The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management
Abstract
:1. Introduction
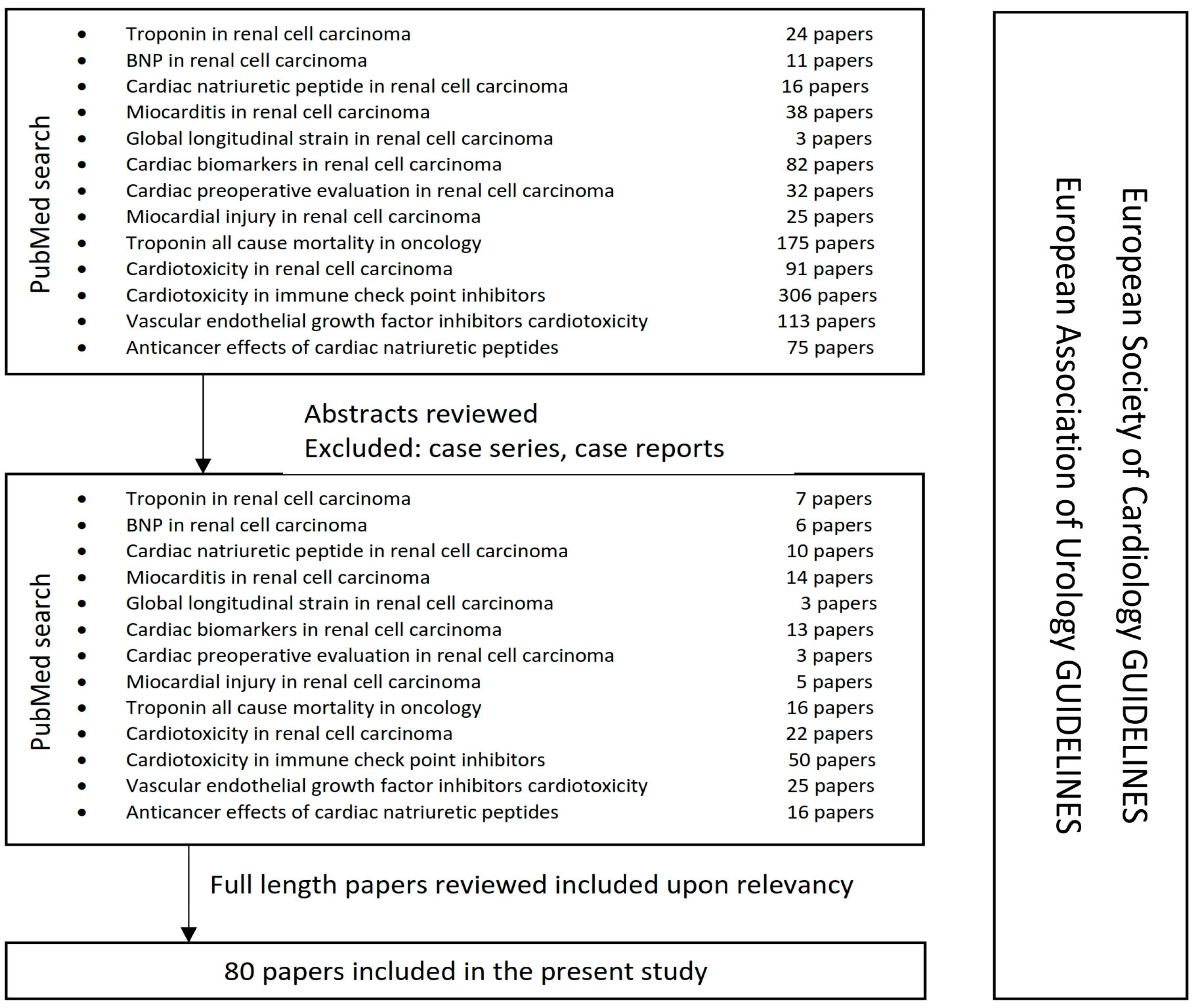
2. Methods
3. Cardiac Biomarkers
4. Serum and Imagistic Cardiac Biomarkers’ Role in Perioperative Evaluation of the RCC Patient
5. Serum and Imagistic Cardiac Biomarkers’ Role in Systemic Therapy of RCC Patient
6. Cardiac Natriuretic Peptides—A Potential Therapeutic Target in RCC
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Graff, R.E.; Sanchez, A.; Tobias, D.K.; Rodríguez, D.; Barrisford, G.W.; Blute, M.L.; Li, Y.; Sun, Q.; Preston, M.A.; Wilson, K.M.; et al. Type 2 Diabetes in Relation to the Risk of Renal Cell Carcinoma Among Men and Women in Two Large Prospective Cohort Studies. Diabetes Care 2018, 41, 1432–1437. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk factors for renal cell carcinoma in the vital study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef]
- Saly, D.L.; Eswarappa, M.S.; Street, S.E.; Deshpande, P. Renal Cell Cancer and Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2021, 28, 460–468.e1. [Google Scholar] [CrossRef]
- Capitanio, U.; Bedke, J.; Albiges, L.; Volpe, A.; Giles, R.H.; Hora, M.; Marconi, L.; Klatte, T.; Abu-Ghanem, Y.; Dabestani, S.; et al. A renewal of the TNM Staging System for patients with Renal Cancer to comply with current decision-making: Proposal from the European Association of Urology Guidelines Panel. Eur. Urol. 2023, 83, 3–5. [Google Scholar] [CrossRef]
- Tabbara, M.M.; González, J.; Martucci, M.; Ciancio, G. Current Approaches in Surgical and Immunotherapy-Based Management of Renal Cell Carcinoma with Tumor Thrombus. Biomedicines 2023, 11, 204. [Google Scholar] [CrossRef]
- Psutka, S.P. Personalizing preoperative risk stratification and refining patient selection for cytoreductive nephrectomy in metastatic renal cell carcinoma. Cancer 2020, 126, 3912–3915. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016, 4, 194. [Google Scholar] [CrossRef]
- Ebashi, S. Third component participating in the superprecipitation of ‘natural actomyosin’. Nature 1963, 200, 1010. [Google Scholar] [CrossRef]
- Ebashi, S.; Ebashi, F.; Kodama, A. Troponin as the Ca++-receptive protein in the contractile system. J. Biochem. 1967, 62, 137–138. [Google Scholar] [CrossRef]
- Ebashi, S.; Endo, M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968, 18, 123–183. [Google Scholar] [CrossRef]
- Greaser, M.L.; Gergely, J. Reconstitution of troponin activity from three protein components. J. Biol. Chem. 1971, 246, 4226–4233. [Google Scholar] [CrossRef]
- Lazar, D.R.; Lazar, F.L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D.M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis. Mrk. 2022, 2022, 9713326. [Google Scholar] [CrossRef]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Xu, R.Y.; Zhu, X.F.; Yang, Y.; Ye, P. High-sensitive cardiac troponin T. J. Geriatr. Cardiol. 2013, 10, 102–109. [Google Scholar]
