Determining an Optimal Oxygen Saturation Target Range Based on Neonatal Maturity: Demonstration of a Decision Tree Analytic
Abstract
:1. Background
2. Methods
2.1. Design
2.2. Population
2.3. Data Collection
2.4. Endpoints
2.5. Statistical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Association between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA 2018, 319, 2190–2201. [Google Scholar] [CrossRef]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef]
- Silverman, W.A. A Cautionary Tale About Supplemental Oxygen: The Albatross of Neonatal Medicine. Pediatrics 2004, 113, 394–396. [Google Scholar] [CrossRef]
- Hagadorn, J.I.; Furey, A.M.; Nghiem, T.-H.; Schmid, C.H.; Phelps, D.L.; Pillers, D.-A.M.; Cole, C.H.; The AVIOx Study Group. Achieved Versus Intended Pulse Oximeter Saturation in Infants Born Less Than 28 Weeks’ Gestation: The AVIOx Study. Pediatrics 2006, 118, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.J.; Polin, R.A.; Watterberg, K.L.; Benitz, W.E.; Eichenwald, E.C.; Poindexter, B.B.; Stewart, D.L.; Aucott, S.W.; Goldsmith, J.P.; Puopolo, K.M.; et al. Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics 2016, 138, e20161576. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef]
- Huizing, M.J.; Villamor-Martínez, E.; Vento, M.; Villamor, E. Pulse oximeter saturation target limits for preterm infants: A survey among European neonatal intensive care units. Eur. J. Pediatr. 2017, 176, 51–56. [Google Scholar] [CrossRef]
- Perrone, S.; on behalf of the National Study Group of Neonatal Clinical Biochemistry of the Italian Society of Neonatology; Giordano, M.; De Bernardo, G.; Lugani, P.; Sarnacchiaro, P.; Stazzoni, G.; Buonocore, G.; Esposito, S.; Tataranno, M.L. Management of oxygen saturation monitoring in preterm newborns in the NICU: The Italian picture. Ital. J. Pediatr. 2021, 47, 1–10. [Google Scholar] [CrossRef]
- Wilinski, M.; Bachman, T.E.; Piwowrczyk, P.; Kostuch, M.; Tousty, J.; Berła, K.; Hajdar, R.; Skrzypek, M. Routine se of automated FiO2 control in Poland: Prospective registry and survey. Front. Pediatr. 2023, 11, 1213310. [Google Scholar] [CrossRef]
