Retrospective Analysis of the Predictive Value of 18F-FDG PET/CT Metabolic Parameters for PD-L1 Expression in Cervical Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 18F-FDG PET/CT Protocol and Image Interpretation
2.3. Immunohistochemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Univariate Analysis of the Relationship between PD-L1 Status and Clinicopathologic Characteristics
3.3. Multivariable Analysis of the Relationship between PD-L1 Status and Clinicopathologic Characteristics
3.4. ROC Curve Analysis of the Predicting Role of PD-L1 Status by Metabolic Parameters
3.5. Comprehensive Predictive Ability of Clinicopathological and Metabolic Parameters to PD-L1 Expression
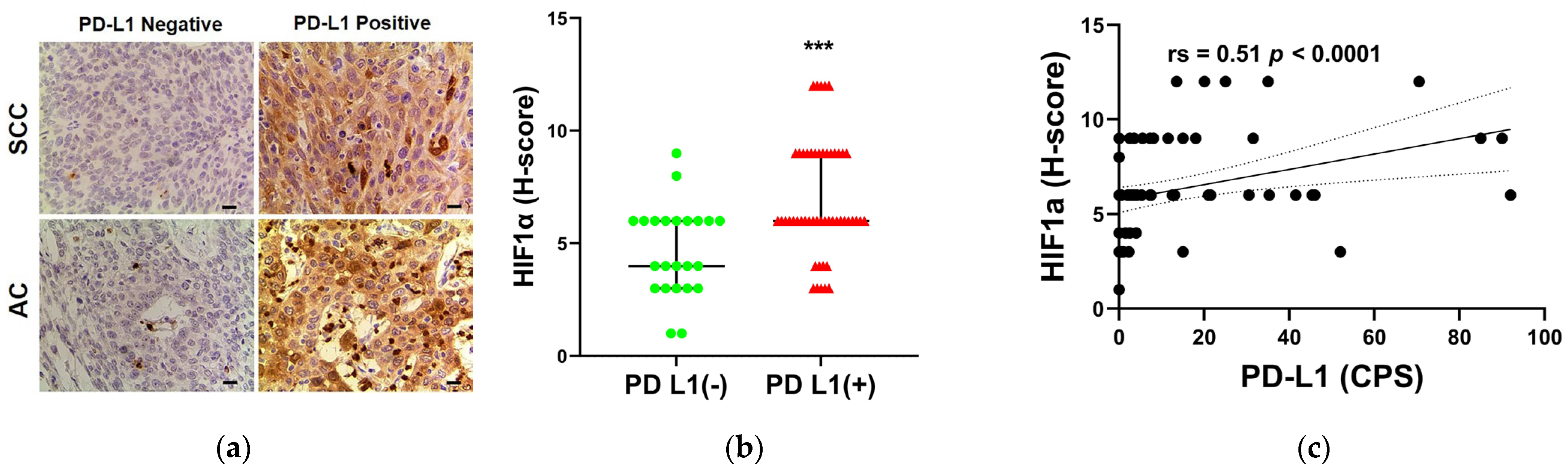
3.6. The Relationship between PD-L1 STATUS and HIF-1α Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G. PD-1/PD-L1 blockade in cervical cancer: Current studies and perspectives. Front. Med. 2019, 13, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Tewari, K.S. Current and emerging immunotherapies for recurrent cervical cancer. Clin. Adv. Hematol. Oncol. HO 2022, 20, 108–115. [Google Scholar]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Salem, A.E.; Shah, H.R.; Covington, M.F.; Koppula, B.R.; Fine, G.C.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology: I. Hematologic Malignancies. Cancers 2022, 14, 5941. [Google Scholar] [CrossRef]
- Covington, M.F.; Koppula, B.R.; Fine, G.C.; Salem, A.E.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology: II. Primary Thoracic and Breast Malignancies. Cancers 2022, 14, 2689. [Google Scholar] [CrossRef]
- Koppula, B.R.; Fine, G.C.; Salem, A.E.; Covington, M.F.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology: III. Gastrointestinal Malignancies. Cancers 2022, 14, 2668. [Google Scholar] [CrossRef]
- Salem, A.E.; Fine, G.C.; Covington, M.F.; Koppula, B.R.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology-IV. Gynecologic and Genitourinary Malignancies. Cancers 2022, 14, 3000. [Google Scholar] [CrossRef]