- White, H.D. Pathobiology of troponin elevations: Do elevations occur with myocardial ischemia as well as necrosis? J. Am. Coll. Cardiol. 2011, 57, 2406–2408. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Ripoli, A.; Masotti, S.; Prontera, C.; Passino, C.; Plebani, M.; on the behalf of the Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry (SIBioC). The 99th percentile of reference population for cTnI and cTnT assay: Methodology, pathophysiology and clinical implications. Clin. Chem. Lab. Med. 2017, 55, 1634–1651. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Aimo, A.; Cardinale, D.M.; Dittadi, R.; Sandri, M.T.; Perrone, M.A.; Belloni, L.; Fortunato, A.; Trenti, T.; et al. Variability of cardiac troponin levels in normal subjects and in patients with cardiovascular diseases: Analytical considerations and clinical relevance. Clin. Chem. Lab. Med. 2023, 61, 1209–1229. [Google Scholar] [CrossRef]
- Jeremias, A.; Gibson, C.M. Narrative review: Alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann. Intern Med. 2005, 142, 786–791. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.; Cha, W.C.; Yoon, H.; Hwang, S.Y.; Shin, T.G.; Sim, M.S.; Jo, I.; Lee, S.H.; Park, H.D.; et al. Cardiac troponin I predicts clinical outcome of patients with cancer at emergency department. Clin. Cardiol. 2020, 43, 1585–1591. [Google Scholar] [CrossRef]
- Finke, D.; Romann, S.W.; Heckmann, M.B.; Hund, H.; Bougatf, N.; Kantharajah, A.; Katus, H.A.; Müller, O.J.; Frey, N.; Giannitsis, E.; et al. High-sensitivity cardiac troponin T determines all-cause mortality in cancer patients: A single-centre cohort study. ESC Heart Fail. 2021, 8, 3709–3719. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J. Thorac. Dis. 2018, 10 (Suppl. S35), S4282–S4295. [Google Scholar] [CrossRef]
- Hua, Y.B.; Li, X.; Wang, D.X. Prevalence and risk factors of myocardial and acute kidney injury following radical nephrectomy with vena cava thrombectomy: A retrospective cohort study. BMC Anesthesiol. 2021, 21, 243. [Google Scholar] [CrossRef]
- Rini, B.I.; Moslehi, J.J.; Bonaca, M.; Schmidinger, M.; Albiges, L.; Choueiri, T.K.; Motzer, R.J.; Atkins, M.B.; Haanen, J.; Mariani, M.; et al. Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients with Advanced Renal Cell Cancer: Data From the Phase III JAVELIN Renal 101 Trial. J. Clin. Oncol. 2022, 40, 1929–1938. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Li, P.; Yang, Y.; Zhang, W.; Zhao, S.; Zeng, Y.; Zhou, X.; Zeng, L.; Yang, G. Modified natriuretic peptides and their potential roles in cancer treatment. Biomed. J. 2022, 45, 118–131. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Burjonroppa, S.C.; Tong, A.T.; Xiao, L.C.; Johnson, M.M.; Yusuf, S.W.; Lenihan, D.J. Cancer Patients with Markedly Elevated B-Type Natriuretic Peptide May Not Have Volume Overload. Am. J. Clin. Oncol. 2007, 30, 287–293. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Kontovinis, L.F.; Papandreou, C.N.; Kouvatseas, G.; Lafaras, C.; Antonakis, E.; Christopoulou, M.; Andreadis, C.; Mouratidou, D.; Kortsaris, A.H. Brain natriuretic peptide precursor (NT-pro-BNP) levels predict for clinical benefit to sunitinib treatment in patients with metastatic renal cell carcinoma. BMC Cancer 2010, 10, 489. [Google Scholar] [CrossRef]