- Hagadorn, J.I.; Sink, D.W.; Buus-Frank, M.E.; Edward, E.M.; Morrow, K.A.; Horbar, J.D.; Ferrelli, K.; Soll, R.F. Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 collaborative. J. Perinatol. 2017, 37, 270–276. [Google Scholar] [CrossRef]
- Askie, L.M.; Henderson-Smart, D.J.; Irwig, L.; Simpson, J.M. Oxygen-Saturation Targets and Outcomes in Extremely Preterm Infants. N. Engl. J. Med. 2003, 349, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Li, F.Y.; Katz, K.; Shabanova, V.; Ehrenkranz, R.A.; Bhandari, V. Temporal quantification of oxygen saturation ranges: An effort to reduce hyperoxia in the neonatal intensive care unit. J. Perinatol. 2014, 34, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Sola, A.; Baquero, H.; Neira, F.; Alvis, R.; Deulofeut, R.; Critz, A. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: Is 85% to 93% an acceptable range? Pediatrics 2008, 121, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Golombek, S.G.; Montes Bueno, M.T.; Lemus-Varela, L.; Zuluaga, C.; Domínguez, F.; Baquero, H.; Young Sarmiento, A.E.; Natta, D.; Rodriguez Perez, J.M.; et al. Safe oxygen saturation targeting and monitoring in preterm infants: Can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014, 103, 1009–1018. [Google Scholar] [CrossRef]
- Kelly, R.; Quine, D.; Stenson, B.J. Exploring the risk of hyperoxia in oxygen-dependent very low birthweight infants in the first week of life to plan future trials of oxygen targeting. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Quine, D.; Stenson, B.J. Arterial oxygen tension (PaO2) values in infants <29 weeks of gestation at currently targeted saturations. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 94, F51–F53. [Google Scholar]
- Di Fiore, J.M.; MacFarlane, P.M.; Martin, R.J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 2019, 46, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Bachman, T.E.; Iyer, N.P.; Newth, C.J.L.; Ross, P.A.; Khemani, R.G. Thresholds for oximetry alarms and target range in the NICU: An observational assessment based on likely oxygen tension and maturity. BMC Pediatr. 2020, 20, 317. [Google Scholar] [CrossRef]
- Engle, W.A.; American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics 2004, 114, 1362–1364. [Google Scholar]
