Enterobacterales Biofilm-Specific Genes and Antimicrobial and Anti-Inflammatory Biomarkers in the Blood of Patients with Ischemic Heart Disease
Abstract
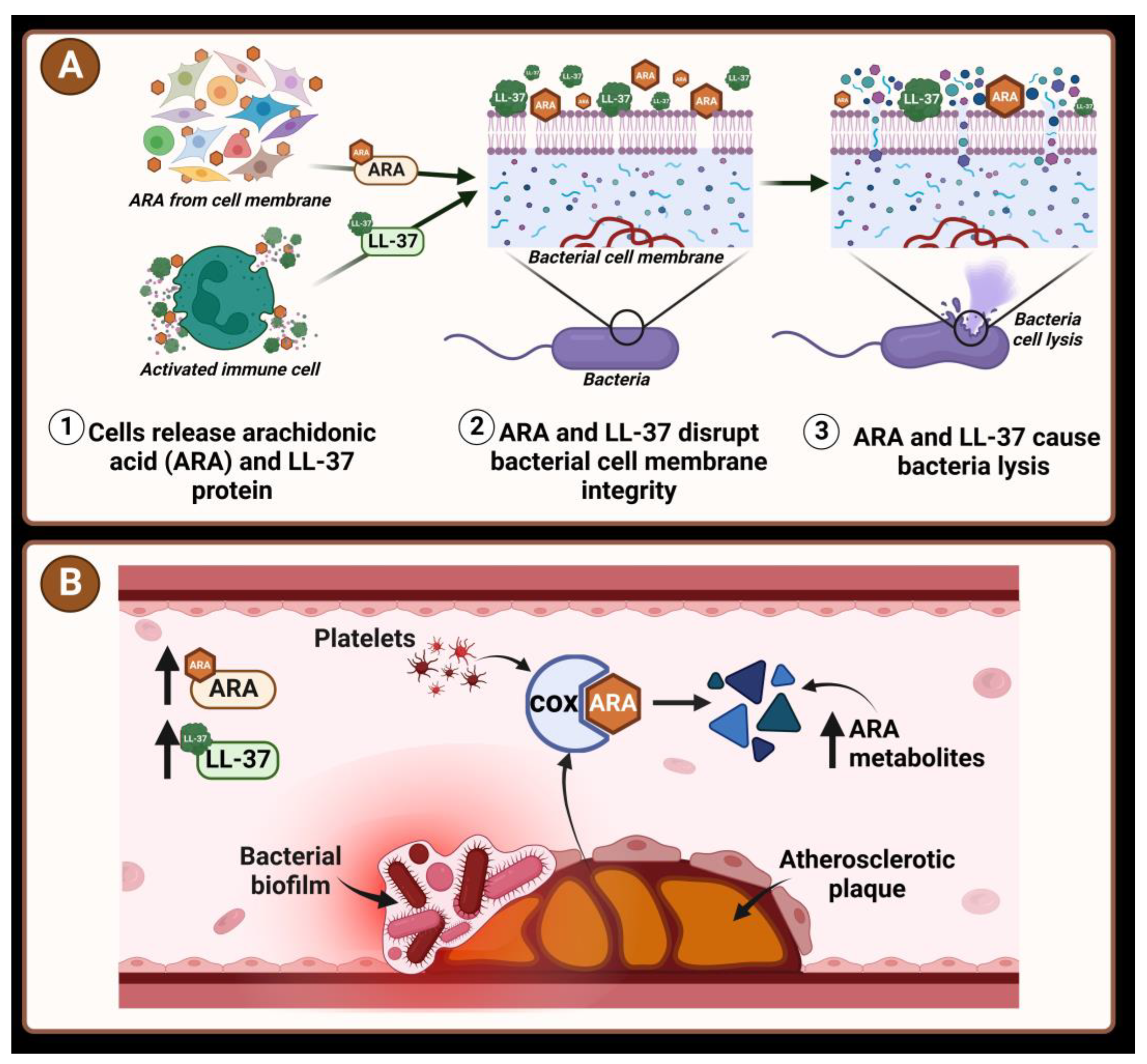
:1. Introduction
2. Materials and Methods
2.1. Study Population and Inclusion Criteria
2.2. Preparation of Blood Samples
2.3. Extraction of Bacterial DNA
2.4. Confirmation of Bacterial DNA with Sanger Sequencing
2.5. Detection of LL-37
2.6. Detection of ARA Metabolites in Blood Plasma
2.7. Statistical Analysis
3. Results
3.1. Bacterial DNA Sequencing Results
3.2. Enterobacterales Genes in Patient Blood
3.3. Protein LL-37 Concentration and Bacterial Genes
3.4. Concentration of Arachidonic Acid Metabolites and Presence of Enterobacterales Genes in Blood Samples
3.5. LL-37 and Arachidonic acid Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Factor | Enterobacterales Genes Detected | Enterobacterales Genes Not Detected | Pearson χ2 p-Value or p-Value between Medians | Total |
|---|---|---|---|---|
| Sex | ||||
| Males n (%) | 11 (28.2) | 28 (71.8) | 3.419 p = 0.086 | 39 (100) |
| Females n (%) | 4 (11.1) | 32 (88.9) | 36 (100) | |
| Antropometric parameters | ||||
| Less than 65 years old n (%) | 10 (34.5) | 19 (65.5) | 6.198 p = 0.018 | 29 (100) |
| 65 years and older n (%) | 5 (10.9) | 41 (89.1) | 46 (100) | |
| Age in years median (min–max) | 64.3 (44.5–79.3) | 70.1 (42.5–87.5) | p = 0.039 | 68.9 (42.5–87.5) |
| Patient weight in kg median (min–max) | 88 (53–113) | 78 (45–134) | p = 0.378 | 80 (45–134) |
| Body weight index in kg/m2 median (min–max) | 26.9 (18.1–45.3) | 27.7 (17.3–49.8) | p = 0.624 | 27.3 (17.3–49.8) |
| Waist circumference in cm median (min–max) | 92 (67–110) | 92 (70–132) | p = 0.886 | 92 (67–132) |
| Left ventricle ejection fraction | ||||
| Left ventricle ejection fraction in % median (min–max) | 45 (20–55) | 50 (25–55) | p = 0.668 | 49 (20–55) |
| ST-elevation MI | ||||
| STEMI n (%) | 4 (14.3) | 24 (85.7) | 0.912 p = 0.388 | 28 (100) |
| NSTEMI n (%) | 11 (23.4) | 36 (76.6) | 47 (100) | |
| MI in anamnesis | ||||
| First n (%) | 11 (19.3) | 46 (80.7) | 0.073 p = 0.747 | 57 (100) |
| Recurrent n (%) | 4 (22.2) | 14 (77.8) | 18 (100) | |
| Hypertension | ||||
| Yes n (%) | 7 (15.2) | 39 (84.8) | 1.701 p = 0.241 | 46 (100) |
| No n (%) | 8 (27.6) | 21 (72.4) | 29 (100) | |
| Diabetes | ||||
| Yes n (%) | 7 (36.8) | 12 (63.2) | 4.511 p = 0.048 | 19 (100) |
| No n (%) | 8 (14.3) | 48 (85.7) | 56 (100) | |
| Smoking | ||||
| Smokers n (%) | 3 (16.7) | 15 (83.3) | 0.079 p = 1 | 18 (100) |
| Non-smokers n (%) | 11 (19.6) | 45 (80.4) | 56 (100) | |
| Concomitant drug users, n (%) | ||||