- Wiggins, R.H.; Hoffman, J.M.; Fine, G.C.; Covington, M.F.; Salem, A.E.; Koppula, B.R.; Morton, K.A. PET-CT in Clinical Adult Oncology-V. Head and Neck and Neuro Oncology. Cancers 2022, 14, 2726. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.C.; Covington, M.F.; Koppula, B.R.; Salem, A.E.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology-VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors. Cancers 2022, 14, 2835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Li, J.L.; Wang, Y.H.; Tai, X.H.; Liu, L.; Zhang, X.X.; An, Y.W.; Li, H.L. The Correlation Between (18)F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Semiquantitative Parameters and the Clinical Features and Pathological Biological Indexes of Gastric Cancer. Cancer Biother. Radiopharm. 2021; Ahead of print. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, J.; Xu, J.; Wu, F.; Huang, Q.; Guo, M.; Liao, T.; Liu, S.; Lan, X.; Liao, S.; et al. Value of (18)F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: A retrospective analysis of 849 Chinese patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 735–750. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhou, X.; Liu, J.; Huang, G. Relationship between the expression of PD-1/PD-L1 and (18)F-FDG uptake in bladder cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chen, W.; Zhang, Y.; Shi, H.; Cheng, D.; Xiu, Y. (18)F-FDG maximum standard uptake value predicts PD-L1 expression on tumor cells or tumor-infiltrating immune cells in non-small cell lung cancer. Ann. Nucl. Med. 2020, 34, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, T.; Fujii, T.; Kurozumi, S.; Katayama, A.; Honda, C.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; et al. FDG uptake reflects breast cancer immunological features: The PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res. Treat. 2020, 181, 331–338. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet. Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Voss, M.H.; Azad, A.A.; Hansen, A.R.; Gray, J.E.; Welsh, S.J.; Song, X.; Kuziora, M.; Meinecke, L.; Blando, J.; Achour, I.; et al. A Randomized Phase II Study of MEDI0680 in Combination with Durvalumab versus Nivolumab Monotherapy in Patients with Advanced or Metastatic Clear-cell Renal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 3032–3041. [Google Scholar] [CrossRef]
- Basile, G.; Bandini, M.; Gibb, E.A.; Ross, J.S.; Raggi, D.; Marandino, L.; Costa de Padua, T.; Crupi, E.; Colombo, R.; Colecchia, M.; et al. Neoadjuvant Pembrolizumab and Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Cancer: 3-Year Median Follow-Up Update of PURE-01 Trial. Clin. Cancer Res. 2022, 28, 5107–5114. [Google Scholar] [CrossRef]
- Long, G.V.; Luke, J.J.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet. Oncol. 2022, 23, 1378–1388. [Google Scholar] [CrossRef]
- Kaira, K.; Kuji, I.; Kagamu, H. Value of (18)F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2021, 21, 11. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jo, K.; Hwang, S.H.; Jeong, Y.; Lee, J.Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Kang, W.J. Association between PD-L1 expression and (18)F-FDG uptake in ovarian cancer. Ann. Nucl. Med. 2021, 35, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Y.; Zhao, Q.; Wang, L.; Song, X.; Li, Y.; Jiang, L. PD-L1 expression correlation with metabolic parameters of FDG PET/CT and clinicopathological characteristics in non-small cell lung cancer. EJNMMI Res. 2020, 10, 51. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Y.; Huang, G.; Liu, J. Relationship between PD-L1 expression and (18)F-FDG uptake in gastric cancer. Aging 2019, 11, 12270–12277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhuang, Y.; Fu, K.; Chen, P.; Wang, Y.; Zhuo, J.; Liao, X.; Chen, H.; Lin, Q. Usefulness of [(18)F]fluorodeoxyglucose PET/CT for evaluating the PD-L1 status in nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1065–1074. [Google Scholar] [CrossRef]