- Wu, X.; Kumar, V.; Ross Quinlan, J.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, A.; Liu, B.; Yu, P.S.; et al. Top 10 algorithms in data mining. Knowl. Inf. Syst. 2008, 14, 1–37. [Google Scholar] [CrossRef]
- Kass, G.V. An Exploratory Technique for Investigating Large Quantities of Categorical Data. Appl. Stat. 1980, 29, 119–127. [Google Scholar] [CrossRef]
- Kelman, G.R.; Elliott, J.E.; Laurie, S.S.; Kern, J.P.; Beasley, K.M.; Goodman, R.D.; Kayser, B.; Subudhi, A.W.; Roach, R.C.; Lovering, A.T.; et al. Digital computer subroutine for the conversion of oxygen tension into saturation. J. Appl. Physiol. 1966, 21, 1375–1376. [Google Scholar] [CrossRef]
- McEvoy, C.; Durand, M.; Hewlett, V. Episodes of spontaneous desaturations in infants with chronic lung disease at two different levels of oxygenation. Pediatr. Pulmonol. 1993, 15, 140–144. [Google Scholar] [CrossRef]
- Kurtom, W.; Dormishian, A.; Jain, D.; Schott, A.; Aguilar, A.C.; Grieb, G.; Bancalari, E.; Claure, N. Effect of the Target Range on Arterial Oxygen Saturation Stability in Extremely Premature Infants. Neonatology 2022, 119, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Loganathan, P.; Lal, M.K.; Bachman, T. Automated Oxygen Delivery in Neonatal Intensive Care. Front. Pediatr. 2022, 10, 915312. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, H.A.; Tan, R.N.; Thio, M.; De Man-Van Ginkel, J.M.; Van Zwet, E.W.; Lopriore, E.; te Pas, A.B. The risk for hyperoxaemia after apnoea, bradycardia and hypoxaemia in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F269–F273. [Google Scholar] [CrossRef] [PubMed]
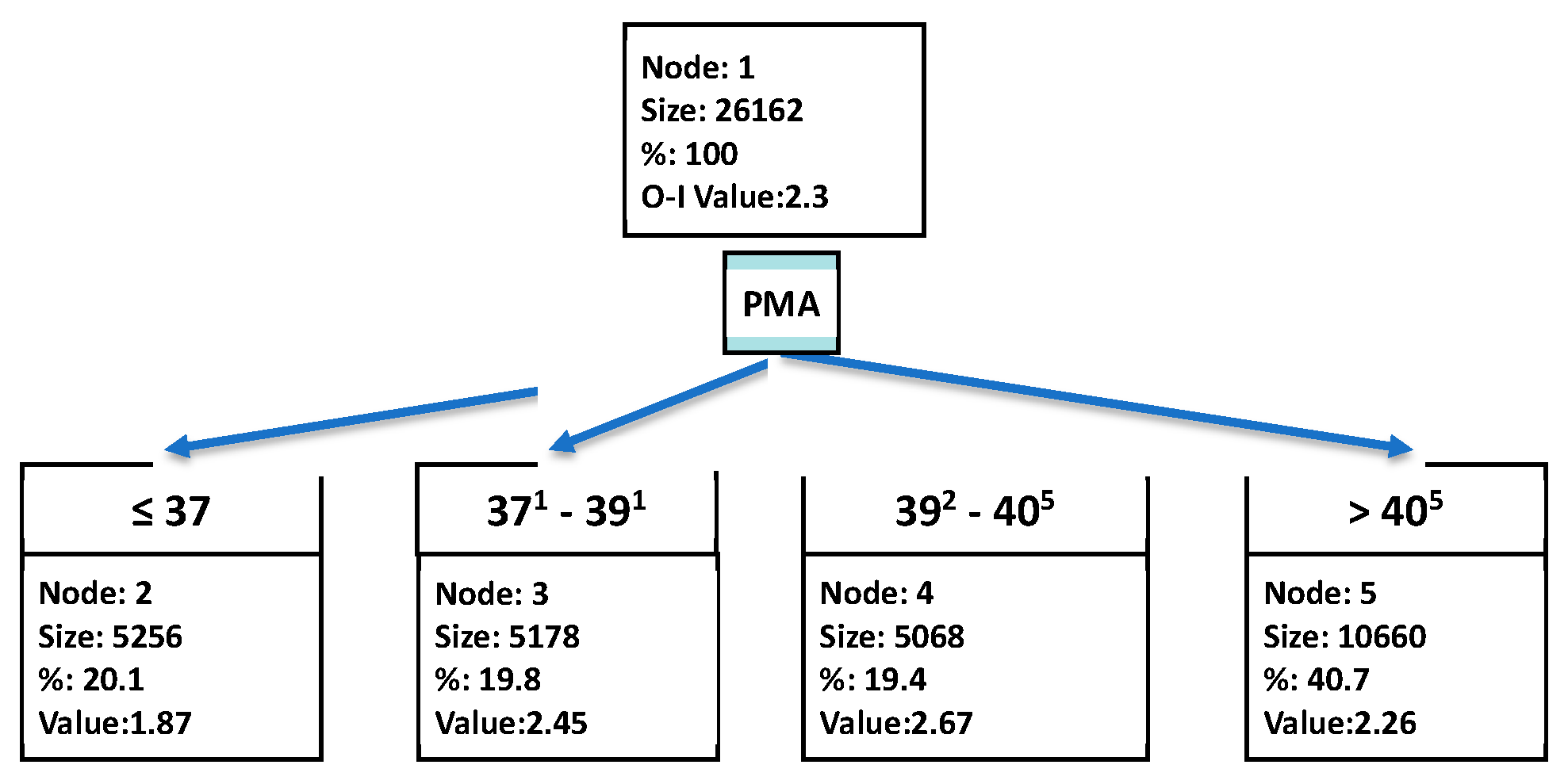
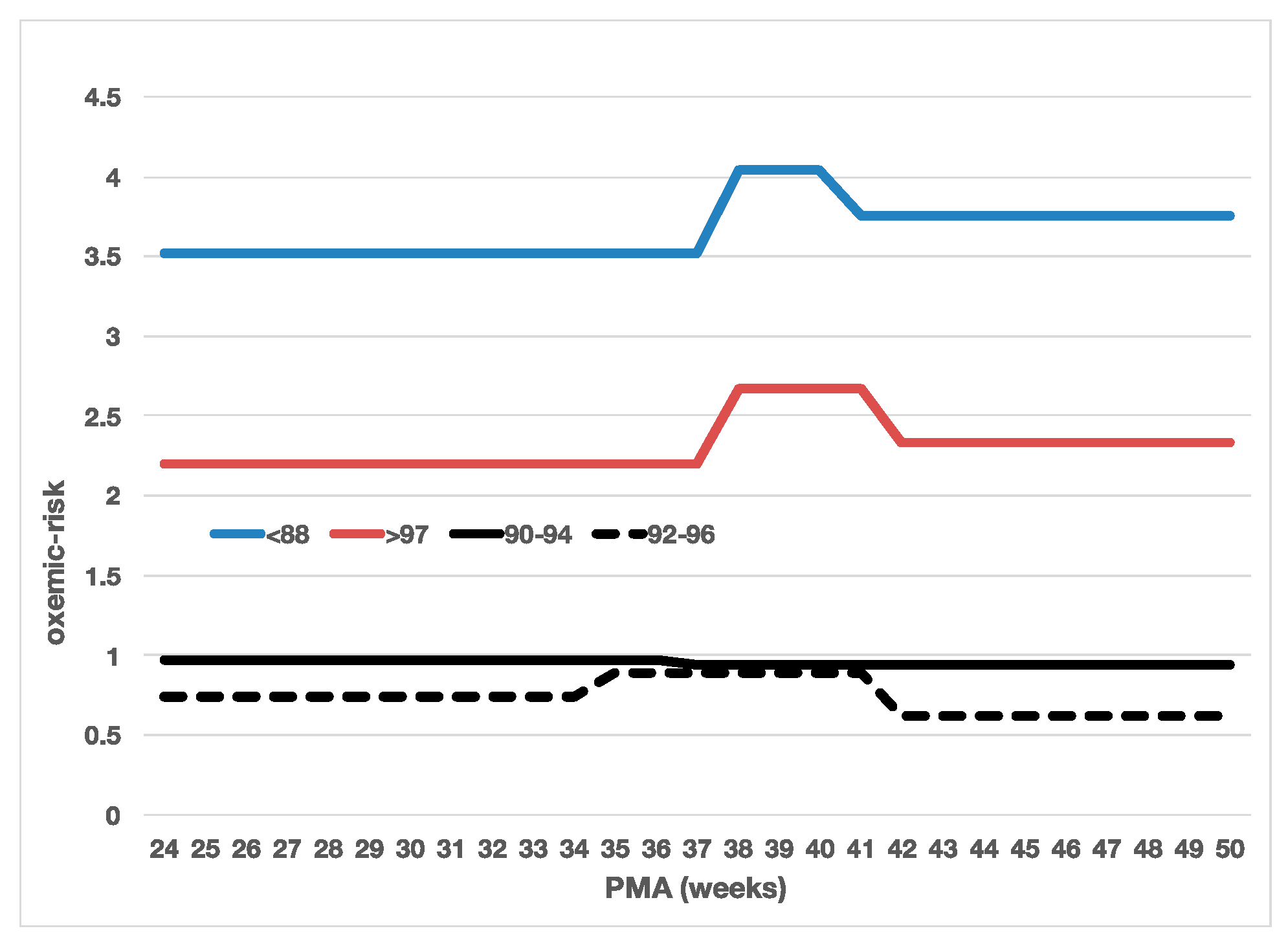
| SpO2 Strata | n | PMA (Weeks) | PaO2 (mmHg) | SpO2 (%) |
|---|---|---|---|---|
| 88–97% | 7500 | 40 ± 7 | 74 ± 40 | 94 ± 3 |
| <88% | 6667 | 41 ± 8 | 42 ± 16 | 80 ± 4 |
| >97% | 10,652 | 40 ± 7 | 129 ± 66 | 99 ± 1 |
| PaO2 Strata | n | % | SpO2 | PaO2 | Oxemic-Risk |
|---|---|---|---|---|---|
| ≤40 | 381 | 5.1% | 90 ± 2 | 37 ± 4 | 6 |
| 41–49 | 1398 | 19% | 91 ± 2 | 46 ± 2 | 2 |
| 50–80 | 4367 | 58% | 93 ± 3 | 62 ± 8 | 0 |
| 81–99 | 731 | 9.7% | 95 ± 2 | 88 ± 5 | 1 |