| Antidiabetic drugs | 6 (35.3) | 11 (64.7) | 3.214 p = 0.091 | 17 (100) |
| Insulin | 3 (42.9) | 4 (57.1) | 2.521 p = 0.138 | 7 (100) |
| ACE inhibitors | 15 (21.4) | 55 (78.6) | 1.339 p = 0.576 | 70 (100) |
| Angiotensin II receptor antagonists | 1 (25) | 3 (75) | 0.066 p = 1 | 4 (100) |
| Beta adrenoblockers | 15 (21.1) | 56 (78.9) | 1.056 p = 0.578 | 71 (100) |
| Statins | 15 (20.3) | 59 (79.7) | 0.253 p = 1 | 74 (100) |
| Calcium Channel Blockers | 1 (20) | 4 (80) | 0.000 p = 1 | 5 (100) |
| Diuretics | 4 (25) | 12 (75) | 0.318 p = 0.725 | 16 (100) |
| Proton pump inhibitors | 2 (50) | 2 (50) | 2.377 p = 0.176 | 4 (100) |
| Blood test parameters | ||||
| Platelet count (×109/L) median (min–max) | 224 (94–926) | 220 (112–1000) | p = 0.632 | 222 (94–926) |
| WBC (×109/L) median (min–max) | 9.43 (5.3–19.9) | 8.67 (4.34–16.9) | p = 0.251 | 8.77 (4.34–19.9) |
| C-reactive protein (mg/L) median (min–max) | 1.68 (1–249.5) | 6.33 (1–275.6) | p = 0.185 | 5.88 (1–275.6) |
| Creatinine (µmol/L) median (min–max) | 89 (73–303) | 86.5 (54–262) | p = 0.672 | 87 (54–303) |
| Hemoglobin (g/L) median (min–max) | 134 (107–153) | 130.5 (89–160) | p = 0.543 | 132 (89–160) |
| Platelet aggregation after induction with ADP (%) | 18 (6–59) | 18.5 (6–68) | p = 0.791 | 18 (6–68) |
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- European Cardiovascular Disease Statistics. 2017. Available online: https://ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 15 March 2022).
- An Official Website of the European Union. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20200928-1 (accessed on 23 April 2022).
- Ou, J.S.; Li, H.M.; Shi, M.M.; Ou, Z.J. Ischemic Heart Disease. In Encyclopedia of Gerontology and Population Aging; Gu, D., Dupre, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Pahwa, R.; Jialal, I. Atherosclerosis. [Updated 8 August 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK507799/ (accessed on 1 March 2022).
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Buja, L.M.; Vander Heide, R.S. Pathobiology of Ischemic Heart Disease: Past, Present and Future. Cardiovasc. Pathol. 2016, 25, 214–220. [Google Scholar] [CrossRef]
- Jonsson, A.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Six, I.; Guillaume, N.; Jacob, V.; Mentaverri, R.; Kamel, S.; Boullier, A.; Slama, M. The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19. Int. J. Mol. Sci. 2022, 23, 6196. [Google Scholar] [CrossRef]
- Xu, S.; Jin, T.; Weng, J. Endothelial Cells as a Key Cell Type for Innate Immunity: A Focused Review on RIG-I Signaling Pathway. Front. Immunol. 2022, 13, 951614. [Google Scholar] [CrossRef] [PubMed]
- Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 2017, 38, 3195–3201. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Petrozza, V.; Cammisotto, V.; Pacini, L.; Sorrentino, V.; Martinelli, O.; Irace, L.; Sciarretta, S.; Frati, G.; et al. Localization of lipopolysaccharide from Escherichia Coli into human atherosclerotic plaque. Sci. Rep. 2018, 8, 3598. [Google Scholar] [CrossRef] [PubMed]
- Zdimal, A.M.; Davies, D.G. Laboratory Grown Biofilms of Bacteria Associated with Human Atherosclerotic Carotid Arteries Release Collagenases and Gelatinases during Iron-Induced Dispersion. Microbiol. Spectr. 2022, 10, e0100121. [Google Scholar] [CrossRef]
- Lanter, B.B.; Sauer, K.; Davies, D.G.; Cassone, A. Bacteria Present in Carotid Arterial Plaques Are Found as Biofilm Deposits Which May Contribute to Enhanced Risk of Plaque Rupture. mBio 2014, 5, e01206-14. [Google Scholar] [CrossRef]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. CMI 2014, 20, P981–P990. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Niba, E.T.; Naka, Y.; Nagase, M.; Mori, H.; Kitakawa, M. A genome-wide approach to identify the genes involved in biofilm formation in E. coli. DNA Res. 2007, 14, 237–246. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J.M. Escherichia coli biofilms. Curr. Top Microbiol. Immunol. 2008, 322, 249–289. [Google Scholar] [PubMed]
- Scott, P.M.; Erickson, K.M.; Troutman, J.M. Identification of the Functional Roles of Six Key Proteins in the Biosynthesis of Enterobacteriaceae Colanic Acid. Biochemistry 2019, 58, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpour, Z.; Tadjrobehkar, O.; Shahkhah, M. Significant association between genes encoding virulence factors with antibiotic resistance and phylogenetic groups in community acquired uropathogenic Escherichia coli isolates. BMC Microbiol. 2020, 20, 241. [Google Scholar] [CrossRef]
- Qin, Y.; He, Y.; She, Q.; Larese-Casanova, P.; Li, P.; Chai, Y. Heterogeneity in respiratory electron transfer and adaptive iron utilization in a bacterial biofilm. Nat. Commun. 2019, 10, 3702. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Fida, M.; Wolf, M.J.; Hamdi, A.; Vijayvargiya, P.; Esquer Garrigos, Z.; Khalil, S.; Greenwood-Quaintance, K.E.; Thoendel, M.J.; Patel, R. Detection of Pathogenic Bacteria from Septic Patients Using 16S Ribosomal RNA Gene-Targeted Metagenomic Sequencing. Clin. Infect. Dis. 2021, 73, 1165–1172. [Google Scholar] [CrossRef]
- Tsukuda, M.; Kitahara, K.; Miyazaki, K. Comparative RNA function analysis reveals high functional similarity between distantly related bacterial 16 S rRNAs. Sci. Rep. 2017, 7, 9993. [Google Scholar] [CrossRef]