- Tuñón, J.; Higueras, J.; Tarín, N.; Cristóbal, C.; Lorenzo, Ó.; Blanco-Colio, L.; Martín-Ventura, J.L.; Huelmos, A.; Alonso, J.; Aceña, Á.; et al. N-Terminal Pro-Brain Natriuretic Peptide Is Associated with a Future Diagnosis of Cancer in Patients with Coronary Artery Disease. PLoS ONE 2015, 10, e0126741. [Google Scholar] [CrossRef]
- Shehata, I.M.; Odell, T.D.; Elhassan, A.; Urits, I.; Viswanath, O.; Kaye, A.D. Global Longitudinal Strain: Is It Time to Change the Preoperative Cardiac Assessment of Oncology Patients? Oncology 2021, 9, 13–19. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Edvardsen, T. Global longitudinal strain: The best biomarker for predicting prognosis in heart failure? Eur. J. Heart Fail. 2016, 18, 1340–1341. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Deblois, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; Vinereanu, D.; Cho, G.Y.; et al. Precision and stability of parameters for assessment of left ventricular systolic function in clinical trials: Lessons from the SUCCOUR trial. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 1514. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Semeraro, G.C.; Cipolla, C.M.; Cardinale, D.M. Role of Cardiac Biomarkers in Cancer Patients. Cancers 2021, 13, 5426. [Google Scholar] [CrossRef]
- Esdaille, A.R.; Abel, E.J.; Bell, D.W. Evolution of risk stratification systems is critical for improving patient selection for cytoreductive nephrectomy. Cancer 2021, 127, 3920–3923. [Google Scholar] [CrossRef]
- Chapman, E.; Pichel, A.C. Anaesthesia for nephrectomy. BJA Educ. 2016, 16, 98–101. [Google Scholar] [CrossRef]
- Nasrallah, A.A.; Dakik, H.A.; Abou Heidar, N.F.; Najdi, J.A.; Nasrallah, O.G.; Mansour, M.; Tamim, H.; Hajj, A.E. Major adverse cardiovascular events following partial nephrectomy: A procedure-specific risk index. Adv. Urol. 2022, 14, 17562872221084847. [Google Scholar] [CrossRef]
- Kusunose, K.; Torii, Y.; Yamada, H.; Nishio, S.; Hirata, Y.; Saijo, Y.; Ise, T.; Yamaguchi, K.; Fukuda, D.; Yagi, S.; et al. Association of Echocardiography Before Major Elective Non-Cardiac Surgery with Improved Postoperative Outcomes—Possible Implications for Patient Care. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 2512–2519. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Moncur, R.A.; Levine, O.; Heels-Ansdell, D.; Chan, M.T.; Alonso-Coello, P.; Yusuf, S.; Sessler, D.; Villar, J.C.; Berwanger, O.; et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J. Am. Coll. Cardiol. 2009, 54, 1599–1606. [Google Scholar]