| 100–199 | 501 | 6.7% | 95 ± 2 | 127 ± 27 | 3 |
| ≥200 | 122 | 1.6% | 94 ± 2 | 273 ± 64 | 6 |
| 88–92% | 89–93% | 90–94% | 91–95% | 92–96% | 93–97% | |
|---|---|---|---|---|---|---|
| PaO2 (mmHg) | 56 ± 24 | 59 ± 26 | 63 ± 31 | 67 ± 32 | 73 ± 37 | 80 ± 41 |
| Oxemic-risk (95% CI) | 1.24 (1.18–1.29) | 1.12 (1.07–1.18) | 0.95 (0.90–1.00) | 0.83 (0.78–0.87) | 0.80 (0.76–0.85) | 0.88 (0.83–0.92) |
| SpO2 Target Range | Overall | Low-PMA | Middle-PMA | High-PMA |
|---|---|---|---|---|
| 88–92% | ||||
| PMA break | <395 | 395–421 | >421 | |
| Oxemic-risk | 1.24 | 1.26 | 1.38 | 1.02 |
| 89–93% | ||||
| PMA break | ≤422 | * | >422 | |
| Oxemic-risk | 1.12 | 1.15 | 1.01 | |
| 90–94% | ||||
| PMA break | ≤423 | * | >423 | |
| Oxemic-risk | 0.95 | 1.02 | 0.67 | |
| 91–95% | ||||
| PMA break | ≤422 | * | >422 | |
| Oxemic-risk | 0.83 | 0.89 | 0.59 | |
| 92–96% | ||||
| PMA break | ≤343 | 343–423 | >423 | |
| Oxemic-risk | 0.80 | 0.74 | 0.89 | 0.62 |
| 93–97% | ||||
| PMA break | ≤383 | 384–426 | >426 | |
| Oxemic-risk | 0.88 | 0.83 | 1.00 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachman, T.E.; Iyer, N.P.; Newth, C.J.L.; LeMoyne, R. Determining an Optimal Oxygen Saturation Target Range Based on Neonatal Maturity: Demonstration of a Decision Tree Analytic. Diagnostics 2023, 13, 3312. https://doi.org/10.3390/diagnostics13213312
Bachman TE, Iyer NP, Newth CJL, LeMoyne R. Determining an Optimal Oxygen Saturation Target Range Based on Neonatal Maturity: Demonstration of a Decision Tree Analytic. Diagnostics. 2023; 13(21):3312. https://doi.org/10.3390/diagnostics13213312
Chicago/Turabian StyleBachman, Thomas E., Narayan P. Iyer, Christopher J. L. Newth, and Robert LeMoyne. 2023. "Determining an Optimal Oxygen Saturation Target Range Based on Neonatal Maturity: Demonstration of a Decision Tree Analytic" Diagnostics 13, no. 21: 3312. https://doi.org/10.3390/diagnostics13213312
APA StyleBachman, T. E., Iyer, N. P., Newth, C. J. L., & LeMoyne, R. (2023). Determining an Optimal Oxygen Saturation Target Range Based on Neonatal Maturity: Demonstration of a Decision Tree Analytic. Diagnostics, 13(21), 3312. https://doi.org/10.3390/diagnostics13213312