- Narayana Iyengar, S.; Dietvorst, J.; Ferrer-Vilanova, A.; Guirado, G.; Muñoz-Berbel, X.; Russom, A. Toward Rapid Detection of Viable Bacteria in Whole Blood for Early Sepsis Diagnostics and Susceptibility Testing. ACS Sens. 2021, 6, 3357–3366. [Google Scholar] [CrossRef]
- Liu, C.F.; Shi, X.P.; Chen, Y.; Jin, Y.; Zhang, B. Rapid diagnosis of sepsis with TaqMan-Based multiplex real-time PCR. J. Clin. Lab Anal. 2018, 32, e22256. [Google Scholar] [CrossRef]
- Klyachkin, Y.M.; Idris, A.; Rodell, C.B.; Tripathi, H.; Ye, S.; Nagareddy, P.; Asfour, A.; Gao, E.; Annabathula, R.; Ratajczak, M.; et al. Cathelicidin Related Antimicrobial Peptide (CRAMP) Enhances Bone Marrow Cell Retention and Attenuates Cardiac Dysfunction in a Mouse Model of Myocardial Infarction. Stem. Cell Rev. Rep. 2018, 14, 702–714. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Yamashita, S.; Li, W.; Liu, C.; Chen, Y.; Zhou, P.; Chi, Y.; Wang, S.; Zhao, B.; et al. Acute ST-segment elevation myocardial infarction is associated with decreased human antimicrobial peptide LL-37 and increased human neutrophil peptide-1 to 3 in plasma. J. Atheroscler. Thromb. 2012, 19, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, H.; Zhou, J.; Wang, Y.; Li, J.; Zhao, X.; Li, N.; Liu, C.; Zhou, P.; Chen, Y.; et al. Prognostic Impacts of LL-37 in Relation to Lipid Profiles of Patients with Myocardial Infarction: A Prospective Cohort Study. Biomolecules 2022, 12, 1482. [Google Scholar] [CrossRef]
- Bei, Y.; Pan, L.L.; Zhou, Q.; Zhao, C.; Xie, Y.; Wu, C.; Meng, X.; Gu, H.; Xu, J.; Zhou, L.; et al. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sheng, Z.; Tan, Y.; Chen, R.; Zhou, J.; Li, J.; Zhao, Q.; Wang, Y.; Zhao, X.; Chen, Y.; et al. High Human Antimicrobial Peptide LL-37 Level Predicts Lower Major Adverse Cardiovascular Events after an Acute ST-Segment Elevation Myocardial Infarction. J. Atheroscler. Thromb. 2022, 29, 1499–1510. [Google Scholar] [CrossRef]
- Eberhard, J.; Jepsen, S.; Pohl, L.; Albers, H.K.; Açil, Y. Bacterial challenge stimulates formation of arachidonic acid metabolites by human keratinocytes and neutrophils in vitro. Clin. Diagn. Lab Immunol. 2002, 9, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tatarunas, V.; Kupstyte-Kristapone, N.; Zvikas, V.; Jakstas, V.; Zaliunas, R.; Lesauskaite, V. Factors associated with platelet reactivity during dual antiplatelet therapy in patients with diabetes after acute coronary syndrome. Sci. Rep. 2020, 10, 3175. [Google Scholar] [CrossRef]
- Tatarunas, V.; Kupstyte-Kristapone, N.; Norvilaite, R.; Tamakauskas, V.; Skipskis, V.; Veikutiene, A.; Jurgaityte, J.; Stuoka, M.; Lesauskaite, V. The impact of CYP2C19 and CYP4F2 variants and clinical factors on treatment outcomes during antiplatelet therapy. Pharmacogenomics 2019, 20, 483–492. [Google Scholar] [CrossRef]
