Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders
Abstract
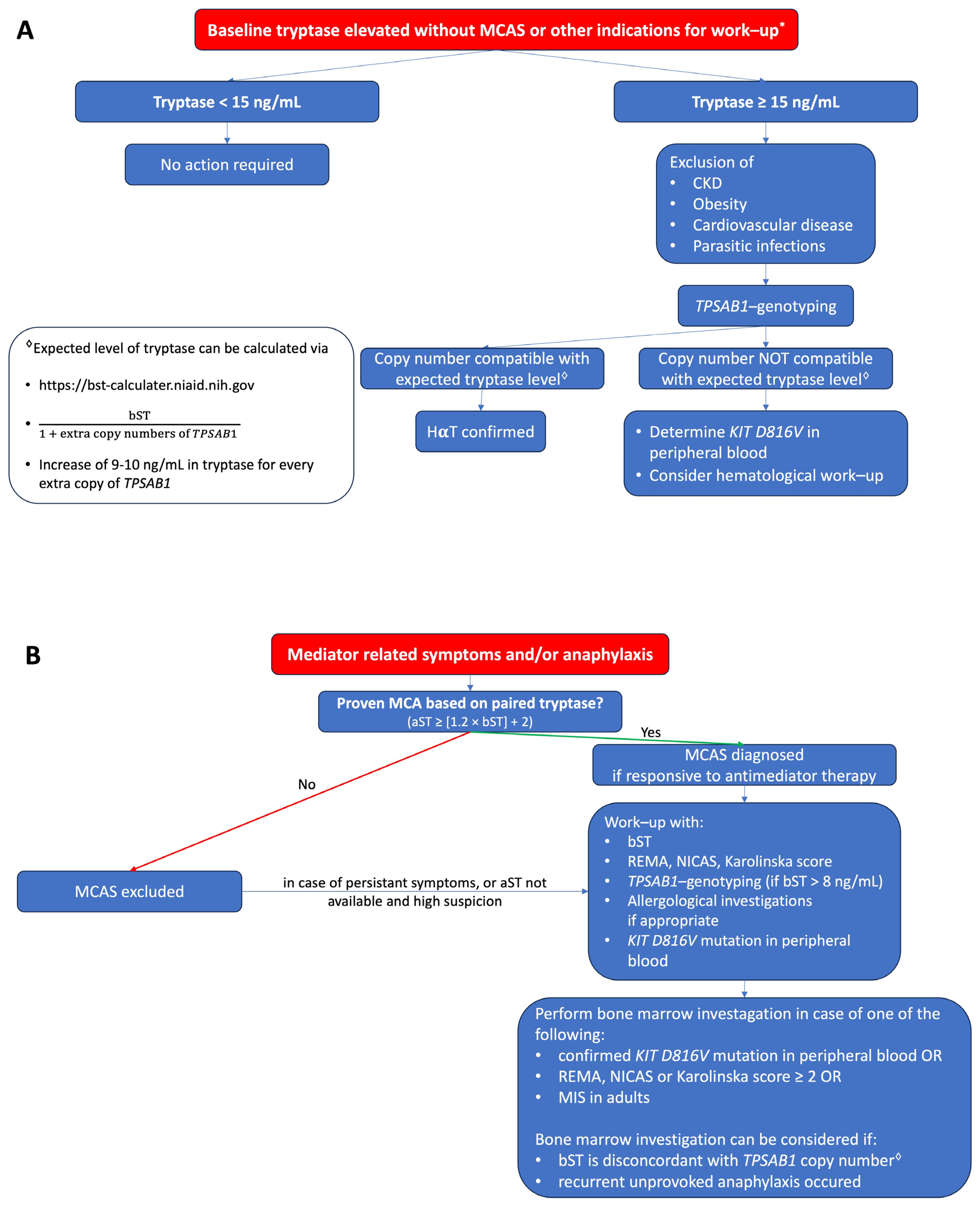
:1. Introduction
2. Clinical Vignettes
2.1. Venom-Induced Anaphylaxis with Elevated bST
2.2. Venom-Induced Anaphylaxis with bST within the Normal Range
2.3. Anaphylaxis Mimicking Epilepsy
2.4. Recurrent Anaphylactic Episodes without Elevated bST
2.5. Anaphylaxis in a Patient with a Relatively Elevated Baseline Serum Tryptase
2.6. Recurrent Angioedema and Relatively Elevated Baseline Serum Tryptase
2.7. Elevated Basal Tryptase without Mediator Related Symptoms
3. Discussion
4. Conclusions
- Acute tryptase is determined within 4 h after the onset of symptoms (ideally between 30 and 120 min after the start of symptoms) and should always be paired with a baseline sample at least 24 h later.