- Rodseth, R.N.; Biccard, B.M.; Le Manach, Y.; Sessler, D.I.; Lurati Buse, G.A.; Thabane, L.; Schutt, R.C.; Bolliger, D.; Cagini, L.; Cardinale, D.; et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: A systematic review and individual patient data meta-analysis. J. Am. Coll. Cardiol. 2014, 63, 170–180. [Google Scholar]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Drăgan, A.; Sinescu, I. AKI3-Risk Predictors and Scores in Radical Nephrectomy with High Thrombectomy under Extracorporeal Circulation for Renal Cell Carcinoma with Supradiaphragmatic Inferior Vena Cava/Right Atrial Thrombus: A Single-Centre Retrospective Study. Medicina 2023, 59, 386. [Google Scholar] [CrossRef]
- Bando, S.; Soeki, T.; Matsuura, T.; Tobiume, T.; Ise, T.; Kusunose, K.; Yamaguchi, K.; Yagi, S.; Fukuda, D.; Iwase, T.; et al. Plasma brain natriuretic peptide levels are elevated in patients with cancer. PLoS ONE 2017, 12, e0178607. [Google Scholar] [CrossRef]
- Kamai, T.; Tokura, Y.; Uematsu, T.; Sakamoto, K.; Suzuki, I.; Takei, K.; Narimatsu, T.; Kambara, T.; Yuki, H.; Betsunoh, H.; et al. Elevated serum levels of cardiovascular biomarkers are associated with progression of renal cancer. Open Heart 2018, 5, e000666. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, H.M.; Choi, E.Y.; Lee, H.S.; Park, G.; Han, D.W.; Lee, S.E.; Park, C.S.; Hwang, J.W.; Choi, J.H.; et al. PRE-OPerative ECHOcardiograhy for prevention of cardiovascular events after non-cardiac surgery in intermediate- and high-risk patients: Protocol for a low-interventional, mixed-cohort prospective study design (PREOP-ECHO). Trials 2022, 23, 776. [Google Scholar] [CrossRef]
- Kim, M.; Moon, I.; Bae, S.; Seo, H.; Jung, I.H. Prognostic value of preoperative left ventricular global longitudinal strain for predicting postoperative myocardial injury and mortality in patients undergoing major non-cardiac surgery (SOLOMON study). Int. J. Cardiol. 2023, 378, 151–158. [Google Scholar] [CrossRef]
- Flavin, K.; Vasdev, N.; Ashead, J.; Lane, T.; Hanbury, D.; Nathan, P.; Gowrie-Mohan, S. Perioperative Considerations in Metastatic Renal Cell Carcinoma. Rev. Urol. 2016, 18, 133–142. [Google Scholar]
- McIntosh, A.G.; Umbreit, E.C.; Holland, L.C.; Gu, C.; Tannir, N.M.; Matin, S.F.; Karam, J.A.; Culp, S.H.; Wood, C.G. Optimizing patient selection for cytoreductive nephrectomy based on outcomes in the contemporary era of systemic therapy. Cancer 2020, 126, 3950–3960. [Google Scholar] [CrossRef]
- Öztürk, C.; Validyev, D.; Becher, U.M.; Weber, M.; Nickenig, G.; Tiyerili, V. A novel scoring system to estimate chemotherapy-induced myocardial toxicity: Risk assessment prior to non-anthracycline chemotherapy regimens. Int. J. Cardiol. Heart Vasc. 2021, 33, 100751. [Google Scholar] [CrossRef]
- Cardinale, D.; Biasillo, G.; Salvatici, M.; Sandri, M.T.; Cipolla, C.M. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev. Mol. Diagn. 2017, 17, 245–256. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncology 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Cuomo, G.; Iannone, F.P.; Di Lorenzo, A.; Testa, C.; Ciccarelli, M.; Venturini, E.; Cesaro, A.; Pacileo, M.; Tagliamonte, E.; D’Andrea, A.; et al. Potential Role of Global Longitudinal Strain in Cardiac and Oncological Patients Undergoing Cardio-Oncology Rehabilitation (CORE). Clin. Pract. 2023, 13, 384–397. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Sławiński, G.; Hawryszko, M.; Liżewska-Springer, A.; Nabiałek-Trojanowska, I.; Lewicka, E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers 2023, 15, 986. [Google Scholar] [CrossRef]