- Peasey, A.; Bobak, M.; Kubinova, R.; Malyutina, S.; Pajak, A.; Tamosiunas, A.; Pikhart, H.; Nicholson, A.; Marmot, M. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: Rationale and design of the HAPIEE study. BMC Public Health 2006, 6, 255. [Google Scholar] [CrossRef]
- Sidstedt, M.; Hedman, J.; Romsos, E.L.; Waitara, L.; Wadsö, L.; Steffen, C.R.; Vallone, P.M.; Rådström, P. Inhibition mechanisms of hemoglobin, immunoglobulin G, and whole blood in digital and real-time PCR. Anal. Bioanal. Chem. 2018, 410, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.T.; Hien, T.T.T.; Huyen, T.T.T.; Quyen, D.T.; Van Son, T.; Hoan, P.Q.; Phuong, N.T.K.; Lien, T.T.; Binh, M.T.; Van Tong, H.; et al. Enrichment of bacterial DNA for the diagnosis of blood stream infections. BMC Infect. Dis. 2016, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Shrirao, A.B.; Schloss, R.S.; Fritz, Z.; Shrirao, M.V.; Rosen, R.; Yarmush, M.L. Autofluorescence of blood and its application in biomedical and clinical research. Biotechnol. Bioeng. 2021, 118, 4550–4576. [Google Scholar] [CrossRef] [PubMed]
- Barghouthi, S.A. A Universal Method for the Identification of Bacteria Based on General PCR Primers. Indian J. Microbiol. 2011, 51, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xue, M.; Yu, L.; Qi, K.; Ni, J.; Chen, X.; Deng, R.; Shang, F.; Xue, T. QseBC is involved in the biofilm formation and antibiotic resistance in Escherichia coli isolated from bovine mastitis. Peer J. 2020, 8, e8833. [Google Scholar] [CrossRef]
- Tatarunas, V.; Kupstyte, N.; Giedraitiene, A.; Skipskis, V.; Jakstas, V.; Zvikas, V.; Lesauskaite, V. The impact of CYP2C19*2, CYP4F2*3, and clinical factors on platelet aggregation, CYP4F2 enzyme activity, and 20-hydroxyeicosatetraenoic acid concentration in patients treated with dual antiplatelet therapy. Blood Coagul. Fibrinolysis 2017, 28, 658–664. [Google Scholar] [CrossRef]
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010, 52, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, V.; Rathinavel, A.; Pushpanathan, M.; Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Elevated levels of circulating DNA in cardiovascular disease patients: Metagenomic profiling of microbiome in the circulation. PLoS ONE 2014, 9, e105221. [Google Scholar] [CrossRef]
- Amar, J.; Lange, C.; Payros, G.; Garret, C.; Chabo, C.; Lantieri, O.; Courtney, M.; Marre, M.; Charles, M.A.; Balkau, B.; et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: The D.E.S.I.R. study. PLoS ONE 2013, 8, e54461. [Google Scholar] [CrossRef]
