The Clinical Impact of Metagenomic Next-Generation Sequencing (mNGS) Test in Hospitalized Patients with Suspected Sepsis: A Multicenter Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethics Approval
2.3. Data Collection
2.4. mNGS Detection
2.5. Blood Culture
2.6. Determination of Inflammatory Factors
2.7. Statistical Analysis
3. Results
3.1. Demographic and Basic Clinical Information
3.2. Characteristics of Infection
3.3. Performance of mNGS Test in Comparison with Culture
3.4. Comparison of mNGS Test and Blood Culture in Different Subgroups
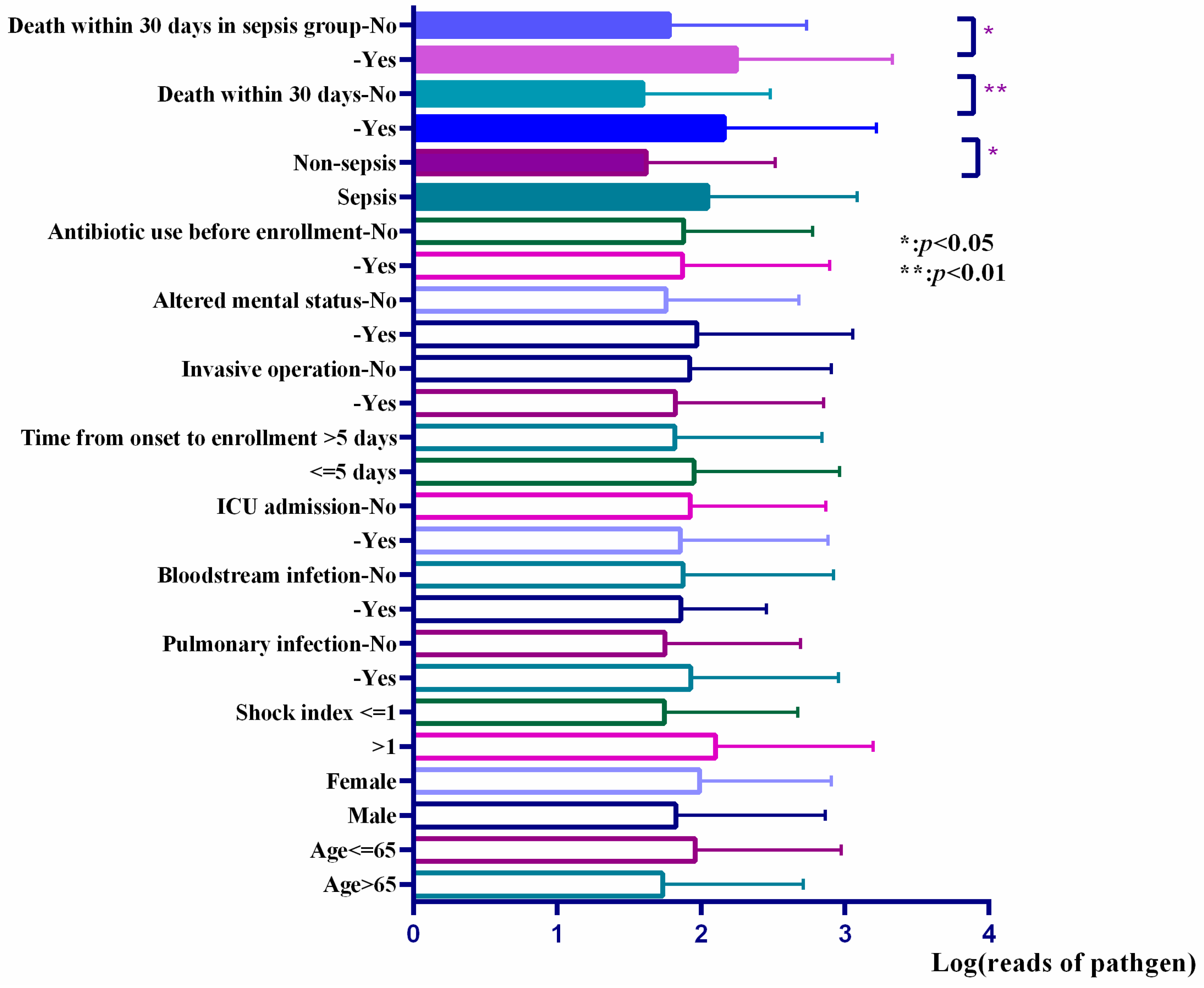
3.5. Reads of Responsible Pathogens in mNGS Reports Were Associated with the Prognosis of Patients and the Level of Inflammatory Factors
3.6. Microbial Detection Facilitated the Modification of Antimicrobial Prescription and Improved the Prognosis of Patients
4. Discussion
5. Conclusions
Registration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Song, J.E.; Ann, H.W.; Jeon, Y.; Ahn, M.Y.; Jung, I.Y.; Kim, M.H.; Jeong, W.; Jeong, S.J.; Ku, N.S.; et al. Effects of Early Exercise Rehabilitation on Functional Recovery in Patients with Severe Sepsis. Yonsei Med. J. 2018, 59, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Kidson, K.M.; Henderson, W.R.; Hutcheon, J.A. Case Fatality and Adverse Outcomes Are Reduced in Pregnant Women with Severe Sepsis or Septic Shock Compared with Age-Matched Comorbid-Matched Nonpregnant Women. Crit. Care Med. 2018, 46, 1775–1782. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; Jiang, Y. The Prognostic Value of Plasma MicroRNA-155 and MicroRNA-146a Level in Severe Sepsis and Sepsis-Induced Acute Lung Injury Patients. Clin. Lab. 2016, 62, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, M.; Jiang, S.; Ma, Y.F. Early goal-directed resuscitation for patients with severe sepsis and septic shock: A meta-analysis and trial sequential analysis. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 23. [Google Scholar] [CrossRef] [Green Version]
- Baharoon, S.; Al-Jahdali, H.; Al Hashmi, J.; Memish, Z.A.; Ahmed, Q.A. Severe sepsis and septic shock at the Hajj: Etiologies and outcomes. Travel Med. Infect. Dis. 2009, 7, 247–252. [Google Scholar] [CrossRef]
- Yan, H.P.; Li, M.; Lu, X.L.; Zhu, Y.M.; Ou-Yang, W.X.; Xiao, Z.H.; Qiu, J.; Li, S.J. Use of plasma mitochondrial DNA levels for determining disease severity and prognosis in pediatric sepsis: A case control study. BMC Pediatr. 2018, 18, 267. [Google Scholar] [CrossRef] [Green Version]