- A baseline tryptase below 8 ng/mL does not exclude primary mast cell disorders.
- In the case of anaphylaxis or recurrent mediator-related symptoms, a baseline tryptase > 8 ng/mL justifies TPSAB1-genotyping.
- The baseline tryptase can be elevated in various situations. The most common cause is hereditary alpha tryptasemia followed by chronic kidney disease.
- Hereditary alpha tryptasemia is a genetic trait characterized by an elevated baseline tryptase, which can be asymptomatic but may lead to mediator-related symptoms in some patients and may increase the risk of (severe) anaphylaxis.
- Tryptase height does not reflect symptom severity in patients with hereditary alpha tryptasemia or systemic mastocytosis.
- The link between hereditary alpha tryptasemia and systemic mastocytosis remains to be elucidated.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jogie-Brahim, S.; Min, H.K.; Fukuoka, Y.; Xia, H.Z.; Schwartz, L.B. Expression of alpha-tryptase and beta-tryptase by human basophils. J. Allergy Clin. Immunol. 2004, 113, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Lewis, R.A.; Austen, K.F. Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 1981, 256, 11939–11943. [Google Scholar] [CrossRef] [PubMed]
- Pallaoro, M.; Fejzo, M.S.; Shayesteh, L.; Blount, J.L.; Caughey, G.H. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J. Biol. Chem. 1999, 274, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Tryptase genetics and anaphylaxis. J. Allergy Clin. Immunol. 2006, 117, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Yasuda, S.; Madhusudhan, M.S.; Li, L.; Yang, Y.; Krilis, S.A.; Sali, A.; Stevens, R.L. Human tryptase epsilon (PRSS22), a new member of the chromosome 16p13.3 family of human serine proteases expressed in airway epithelial cells. J. Biol. Chem. 2001, 276, 49169–49182. [Google Scholar] [CrossRef]
- Sprinzl, B.; Greiner, G.; Uyanik, G.; Arock, M.; Haferlach, T.; Sperr, W.R.; Valent, P.; Hoermann, G. Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. Int. J. Mol. Sci. 2021, 22, 2458. [Google Scholar] [CrossRef]
- Lyons, J.J. Inherited and acquired determinants of serum tryptase levels in humans. Ann. Allergy Asthma Immunol. 2021, 127, 420–426. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Bradford, T.R.; Rouse, C.; Irani, A.M.; Rasp, G.; Van der Zwan, J.K.; Van der Linden, P.W. Development of a new, more sensitive immunoassay for human tryptase: Use in systemic anaphylaxis. J. Clin. Immunol. 1994, 14, 190–204. [Google Scholar] [CrossRef]
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arock, M.; et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51. [Google Scholar] [CrossRef]
- Ebo, D.G.; De Puysseleyr, L.P.; Van Gasse, A.L.; Elst, J.; Poorten, M.V.; Faber, M.A.; Mertens, C.; Van Houdt, M.; Hagendorens, M.M.; Sermeus, L.; et al. Mast Cell Activation During Suspected Perioperative Hypersensitivity: A Need for Paired Samples Analysis. J. Allergy Clin. Immunol. Pract. 2021, 9, 3051–3059.e1. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Gülen, T.; Brockow, K.; Alvarez-Twose, I.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM-Adjusted Proposal of the ECNM-AIM Consortium. J. Allergy Clin. Immunol. Pract. 2022, 10, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int. Arch. Allergy Immunol. 2022, 183, 693–705. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am. J. Hematol. 2023, 98, 1097–1116. [Google Scholar] [CrossRef]