- Hammad, D.B.M.; Hider, S.L.; Liyanapathirana, V.C.; Tonge, D.P. Molecular Characterization of Circulating Microbiome Signatures in Rheumatoid Arthritis. Front. Cell Infect Microbiol. 2020, 9, 440. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Snow, D.E.; Everett, J.; Mayer, G.; Cox, S.B.; Miller, B.; Rumbaugh, K.; Wolcott, R.A.; Wolcott, R.D. The presence of biofilm structures in atherosclerotic plaques of arteries from legs amputated as a complication of diabetic foot ulcers. J. Wound Care 2016, 25, S16–S22. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Jensen, B.A.H.; Varin, T.V.; Servant, F.; Van Blerk, S.; Richard, D.; Marceau, S.; Surette, M.; Biertho, L.; Lelouvier, B.; et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2020, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Madacki-Todorović, K.; Eminović, I.; Mehmedinović, N.I.; Ibrišimović, M. Insulin Acts as Stimulatory Agent in Diabetes-Related Escherichia Coli Pathogenesis. Int. J. Diabetes Clin. Res. 2018, 5, 098. [Google Scholar]
- Patel, N.; Curtis, J.C.; Plotkin, B.J. Insulin Regulation of Escherichia coli Abiotic Biofilm Formation: Effect of Nutrients and Growth Conditions. Antibiotics 2021, 10, 1349. [Google Scholar] [CrossRef]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide production is required for the development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 2000, 182, 3593–3596. [Google Scholar] [CrossRef]
- Zhang, J.; Poh, C.L. Regulating exopolysaccharide gene wcaF allows control of Escherichia coli biofilm formation. Sci. Rep. 2018, 8, 13127. [Google Scholar] [CrossRef]
- Norgren, M.; Båga, M.; Tennent, J.M.; Normark, S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol. Microbiol. 1987, 1, 169–178. [Google Scholar] [CrossRef]
- Harwalkar, A.; Gupta, S.; Rao, A.; Srinivasa, H. Lower prevalence of hlyD, papC and cnf-1 genes in ciprofloxacin-resistant uropathogenic Escherichia coli than their susceptible counterparts isolated from southern India. J. Infect. Public Health. 2014, 7, 413–419. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2016, 66, 872–885. [Google Scholar] [CrossRef]
- Ellermann, M.; Jimenez, A.G.; Pifer, R.; Ruiz, N.; Sperandio, V. The Canonical Long-Chain Fatty Acid Sensing Machinery Processes Arachidonic Acid to Inhibit Virulence in Enterohemorrhagic Escherichia coli. mBio 2021, 12, e03247-20. [Google Scholar] [CrossRef]
- Beavers, W.N.; Monteith, A.J.; Amarnath, V.; Mernaugh, R.L.; Roberts, L.J.; Chazin, W.J.; Davies, S.S.; Skaar, E.P. Arachidonic Acid Kills Staphylococcus aureus through a Lipid Peroxidation Mechanism. mBio 2019, 10, e01333-19. [Google Scholar] [CrossRef]