- Saglam, O.; Zhou, J.; Wang, X.; Conejo-Garcia, J.R. PD-L1 Expression Correlates with Young Age and CD8+ TIL Density in Poorly Differentiated Cervical Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2020, 39, 428–435. [Google Scholar] [CrossRef]
- Blichárová, A.; Tancoš, V.; Benetinová, Z.; Verbóová, Ľ.; Grendár, M.; Mazuráková, A.; Plank, L.; Mechírová, E. Programmed death ligand-1 expression and its association with the degree of differentiation and the presence of necrosis in non-small cell lung carcinoma. Pathol. Res. Pract. 2023, 242, 154296. [Google Scholar] [CrossRef]
- Akhave, N.; Zhang, J.; Bayley, E.; Frank, M.; Chiou, S.H.; Behrens, C.; Chen, R.; Hu, X.; Parra, E.R.; Lee, W.C.; et al. Immunogenomic profiling of lung adenocarcinoma reveals poorly differentiated tumors are associated with an immunogenic tumor microenvironment. Lung Cancer 2022, 172, 19–28. [Google Scholar] [CrossRef]
- Cameselle-García, S.; Abdulkader-Sande, S.; Sánchez-Ares, M.; Rodríguez-Carnero, G.; Garcia-Gómez, J.; Gude-Sampedro, F.; Abdulkader-Nallib, I.; Cameselle-Teijeiro, J.M. PD-L1 expression and immune cells in anaplastic carcinoma and poorly differentiated carcinoma of the human thyroid gland: A retrospective study. Oncol. Lett. 2021, 22, 553. [Google Scholar] [CrossRef]
- Albrecht, T.; Brinkmann, F.; Albrecht, M.; Lonsdorf, A.S.; Mehrabi, A.; Hoffmann, K.; Kulu, Y.; Charbel, A.; Vogel, M.N.; Rupp, C.; et al. Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer. Cancers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Xing, M.; Han, L.; Gan, S.; Ma, J.; Wu, F.; Huang, Y.; Chen, Y.; Tian, W.; An, C.; et al. High PD-L1 expression drives glycolysis via an Akt/mTOR/HIF-1α axis in acute myeloid leukemia. Oncol. Rep. 2020, 43, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Xie, J.; Liu, F.; Duan, Y.; Wu, Y.; Huang, S.; He, X.; Wang, Z.; Wu, X. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin β4/SNAI1/SIRT3 signaling pathway. Oncogene 2018, 37, 4164–4180. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sharma, A.; Mathew, S.P.; Jaganathan, B.G. Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front. Immunol. 2022, 13, 955476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Huo, Y.; Yang, X.; Li, Y.; Xu, H.; Li, X.F. Visualization of hypoxia in cancer cells from effusions in animals and cancer patients. Front. Oncol. 2022, 12, 1019360. [Google Scholar] [CrossRef]
- Elzakra, N.; Kim, Y. HIF-1α Metabolic Pathways in Human Cancer. Adv. Exp. Med. Biol. 2021, 1280, 243–260. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zuo, X.L.; Cai, J.; Zhang, Y.; Han, G.Y.; Zhang, L.; Ding, W.Z.; Wu, J.D.; Wang, X.H. Hypoxia-associated circPRDM4 promotes immune escape via HIF-1α regulation of PD-L1 in hepatocellular carcinoma. Exp. Hematol. Oncol. 2023, 12, 17. [Google Scholar] [CrossRef]
- Yong, J.; Gröger, S.; von Bremen, J.; Meyle, J.; Ruf, S. Immunorthodontics: PD-L1, a Novel Immunomodulator in Cementoblasts, Is Regulated by HIF-1α under Hypoxia. Cells 2022, 11, 2350. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Duan, X.; Liu, C.; Du, Y.; Wang, X.; Luo, Y.; Cui, Y. HIF-1α/IL-8 axis in hypoxic macrophages promotes esophageal cancer progression by enhancing PD-L1 expression. Cancer Gene Ther. 2023, 30, 358–367. [Google Scholar] [CrossRef]

| Variable | All | PD-L1 Negative | PD-L1 Positive | p-Value |
|---|---|---|---|---|
| Number of patients | 74 | 23 | 51 | |
| Age, year; median (IQR) | 54.5 (20.3) | 54.0 (23.0) | 56.0 (18.0) | 0.7962 |
| <55 | 37 | 12 | 25 | 0.8017 |
| ≥55 | 37 | 11 | 26 | |
| Histologic type | ||||
| SCC | 52 | 14 | 38 | 0.2348 |
| AC | 22 | 9 | 13 | |
| Tumor differentiation | ||||
| Moderate–Well | 25 | 13 | 12 | 0.0081 |
| Poor | 49 | 10 | 39 | |
| FIGO stage * | ||||
| I–II | 40 | 12 | 28 | 0.8275 |
| III–IV | 34 | 11 | 23 | |
| Tumor size, cm; median (IQR) | 4.4 (2.0) | 4.0 (2.0) | 4.5 (2.2) | 0.0059 |
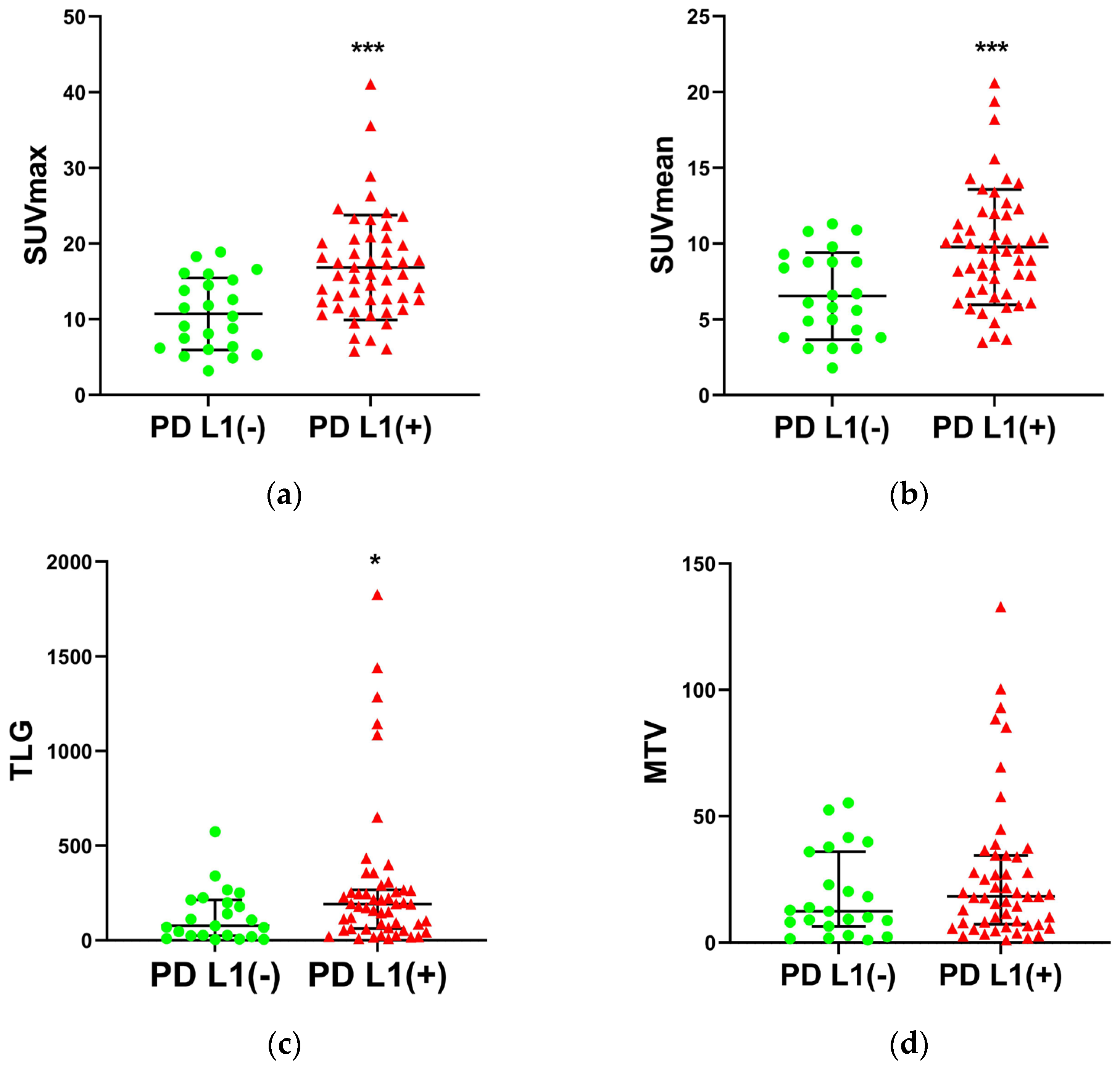
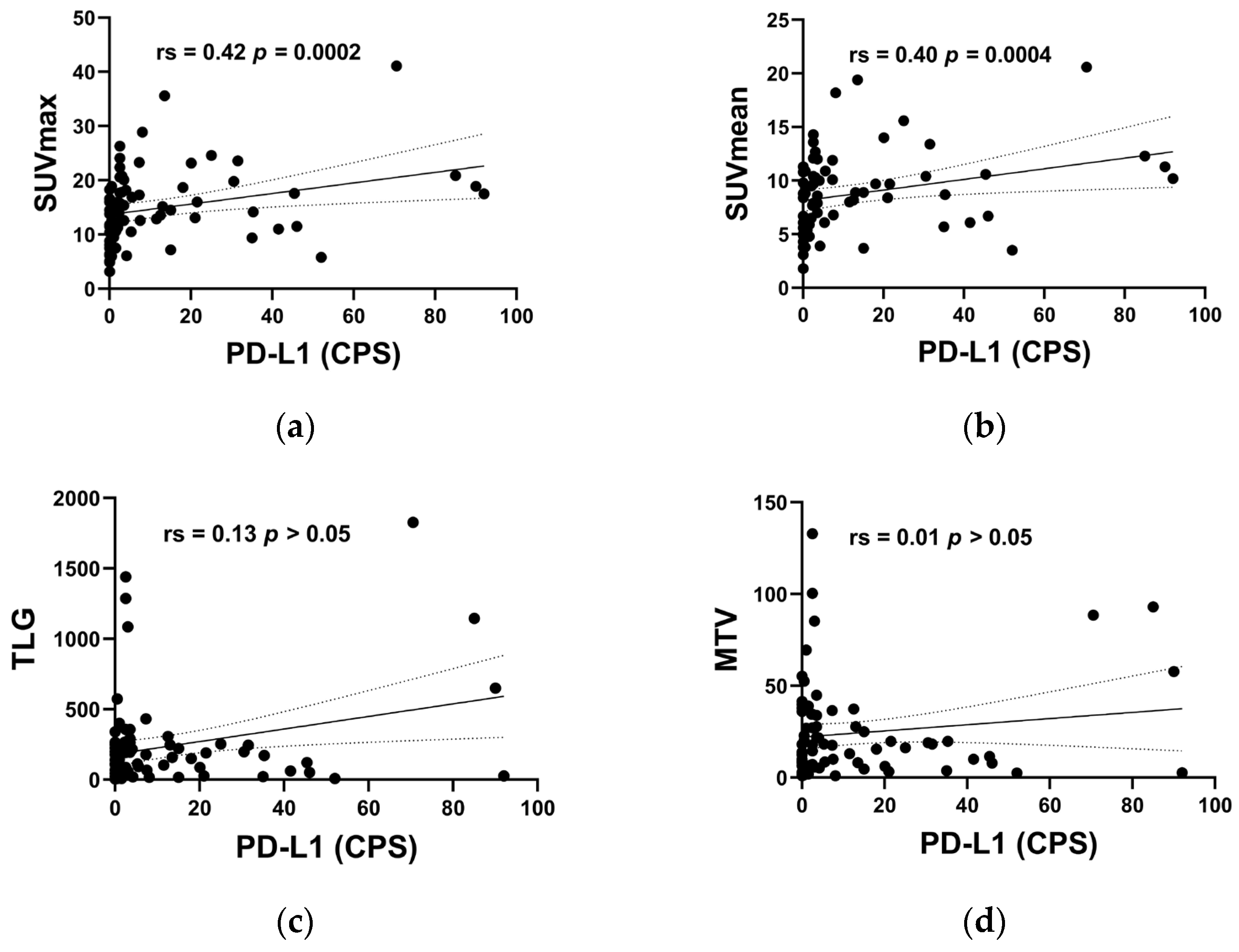
| SUVmax, mean ± SD | 14.9 ± 6.9 | 10.7 ± 4.8 | 16.8 ± 6.9 | 0.0003 |