- Lyons, J.J. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features. Immunol. Allergy Clin. N. Am. 2018, 38, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Chollet, M.B.; Akin, C. Hereditary alpha tryptasemia is not associated with specific clinical phenotypes. J. Allergy Clin. Immunol. 2022, 149, 728–735.e2. [Google Scholar] [CrossRef]
- Robey, R.C.; Wilcock, A.; Bonin, H.; Beaman, G.; Myers, B.; Grattan, C.; Briggs, T.A.; Arkwright, P.D. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J. Allergy Clin. Immunol. Pract. 2020, 8, 3549–3556. [Google Scholar] [CrossRef]
- Beyens, M.; Elst, J.; van der Poorten, M.L.; Van Gasse, A.; Toscano, A.; Verlinden, A.; Vermeulen, K.; Maes, M.B.; Oude Elberink, J.; Ebo, D.; et al. Mastocytosis and related entities: A practical roadmap. Acta Clin. Belg. 2022, 78, 325–335. [Google Scholar] [CrossRef]
- Kačar, M.; Rijavec, M.; Šelb, J.; Korošec, P. Clonal mast cell disorders and hereditary α-tryptasemia as risk factors for anaphylaxis. Clin. Exp. Allergy 2023, 53, 392–404. [Google Scholar] [CrossRef]
- Carter, M.C.; Park, J.; Vadas, P.; Worm, M. Extrinsic and Intrinsic Modulators of Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1998–2006. [Google Scholar] [CrossRef]
- Waters, A.M.; Park, H.J.; Weskamp, A.L.; Mateja, A.; Kachur, M.E.; Lyons, J.J.; Rosen, B.J.; Boggs, N.A. Elevated Basal Serum Tryptase: Disease Distribution and Variability in a Regional Health System. J. Allergy Clin. Immunol. Pract. 2022, 10, 2424–2435.e5. [Google Scholar] [CrossRef]
- Mateja, A.; Wang, Q.; Chovanec, J.; Kim, J.; Wilson, K.J.; Schwartz, L.B.; Glover, S.C.; Carter, M.C.; Metcalfe, D.D.; Brittain, E.; et al. Defining baseline variability of serum tryptase levels improves accuracy in identifying anaphylaxis. J. Allergy Clin. Immunol. 2022, 149, 1010–1017.e10. [Google Scholar] [CrossRef]
- Schwartz, L.B. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463. [Google Scholar] [CrossRef]
- Serrier, J.; Khoy, K.; Petit, G.; Parienti, J.J.; Laroche, D.; Mariotte, D.; Le Mauff, B. Mediators of anaphylactic reactions: Tryptase and histamine stability in whole blood. Allergy 2021, 76, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Gonzalez, C.; Klingebiel, C.; Michel, M. Tryptase and anaphylaxis: The case for systematic paired samples in all settings, from the playground to the COVID-19 vaccination center. Rev. Fr. Allergol. 2022, 62, 287–288. [Google Scholar] [CrossRef]
- Borer-Reinhold, M.; Haeberli, G.; Bitzenhofer, M.; Jandus, P.; Hausmann, O.; Fricker, M.; Helbling, A.; Müller, U. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell-mediated hypersensitivity reaction: A prospective study in Hymenoptera venom allergic patients. Clin. Exp. Allergy 2011, 41, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Egner, W.; Sargur, R.; Shrimpton, A.; York, M.; Green, K. A 17-year experience in perioperative anaphylaxis 1998-2015: Harmonizing optimal detection of mast cell mediator release. Clin Exp Allergy 2016, 46, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Sala-Cunill, A.; Cardona, V.; Labrador-Horrillo, M.; Luengo, O.; Esteso, O.; Garriga, T.; Vicario, M.; Guilarte, M. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int. Arch. Allergy Immunol. 2013, 160, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Sabato, V.; Tacquard, C.; Garvey, L.H.; Michel, M.; Mertes, P.M.; Ebo, D.G.; Schwartz, L.B.; Castells, M.C. Use and Interpretation of Acute and Baseline Tryptase in Perioperative Hypersensitivity and Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2021, 9, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Mendelson, L.; Rosen, J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N. Engl. J. Med. 1992, 327, 380–384. [Google Scholar] [CrossRef]
- Nantanee, R.; Suratannon, N.; Chatchatee, P. Characteristics and Laboratory Findings of Food-Induced Anaphylaxis in Children: Study in an Asian Developing Country. Int. Arch. Allergy Immunol. 2022, 183, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Dua, S.; Dowey, J.; Foley, L.; Islam, S.; King, Y.; Ewan, P.; Clark, A.T. Diagnostic Value of Tryptase in Food Allergic Reactions: A Prospective Study of 160 Adult Peanut Challenges. J. Allergy Clin. Immunol. Pract. 2018, 6, 1692–1698.e1. [Google Scholar] [CrossRef]
- Giannetti, M.P.; Godwin, G.; Weller, E.; Butterfield, J.H.; Castells, M. Differential mast cell mediators in systemic mastocytosis and hereditary α-tryptasemia. J. Allergy Clin. Immunol. 2022, 150, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H. Nontryptase Urinary and Hematologic Biomarkers of Mast Cell Expansion and Mast Cell Activation: Status 2022. J. Allergy Clin. Immunol. Pract. 2022, 10, 1974–1984. [Google Scholar] [CrossRef]
- Fernandez-Bravo, S.; Palacio Garcia, L.; Requena-Robledo, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Esteban, V. Anaphylaxis: Mediators, Biomarkers, and Microenvironments. J. Investig. Allergol. Clin. Immunol. 2022, 32, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Blasco, P.; Gil-Serrano, J.; Sala-Cunill, A. New Biomarkers in Anaphylaxis (Beyond Tryptase). Curr. Treat. Options Allergy 2022, 9, 303–322. [Google Scholar] [CrossRef]
- Elst, J.; van der Poorten, M.M.; Van Gasse, A.L.; Mertens, C.; Hagendorens, M.M.; Ebo, D.G.; Sabato, V. Tryptase release does not discriminate between IgE- and MRGPRX2-mediated activation in human mast cells. Clin. Exp. Allergy 2022, 52, 797–800. [Google Scholar] [CrossRef]
- Noguchi, S.; Takekawa, D.; Saito, J.; Hashiba, E.; Hirota, K. Serum Tryptase Cannot Differentiate Vancomycin-Induced Anaphylaxis From Red Man Syndrome. J. Clin. Immunol. 2019, 39, 855–856. [Google Scholar] [CrossRef]
- Sabato, V.; Chovanec, J.; Faber, M.; Milner, J.D.; Ebo, D.; Lyons, J.J. First Identification of an Inherited TPSAB1 Quintuplication in a Patient with Clonal Mast Cell Disease. J. Clin. Immunol. 2018, 38, 457–459. [Google Scholar] [CrossRef]
- Francois, F.; Mauff, B.L.; Waeckel, L.; de Chaisemartin, L.; Tabary, T.; Dumontet, E.; Lecron, J.C.; Delamare, B.; Boumediene, A.; Chauvineau-Grenier, A.; et al. Basal serum tryptase: A critical reconsideration of reference values. Allergy 2023, 78, 3003–3006. [Google Scholar] [CrossRef]
- Le, Q.T.; Lyons, J.J.; Naranjo, A.N.; Olivera, A.; Lazarus, R.A.; Metcalfe, D.D.; Milner, J.D.; Schwartz, L.B. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J. Exp. Med. 2019, 216, 2348–2361. [Google Scholar] [CrossRef]