- Abot, A.; Wemelle, E.; Laurens, C.; Paquot, A.; Pomie, N.; Carper, D.; Bessac, A.; Orea, X.M.; Fremez, C.; Fontanie, M.; et al. Identification of new enterosynes using prebiotics: Roles of bioactive lipids and mu-opioid receptor signalling in humans and mice. Gut 2021, 70, 1078–1087. [Google Scholar] [CrossRef]
- Kulkarni, A.; Nadler, J.L.; Mirmira, R.G.; Casimiro, I. Regulation of Tissue Inflammation by 12-Lipoxygenases. Biomolecules 2021, 11, 717. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Linde, A.; Lushington, G.H.; Abello, J.; Melgarejo, T. Clinical Relevance of Cathelicidin in Infectious Disease. J. Clin. Cell Immunol. 2013, S13. [Google Scholar] [CrossRef]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Kanitkar, T.; Narouz, M.; Azadian, B.S.; Moore, L.S.; Mughal, N. Clinical utility and cost-effectiveness of bacterial 16S rRNA and targeted PCR based diagnostic testing in a UK microbiology laboratory network. Sci. Rep. 2020, 10, 7965. [Google Scholar] [CrossRef] [PubMed]
- Catchpool, M.; Ramchand, J.; Martyn, M.; Hare, D.L.; James, P.A.; Trainer, A.H.; Knight, J.; Goranitis, I. A cost-effectiveness model of genetic testing and periodical clinical screening for the evaluation of families with dilated cardiomyopathy. Genet. Med. 2019, 21, 2815–2822. [Google Scholar] [CrossRef] [PubMed]

| 16S rDNA Primer | Primer Sequence | Oligomer Location |
|---|---|---|
| F3 (Forward) | 5′-GATACCCTGGTAGTCCA-3′ | 753–769 |
| R3 (Reverse) | 5′-TGGACTACCAGGGTATC-3′ | 769–752 |
| F4 (Forward) | 5′-CCGCCTGGGGAGTACG-3′ | 840–856 |
| R4 (Reverse) | 5′-CGTACTCCCCAGGCGG-3′ | 856–840 |
| F5 (Forward) | 5′-CCTACGGGAGGCAGCAG-3′ | 326–343 |
| F6 (Forward) | 5′-GCAGCCGCGGTAATAC-3′ | 481–497 |
| R1b (Reverse) | 5′-TACCTTGTTACGACTTC-3′ | 1468–1451 |
| General Mixtures | Oligomers Used in Mixtures |
|---|---|
| Reaction one | F5, F6, R1b |
| Reaction two | F5, F6, R3 |
| Reaction two (b) | F5, F6, R3, R4 |
| Reaction three | F3, F4, R1b |
| Step | Temperature (°C) | Time | Cycles |
|---|---|---|---|
| Initialization | 95 | 5 min | 1 |
| Denaturation | 95 | 30 s | 35 |
| Annealing | 48 | 30 s | |
| Elongation | 50 | 30 s |
| Gene | Primer | Primer Sequence | Amplification Product Size (bp) | Reference |
|---|---|---|---|---|
| wcaF | Forward | 5′-TCTCGGTGCCGAAAGGGTTC-3′ | 236 | [40] |
| Reverse | 5′-ATTGACGTCATCGCCGACCC-3′ | |||
| papC | Forward | 5′-GACGGCTGTACTGCAGGGTGTGGCG-3′ | 328 | [31] |
| Reverse | 5′-ATATCCTTTCTGCAGGGATGCAATA-3′ | |||
| sdhC | Forward | 5′-CGCCAGCCGCCCAGCACAG-3′ | 285 | [32] |
| Reverse | 5′-GGTATGGAAGGTCTGTTCCGTCA GATTGGTATTTACAGCCC-3′ |
| Step | Temperature (°C) | Time | Cycles |
|---|---|---|---|
| Initialization | 98 | 5 min | 1 |
| Denaturation | 98 | 5 s | 40 |