| SUVmean, mean ± SD | 8.8 ± 3.8 | 6.5 ± 2.9 | 9.8 ± 3.8 | 0.0006 |
| TLG, median (IQR) | 154.9 (203.8) | 76.4 (189.0) | 191.2 (205.3) | 0.0331 |
| MTV, median (IQR) | 17.0 (27.4) | 12.4 (33.1) | 18.3 (27.3) | 0.3078 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| SUVmax | 2.849 | 1.066–7.615 | 0.037 |
| Tumor differentiation | 0.168 | 0.040–0.703 | 0.015 |
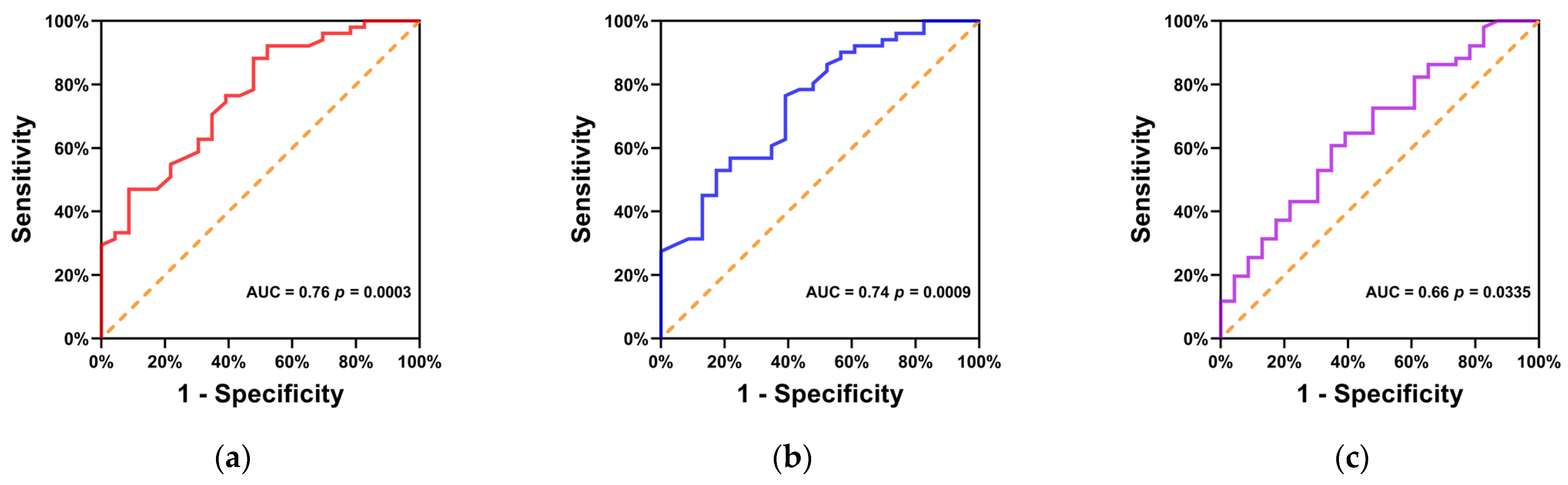
| SUVmax | SUVmean | TLG | |
|---|---|---|---|
| AUC (95% CI) | 0.76 (0.65–0.88) | 0.74 (0.62–0.86) | 0.66 (0.52–0.79) |
| p value | 0.0003 | 0.0009 | 0.0335 |
| cut-off value | 10.45 | 6.75 | 143.4 |
| Sensitivity (95% CI) | 88.2% (76.6–94.5%) | 76.5% (63.2–86.0%) | 60.8% (47.1–73.0%) |
| Specificity (95% CI) | 52.2% (33.0–70.8%) | 60.9% (40.8–77.8%) | 65.2% (44.9–81.2%) |
| Accuracy | 77.0% | 71.6% | 62.2% |
| Probability | Number of Patients | PD-L1 Negative | PD-L1 Positive | p-Value |
|---|---|---|---|---|
| Low | 8 | 8 (100%) | 0 (0%) | p < 0.0001 |
| Moderate | 27 | 9 (33.3%) | 18 (66.7%) | |
| High | 39 | 6 (15.4%) | 33 (84.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Pang, W.; Song, J.; Wang, X.; Tang, H.; Liu, Y.; Yi, H.; Wang, Y.; Gu, Q.; Li, L. Retrospective Analysis of the Predictive Value of 18F-FDG PET/CT Metabolic Parameters for PD-L1 Expression in Cervical Cancer. Diagnostics 2023, 13, 1015. https://doi.org/10.3390/diagnostics13061015
Ji J, Pang W, Song J, Wang X, Tang H, Liu Y, Yi H, Wang Y, Gu Q, Li L. Retrospective Analysis of the Predictive Value of 18F-FDG PET/CT Metabolic Parameters for PD-L1 Expression in Cervical Cancer. Diagnostics. 2023; 13(6):1015. https://doi.org/10.3390/diagnostics13061015
Chicago/Turabian StyleJi, Jianfeng, Weiqiang Pang, Jinling Song, Xiawan Wang, Huarong Tang, Yunying Liu, Heqing Yi, Yun Wang, Qing Gu, and Linfa Li. 2023. "Retrospective Analysis of the Predictive Value of 18F-FDG PET/CT Metabolic Parameters for PD-L1 Expression in Cervical Cancer" Diagnostics 13, no. 6: 1015. https://doi.org/10.3390/diagnostics13061015