- Valent, P.; Hoermann, G.; Bonadonna, P.; Hartmann, K.; Sperr, W.R.; Broesby-Olsen, S.; Brockow, K.; Niedoszytko, M.; Hermine, O.; Chantran, Y.; et al. The Normal Range of Baseline Tryptase Should Be 1 to 15 ng/mL and Covers Healthy Individuals With HαT. J. Allergy Clin. Immunol. Pract. 2023, 11, 3010–3020. [Google Scholar] [CrossRef]
- Chovanec, J.; Tunc, I.; Hughes, J.; Halstead, J.; Mateja, A.; Liu, Y.; O’Connell, M.P.; Kim, J.; Park, Y.H.; Wang, Q.; et al. Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms. Blood Adv. 2023, 7, 1796–1810. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Sun, G.; Stone, K.D.; Nelson, C.; Wisch, L.; O’Brien, M.; Jones, N.; Lindsley, A.; Komarow, H.D.; Bai, Y.; et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J. Allergy Clin. Immunol. 2014, 133, 1471–1474. [Google Scholar] [CrossRef]
- Sabato, V.; Van De Vijver, E.; Hagendorens, M.; Vrelust, I.; Reyniers, E.; Fransen, E.; Bridts, C.; De Clerck, L.; Mortier, G.; Valent, P.; et al. Familial hypertryptasemia with associated mast cell activation syndrome. J. Allergy Clin. Immunol. 2014, 134, 1448–1450.e3. [Google Scholar] [CrossRef]
- Giannetti, M.P.; Akin, C.; Hufdhi, R.; Hamilton, M.J.; Weller, E.; van Anrooij, B.; Lyons, J.J.; Hornick, J.L.; Pinkus, G.; Castells, M.; et al. Patients with mast cell activation symptoms and elevated baseline serum tryptase level have unique bone marrow morphology. J. Allergy Clin. Immunol. 2021, 147, 1497–1501.e1. [Google Scholar] [CrossRef]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef]
- Glover, S.C.; Carter, M.C.; Korošec, P.; Bonadonna, P.; Schwartz, L.B.; Milner, J.D.; Caughey, G.H.; Metcalfe, D.D.; Lyons, J.J. Clinical relevance of inherited genetic differences in human tryptases: Hereditary alpha-tryptasemia and beyond. Ann. Allergy Asthma Immunol. 2021, 127, 638–647. [Google Scholar] [CrossRef]
- Gülen, T.; Akin, C. Anaphylaxis and Mast Cell Disorders. Immunol. Allergy Clin. N. Am. 2022, 42, 45–63. [Google Scholar] [CrossRef]
- Greiner, G.; Sprinzl, B.; Górska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Schwaab, J.; Alvarez-Twose, I.; Brockow, K.; Bonadonna, P.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Personalized Management Strategies in Mast Cell Disorders: ECNM-AIM User’s Guide for Daily Clinical Practice. J. Allergy Clin. Immunol. Pract. 2022, 10, 1999–2012.e6. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Kubala, S.; Grieco, M.C.; Mateja, A.; Pongracic, J.; Liu, Y.; Frischmeyer-Guerrerio, P.A.; Kumar, R.; Lyons, J.J. Severe food allergy reactions are associated with α-tryptase. J Allergy Clin. Immunol. 2023, 152, 933–939. [Google Scholar] [CrossRef] [PubMed]
- González-de-Olano, D.; Navarro-Navarro, P.; Muñoz-González, J.I.; Sánchez-Muñoz, L.; Henriques, A.; de-Andrés-Martín, A.; Peralta-Arjonilla, D.; Mayado, A.; Jara-Acevedo, M.; García-Montero, A.C.; et al. Clinical impact of the TPSAB1 genotype in mast cell diseases: A REMA study in a cohort of 959 individuals. Allergy, 2023; epub ahead of print. [Google Scholar]