| Annealing | 58 | 15 s | |
| Elongation | 72 | 30 s |
| Genes | Gene Detection | Blood Samples from Female Patients, n (%) | Blood Samples from Male Patients, (n) % | p-Value |
|---|---|---|---|---|
| 16S rDNA | Detected | 8 (22.2) | 15 (38.5) | 0.142 |
| Not detected | 28 (77.8) | 24 (61.5) | ||
| wcaF | Detected | 3 (8.3) | 7 (17.9) | 0.176 |
| Not detected | 33 (91.7) | 39 (82.1) | ||
| papC | Detected | 0 (0) | 0 (0) | – |
| Not detected | 36 (100) | 39 (100) | ||
| sdhC | Detected | 1 (2.8) | 5 (12.8) | 0.089 |
| Not detected | 35 (97.2) | 34 (87.2) |
| n (%) | LL-37 Concentration (ng/mL) Median (Min–Max) | p | |
|---|---|---|---|
| 16S rDNA | |||
| Present | 18 (30.5) | 4 (1.3–11.8) | 0.014 |
| Absent | 41 (69.5) | 2.8 (0.8–9.2) | |
| Total | 59 (100) | 2.9 (0.8–11.8) | |
| Biofilm-associated genes | |||
| Present | 10 (16.9) | 4.4 (1.3–8.4) | 0.03 |
| Absent | 49 (83.1) | 2.8 (0.8–11.8) | |
| Total | 59 (100) | 2.9 (0.8–11.8) | |
| Variable | Enterobacterales Genes Detected | Enterobacterales Genes Not Detected | p-Value | Total |
|---|---|---|---|---|
| 5S-HETE (ng/mL) (min–max) | 21 (6.4–68.1) | 12.7 (1.5–57) | 0.077 | 13.7 (1.5–68.1) |
| 9-HETE (ng/mL) (min–max) | 3.9 (1.6–14) | 3.2 (0.1–20.7) | 0.207 | 3.3 (0.1–20.7) |
| 12S-HETE (ng/mL) (min–max) | 6.4 (1–20.9) | 1.9 (0.2–30.5) | 0.046 | 3 (0.2–30.5) |
| 15S-HETE (ng/mL) (min–max) | 5.3 (3.4–80.9) | 8.6 (3–289) | 0.446 | 7.2 (3–289) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giedraitiene, A.; Tatarunas, V.; Kaminskaite, K.; Meskauskaite, U.; Boieva, S.; Ajima, Y.; Ciapiene, I.; Veikutiene, A.; Zvikas, V.; Kupstyte-Kristapone, N.; et al. Enterobacterales Biofilm-Specific Genes and Antimicrobial and Anti-Inflammatory Biomarkers in the Blood of Patients with Ischemic Heart Disease. Diagnostics 2024, 14, 546. https://doi.org/10.3390/diagnostics14050546
Giedraitiene A, Tatarunas V, Kaminskaite K, Meskauskaite U, Boieva S, Ajima Y, Ciapiene I, Veikutiene A, Zvikas V, Kupstyte-Kristapone N, et al. Enterobacterales Biofilm-Specific Genes and Antimicrobial and Anti-Inflammatory Biomarkers in the Blood of Patients with Ischemic Heart Disease. Diagnostics. 2024; 14(5):546. https://doi.org/10.3390/diagnostics14050546
Chicago/Turabian StyleGiedraitiene, Agne, Vacis Tatarunas, Kornelija Kaminskaite, Ugne Meskauskaite, Svitlana Boieva, Yu Ajima, Ieva Ciapiene, Audrone Veikutiene, Vaidotas Zvikas, Nora Kupstyte-Kristapone, and et al. 2024. "Enterobacterales Biofilm-Specific Genes and Antimicrobial and Anti-Inflammatory Biomarkers in the Blood of Patients with Ischemic Heart Disease" Diagnostics 14, no. 5: 546. https://doi.org/10.3390/diagnostics14050546





