ATR | c.2704T>C p.(Ser902Pro) | 3 | chr3:142272170 | SNV | missense | 53.1 | p.Ser902Pro | 2000 | 13 | |||||||||||
| TP53 | c.574C>T p.(Gln192Ter) | 17 | chr17:7578275 | SNV | nonsense | 19.65 | p.Gln192Ter | 682 | 6 | |||||||||||
| 6 | 70% | 0.448 | 5.38 | Oncomine™ FA | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 44 | p.Gly12Val | 1988 | 2 | 890,131 | 88.42% | 2756 | 86.74% | 73,047 | 95.61% |
| PIK3CA | c.2078G>A p.(Arg693His) | 3 | chr3:178938836 | SNV | missense | 6 | p.Arg693His | 2000 | 14 | |||||||||||
| 7 | 70% | 0.596 | 8.94 | Oncomine™ BRCA RA | X | X | X | X | X | X | X | X | X | 1,155,958 | 99.51% | 5203 | 98.26% | NA | NA | |
| 8 | 40% | TOO LOW * | Oncomine™ FA | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 17 | p.Gly12Val | 1988 | 2 | 1,361,477 | 96.50% | 4.652 | 92.24% | 292,911 | 84.88% | |
| 9 | 20% | TOO LOW * | Oncomine™ FA | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 14 | p.Gly12Val | 1999 | 2 | 1,332,844 | 98.03% | 4695 | 94.05% | 213,722 | 87.67% | |
| 10 | 30% | 0.268 | 3.22 | Oncomine™ FA | KRAS | c.35G>T p.(Gly12Val) | 12 | chr12:25398284 | SNV | missense | 14 | p.Gly12Val | 1999 | 2 | 199,900 | 97.57% | 703.9 | 94.96% | 30,188 | 85.86% |
| 11 | 40% | TOO LOW * | 4.00 | Oncomine™ FA | NRAS | c.182A>G p.(Gln61Arg) | 1 | chr1:115256529 | SNV | missense | 13 | p.Gln61Arg | 1998 | 3 | 353,198 | 92.28% | 1125 | 93.02% | 34,968 | 67.76% |
| JAK1 | c.1978G>A p.(Asp660Asn) | 1 | chr1:65312339 | SNV | missense | 5 | p.Asp660Asn | 734 | 14 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza da Silva, R.; Pina, M.J.; Cirnes, L.; Gouveia, L.; Albergaria, A.; Schmitt, F. Comprehensive Genomic Studies on the Cell Blocks of Pancreatic Cancer. Diagnostics 2024, 14, 906. https://doi.org/10.3390/diagnostics14090906
Souza da Silva R, Pina MJ, Cirnes L, Gouveia L, Albergaria A, Schmitt F. Comprehensive Genomic Studies on the Cell Blocks of Pancreatic Cancer. Diagnostics. 2024; 14(9):906. https://doi.org/10.3390/diagnostics14090906
Chicago/Turabian StyleSouza da Silva, Ricella, Maria João Pina, Luís Cirnes, Luís Gouveia, André Albergaria, and Fernando Schmitt. 2024. "Comprehensive Genomic Studies on the Cell Blocks of Pancreatic Cancer" Diagnostics 14, no. 9: 906. https://doi.org/10.3390/diagnostics14090906