- Couto, M.L.; Silva, M.; Barbosa, M.J.; Ferreira, F.; Fragoso, A.S.; Azenha Rama, T. Defining hereditary alpha-tryptasemia as a risk/modifying factor for anaphylaxis: Are we there yet? Eur. Ann. Allergy Clin. Immunol. 2023, 55, 152–160. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; González-de-Olano, D.; Sánchez-Muñoz, L.; Matito, A.; Jara-Acevedo, M.; Teodosio, C.; García-Montero, A.; Morgado, J.M.; Orfao, A.; Escribano, L. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int. Arch. Allergy Immunol. 2012, 157, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Desai, A.; Komarow, H.D.; Bai, Y.; Clayton, S.T.; Clark, A.S.; Ruiz-Esteves, K.N.; Long, L.M.; Cantave, D.; Wilson, T.M.; et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J. Allergy Clin. Immunol. 2018, 141, 180–188.e3. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Sander, B.; Dahlén, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- González de Olano, D.; Cain, W.V.; Bernstein, J.A.; Akin, C. Disease Spectrum of Anaphylaxis Disorders. J. Allergy Clin. Immunol. Pract. 2023, 11, 1989–1996. [Google Scholar] [CrossRef]
- Gülen, T.; Ljung, C.; Nilsson, G.; Akin, C. Risk Factor Analysis of Anaphylactic Reactions in Patients With Systemic Mastocytosis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1248–1255. [Google Scholar] [CrossRef]
- Dölle-Bierke, S.; Siebenhaar, F.; Burmeister, T.; Worm, M. Detection of KIT D816V mutation in patients with severe anaphylaxis and normal basal tryptase-first data from the Anaphylaxis Registry (NORA). J. Allergy Clin. Immunol. 2019, 144, 1448–1450.e1. [Google Scholar] [CrossRef] [PubMed]
- van Anrooij, B.; van der Veer, E.; de Monchy, J.G.; van der Heide, S.; Kluin-Nelemans, J.C.; van Voorst Vader, P.C.; van Doormaal, J.J.; Oude Elberink, J.N. Higher mast cell load decreases the risk of Hymenoptera venom-induced anaphylaxis in patients with mastocytosis. J. Allergy Clin. Immunol. 2013, 132, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Šelb, J.; Rijavec, M.; Eržen, R.; Zidarn, M.; Kopač, P.; Škerget, M.; Bajrović, N.; Luzar, A.D.; Park, Y.H.; Liu, Y.; et al. Routine KIT p.D816V screening identifies clonal mast cell disease in patients with Hymenoptera allergy regularly missed using baseline tryptase levels alone. J. Allergy Clin. Immunol. 2021, 148, 621–626.e7. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, R.; Lombardo, C.; Passalacqua, G.; Caimmi, C.; Bonifacio, M.; De Matteis, G.; Perbellini, O.; Rossini, M.; Schena, D.; Busa, M.; et al. Clonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levels. J. Allergy Clin. Immunol. 2015, 136, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, J.; Gerds, A.T.; Bose, P.; Castells, M.C.; Deininger, M.W.; Gojo, I.; Gundabolu, K.; Hobbs, G.; Jamieson, C.; McMahon, B.; et al. Systemic Mastocytosis, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1500–1537. [Google Scholar] [CrossRef]
- Greiner, G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Simonitsch-Klupp, I.; Mitterbauer-Hohendanner, G.; Mayerhofer, M.; Müllauer, L.; Sperr, W.R.; Valent, P.; et al. Digital PCR: A Sensitive and Precise Method for KIT D816V Quantification in Mastocytosis. Clin. Chem. 2018, 64, 547–555. [Google Scholar] [CrossRef]