- Grumaz, S.; Stevens, P.; Grumaz, C.; Decker, S.O.; Weigand, M.A.; Hofer, S.; Brenner, T.; von Haeseler, A.; Sohn, K. Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med. 2016, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Grumaz, S.; Grumaz, C.; Vainshtein, Y.; Stevens, P.; Glanz, K.; Decker, S.O.; Hofer, S.; Weigand, M.A.; Brenner, T.; Sohn, K. Enhanced Performance of Next-Generation Sequencing Diagnostics Compared with Standard of Care Microbiological Diagnostics in Patients Suffering from Septic Shock. Crit. Care Med. 2019, 47, e394–e402. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.; Raangs, E.C.; Rosema, S.; Veloo, A.C.; et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Suarez, C.J.; Banaei, N.; Pinsky, B.A. Next-Generation Sequencing for Infectious Disease Diagnosis and Management. J. Mol. Diagn. 2015, 17, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Hu, H.; Fang, W.; Shi, D.; Liang, C.; Sun, Y.; Gao, G.; Wang, H.; Zhang, Q.; Wang, L.; et al. Detection of pathogens from resected heart valves of patients with infective endocarditis by next-generation sequencing. Int. J. Infect. Dis. 2019, 83, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.; Ren, H.; Wei, Y.; Mao, C.; Ma, Z.; Zhang, L.; Wang, L.; Ge, Y.; Li, T.; Cui, L.; et al. Next-generation sequencing of the cerebrospinal fluid in the diagnosis of neurobrucellosis. Int. J. Infect. Dis. 2018, 67, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, T.; Wilson, M.R.; Crawford, E.D.; Chow, E.D.; Khan, L.M.; Knopp, K.A.; O’Donovan, B.D.; Xia, D.; Hacker, J.K.; Stewart, J.M.; et al. Illuminatinguveitis: Metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racsa, L.D.; DeLeon-Carnes, M.; Hiskey, M.; Guarner, J. Identification of bacterial pathogens from formalin-fixed, paraffin-embedded tissues by using 16S sequencing: Retrospective correlation of results to clinicians’ responses. Hum. Pathol. 2017, 59, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin. Infect. Dis. 2018, 67, S231–S240. [Google Scholar] [CrossRef] [PubMed]
- Irving, S.Y.; Daly, B.; Verger, J.; Typpo, K.V.; Brown, A.M.; Hanlon, A.; Weiss, S.L.; Fitzgerald, J.C.; Nadkarni, V.M.; Thomas, N.J.; et al. The Association of Nutrition Status Expressed as Body Mass Index z Score with Outcomes in Children with Severe Sepsis: A Secondary Analysis from the Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study. Crit. Care Med. 2018, 46, e1029–e1039. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Z.; Zhuo, Y.; Cui, L.; Li, C.; Li, D.; Zhang, S.; Cui, N.; Wang, X.; Gao, H. Resveratrol alleviates sepsis-induced acute lung injury by suppressing inflammation and apoptosis of alveolar macrophage cells. Am. J. Transl. Res. 2018, 10, 1961–1975. [Google Scholar]