- De Puysseleyr, L.P.; Ebo, D.G.; Elst, J.; Faber, M.A.; Poorten, M.V.; Van Gasse, A.L.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Hagendorens, M.M.; et al. Diagnosis of Primary Mast Cell Disorders in Anaphylaxis: Value of KIT D816V in Peripheral Blood. J. Allergy Clin. Immunol. Pract. 2021, 9, 3176–3187.e3. [Google Scholar] [CrossRef]
- Onnes, M.C.; Alheraky, A.; Nawijn, M.C.; Sluijter, T.E.; Mulder, A.B.; Arends, S.; Oude Elberink, H.N.G. Detection of clonal mast cell disease in wasp venom allergic patients with normal tryptase. Clin. Transl. Allergy 2022, 12, e12174. [Google Scholar] [CrossRef]
- Gülen, T.; Hägglund, H.; Dahlén, B.; Nilsson, G. Mastocytosis: The puzzling clinical spectrum and challenging diagnostic aspects of an enigmatic disease. J. Intern. Med. 2016, 279, 211–228. [Google Scholar] [CrossRef]
- Pyatilova, P.; Akin, C.; Alvarez-Twose, I.; Arock, M.; Bonadonna, P.; Brockow, K.; Butterfield, J.H.; Broesby-Olsen, S.; Carter, M.C.; Castells, M.; et al. Refined Treatment Response Criteria for Indolent Systemic Mastocytosis Proposed by the ECNM-AIM Consortium. J. Allergy Clin. Immunol. Pract. 2022, 10, 2015–2024. [Google Scholar] [CrossRef]
- Sperr, W.R.; Jordan, J.-H.; Fiegl, M.; Escribano, L.; Bellas, C.; Dirnhofer, S.; Semper, H.; Simonitsch-Klupp, I.; Horny, H.-P.; Valent, P. Serum tryptase levels in patients with mastocytosis: Correlation with mast cell burden and implication for defining the category of disease. Int. Arch. Allergy Immunol. 2002, 128, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, A.E.; González, C.; Enríquez, R.; Fernández, J.; Millán, I.; Barber, X.; Amorós, F. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J. Nephrol. 2010, 23, 282–290. [Google Scholar]
- Simon, M.R.; Jan, M.; Yee, J.; Nori, U.S.; Hu, J.; Akin, C.; Schwartz, L.B. Tryptase is not cleared by the kidneys into the urine. Int. Arch. Allergy Immunol. 2010, 152, 28–31. [Google Scholar] [CrossRef]
- Costa, J.J.; Demetri, G.D.; Harrist, T.J.; Dvorak, A.M.; Hayes, D.F.; Merica, E.A.; Menchaca, D.M.; Gringeri, A.J.; Schwartz, L.B.; Galli, S.J. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 1996, 183, 2681–2686. [Google Scholar] [CrossRef]
- Slot, M.C.; Claessen, L.H.J.; Bons, J.A.P.; Menheere, P.; Nieuwhof, C.M.G.; de Boer, D. Tryptase reference ranges are age-dependent in a large population-based cohort. Allergy 2022, 77, 2833–2834. [Google Scholar] [CrossRef]
- Vos, B.J.; van der Veer, E.; van Voorst Vader, P.C.; Mulder, A.B.; van der Heide, S.; Arends, S.; Kluin-Nelemans, J.C.; de Monchy, J.G.; van Doormaal, J.J.; Oude Elberink, J.N. Diminished reliability of tryptase as risk indicator of mastocytosis in older overweight subjects. J. Allergy Clin. Immunol. 2015, 135, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S. Elevated Serum Tryptase in Non-Anaphylaxis Cases: A Concise Review. Int. Arch. Allergy Immunol. 2020, 181, 357–364. [Google Scholar] [CrossRef]
- Lange, L.; Rietschel, E.; Hunzelmann, N.; Hartmann, K. Elevated levels of tryptase in children with nummular eczema. Allergy 2008, 63, 947–949. [Google Scholar] [CrossRef]
- Beceiro, C.; Campos, J.; Valcarcel, M.A.; Fenger, R.V.; Lojo, S.; Linneberg, A.; Vidal, C.; Gonzalez-Quintela, A. Serum concentrations of mast cell tryptase are reduced in heavy drinkers. Alcohol. Clin. Exp. Res. 2015, 39, 672–678. [Google Scholar] [CrossRef]