- Mwaigwisya, S.; Assiri, R.A.M.; O’Grady, J. Emerging commercial molecular tests for the diagnosis of bloodstream infection. Expert Rev. Mol. Diagn. 2015, 15, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhang, Y.; Gong, Y.; Sun, R.; Su, L.; Lin, X.; Shen, A.; Zhou, J.; Caiji, Z.; Wang, X.; et al. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch. Med. Res. 2016, 47, 365–371. [Google Scholar] [CrossRef]
- Florio, W.; Morici, P.; Ghelardi, E.; Barnini, S.; Lupetti, A. Recent advances in the microbiological diagnosis of bloodstream infections. Crit. Rev. Microbiol. 2018, 44, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.O.; Sigl, A.; Grumaz, C.; Stevens, P.; Vainshtein, Y.; Zimmermann, S.; Weigand, M.A.; Hofer, S.; Sohn, K.; Brenner, T. Immune-Response Patterns and Next Generation Sequencing Diagnostics for the Detection of Mycoses in Patients with Septic Shock—Results of a Combined Clinical and Experimental Investigation. Int. J. Mol. Sci. 2017, 18, 1796. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Kang, L.; Xu, M.X. The application value of metagenomic next-generation sequencing in children with invasive pneumococcal disease. Transl. Pediatr. 2021, 10, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.; Breitwieser, F.P.; Hao, S.; Opene, B.N.A.; Workman, R.E.; Tamma, P.D.; Dien-Bard, J.; Timp, W.; Simner, P.J. Metagenomic next-generation sequencing of rectal swabs for the surveillance of antimicrobial-resistant organisms on the Illumina Miseq and Oxford MinION platforms. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 95–102. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Zhou, Y.; Li, Y.; Guo, Q.; Chen, J.; Quan, S.; Zhang, A.; Zheng, H.; Zhu, X.; Lin, J.; et al. The feasibility study of non-invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS ONE 2014, 9, e110240. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]

| Basic Information | Sepsis Group (n = 162) | Non-Sepsis Group (n = 115) | p-Value |
|---|---|---|---|
| Gender, male/female | 114/48 | 81/34 | 0.991 |
| Age, median (q1, q3) | 63 (50, 69) | 60 (43, 72) | 0.451 |
| Comorbidities (n (%)) | |||
| Pulmonary disease a | 13 (8.02%) | 12 (10.43%) | 0.490 |
| Congestive heart failure | 11 (6.79%) | 4 (3.48%) | 0.230 |
| Cerebrovascular disease | 19 (11.73%) | 11 (9.57%) | 0.568 |
| Diabetes | 34 (20.99%) | 14 (12.17%) | 0.056 |
| Hepatic cirrhosis | 3 (1.85%) | 0 (0%) | 0.269 |
| Acute/chronic renal failure | 23 (14.20%) | 15 (13.04%) | 0.783 |
| Smoking history (n (%)) | 56 (34.57) | 32 (27.83%) | 0.235 |
| Antibiotic use before enrollment b (n (%)) | 144 (88.89%) | 104 (92.04%) | 0.679 |
| Invasive procedures before onset of symptoms c (n (%)) | 92 (56.79%) | 55 (47.83%) | 0.141 |
| Corticosteroids/immunosuppressive drug/cytotoxic chemotherapy before onset (n (%)) | 38 (23.46%) | 48 (41.74%) | 0.001 |
| Recent surgery/trauma history d (n (%)) | 54 (33.33%) | 39 (33.91%) | 0.920 |
| ICU admission (n (%)]) | 141 (87.04%) | 71 (61.74%) | <0.001 |
| Infection Characteristics | Sepsis Group (n = 162) | Non-Sepsis Group (n = 115) | p-Value |
|---|---|---|---|
| Time from symptom onset to enrollment (d), median (q1, q3) | 5.5 (2, 15) | 11 (5, 22) | 0.008 |
| Site of infection (n (%)) | |||
| Pulmonary infection | 114 (80.28%) | 70 (60.87%) | 0.100 |
| Extrapulmonary infection | 18 (11.11%) | 8 (6.96%) | 0.247 |
| APACHE II (means ± SD) | 19.97 ± 8.33 | 14.87 ± 8.28 | <0.001 |
| Shock index (means ± SD) | 1.04 ± 0.36 | 0.81 ± 0.19 | <0.001 |
| Fever (n (%)) | 117 (72.22%) | 82 (71.30%) | 0.867 |
| Altered mental status a (n (%)) | 115 (70.99%) | 20 (17.39%) | <0.001 |
| Death within 30 days (n (%)) | 82, 51.57% | 39, 35.14% | 0.008 |
| PCT (ug/L) [median (q1, q3)] | 1.92 (0.36, 11.17) | 0.53 (0.22, 3.82) | 0.214 |
| WBC count (×109/L) (median (q1, q3)) | 10.83 (6.93, 15.65) | 9.79 (6.10, 15.76) | 0.410 |
| Neutrophil count (×109/L) (median (q1, q3)) | 9.07 (5.73, 13.42) | 8.84 (5.00, 13.03) | 0.475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.-H.; Wu, Y.-X.; Hu, W.-P.; Chen, Y.; Li, Y.-P.; Song, Z.-J.; Luo, Z.; Ju, M.-J.; Shi, M.-H.; Xu, S.-Y.; et al. The Clinical Impact of Metagenomic Next-Generation Sequencing (mNGS) Test in Hospitalized Patients with Suspected Sepsis: A Multicenter Prospective Study. Diagnostics 2023, 13, 323. https://doi.org/10.3390/diagnostics13020323
Zuo Y-H, Wu Y-X, Hu W-P, Chen Y, Li Y-P, Song Z-J, Luo Z, Ju M-J, Shi M-H, Xu S-Y, et al. The Clinical Impact of Metagenomic Next-Generation Sequencing (mNGS) Test in Hospitalized Patients with Suspected Sepsis: A Multicenter Prospective Study. Diagnostics. 2023; 13(2):323. https://doi.org/10.3390/diagnostics13020323
Chicago/Turabian StyleZuo, Yi-Hui, Yi-Xing Wu, Wei-Ping Hu, Yan Chen, Yu-Ping Li, Zhen-Ju Song, Zhe Luo, Min-Jie Ju, Min-Hua Shi, Shu-Yun Xu, and et al. 2023. "The Clinical Impact of Metagenomic Next-Generation Sequencing (mNGS) Test in Hospitalized Patients with Suspected Sepsis: A Multicenter Prospective Study" Diagnostics 13, no. 2: 323. https://doi.org/10.3390/diagnostics13020323