- Small-Howard, A.; Turner, H. Exposure to tobacco-derived materials induces overproduction of secreted proteinases in mast cells. Toxicol. Appl. Pharmacol. 2005, 204, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Schussler, E.; Yang, A.; Lyons, J.J.; Milner, J.D.; Wang, J. Persistent tryptase elevation in a patient with Gaucher disease. J. Allergy Clin. Immunol. Pract. 2018, 6, 697–699. [Google Scholar] [CrossRef]
- van Toorenenbergen, A.W.; van Daele, P.L.; Boonstra, J.G. False-elevated serum tryptase assay result caused by heterophilic antibodies. J. Allergy Clin. Immunol. 2005, 116, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Akin, C.; Bonadonna, P.; Siebenhaar, F.; Broesby-Olsen, S.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, H.N.G.; Butterfield, J.H.; et al. Selecting the Right Criteria and Proper Classification to Diagnose Mast Cell Activation Syndromes: A Critical Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 3918–3928. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C. Doctor, I Think I Am Suffering from MCAS: Differential Diagnosis and Separating Facts from Fiction. J. Allergy Clin. Immunol. Pract. 2019, 7, 1109–1114. [Google Scholar] [CrossRef]
- Gulen, T. Management of Mediator Symptoms, Allergy, and Anaphylaxis in Mastocytosis. Immunol. Allergy Clin. N. Am. 2023, 43, 681–698. [Google Scholar] [CrossRef]
- Butterfield, J.H. Increased Excretion of Mast Cell Mediator Metabolites During Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2023, 11, 2542–2546. [Google Scholar] [CrossRef]
- Boehm, T.; Reiter, B.; Ristl, R.; Petroczi, K.; Sperr, W.; Stimpfl, T.; Valent, P.; Jilma, B. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy 2019, 74, 583–593. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: A consensus proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef]
| HαT |
|---|
| Chronic renal failure |
| Obesity |
| Hematologic malignancy (especially myeloid neoplasms) |
| SM |
| Chronic parasitic infections (e.g., helminthic infections) |
| Administration of SCF |
| Rare genetic mutations (e.g., GATA2 or PLCG2) |
| Elderly |
| Cardiovascular disease |
| False positive (due to interference with the immunoassay) |
| Criterion A | Clinical signs of recurrent or severe MCA with involvement of at least two organ systems |
| Criterion B | Proof of MCA with consensus formula of aST and bST (an increase of 20% + 2 ng/mL) or other biomarkers such as histamine, prostaglandins, leukotrienes, and metabolites |
| Criterion C | Response to MC-stabilizing drugs, drugs directed against MC mediator production, or drugs inhibiting MC mediator release or inhibiting MC mediator effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyens, M.; Toscano, A.; Ebo, D.; Gülen, T.; Sabato, V. Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders. Diagnostics 2023, 13, 3662. https://doi.org/10.3390/diagnostics13243662
Beyens M, Toscano A, Ebo D, Gülen T, Sabato V. Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders. Diagnostics. 2023; 13(24):3662. https://doi.org/10.3390/diagnostics13243662
Chicago/Turabian StyleBeyens, Michiel, Alessandro Toscano, Didier Ebo, Theo Gülen, and Vito Sabato. 2023. "Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders" Diagnostics 13, no. 24: 3662. https://doi.org/10.3390/diagnostics13243662