- Schutz, F.A.; Je, Y.; Richards, C.J.; Choueiri, T.K. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J. Clin. Oncol. 2012, 30, 871–877. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Haas, N.B.; Manola, J.; Ky, B.; Flaherty, K.T.; Uzzo, R.G.; Kane, C.J.; Jewett, M.; Wood, L.; Wood, C.G.; Atkins, M.B.; et al. Effects of Adjuvant Sorafenib and Sunitinib on Cardiac Function in Renal Cell Carcinoma Patients without Overt Metastases: Results from ASSURE, ECOG 2805. Clin. Cancer Res. 2015, 21, 4048–4054. [Google Scholar] [CrossRef]
- Telli, M.; Witteles, R.; Fisher, G.; Srinivas, S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol. 2008, 19, 1613–1618. [Google Scholar] [CrossRef]
- Catino, A.B.; Hubbard, R.A.; Chirinos, J.A.; Townsend, R.; Keefe, S.; Haas, N.B.; Puzanov, I.; Fang, J.C.; Agarwal, N.; Hyman, D.; et al. Longitudinal Assessment of Vascular Function with Sunitinib in Patients with Metastatic Renal Cell Carcinoma. Circ. Heart Fail. 2018, 11, e004408. [Google Scholar] [CrossRef]
- Franczyk, B.; Rysz, J.; Ławiński, J.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Cardiotoxicity of Selected Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Renal Cell Carcinoma. Biomedicines 2023, 11, 181. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Fornarini, G.; Massari, F.; Bimbatti, D.; Mosillo, C.; Rebuzzi, S.E.; Di Nunno, V.; Grassi, M.; Fantinel, E.; et al. Cabozantinib-related cardiotoxicity: A prospective analysis in a real-world cohort of metastatic renal cell carcinoma patients. Br. J. Clin. Pharmacol. 2019, 85, 1283–1289. [Google Scholar] [CrossRef]
- Hall, P.S.; Harshman, L.C.; Srinivas, S.; Witteles, R.M. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013, 1, 72–78. [Google Scholar] [CrossRef]
- Ghatalia, P.; Morgan, C.J.; Je, Y.; Nguyen, P.L.; Trinh, Q.-D.; Choueiri, T.K.; Sonpavde, G. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit. Rev. Oncol. Hematol. 2015, 94, 228–237. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Shalata, W.; Abu-Salman, A.; Steckbeck, R.; Mathew Jacob, B.; Massalha, I.; Yakobson, A. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review. Cancers 2021, 13, 5218. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: A meta-analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Vesely, D.L. Heart Peptide Hormones: Adjunct and Primary Treatments of Cancer. Anticancer Res. 2016, 36, 5693–5700. [Google Scholar] [CrossRef]
- Skelton, W.P., IV; Pi, G.E.; Vesely, D.L. Four cardiac hormones cause death of human cancer cells but not of healthy cells. Anticancer Res. 2011, 31, 395–402. [Google Scholar]
- Vesely, B.A.; Eichelbaum, E.J.; Alli, A.A.; Sun, Y.; Gower, W.R., Jr.; Vesely, D.L. Urodilatin and four cardiac hormones decrease human renal carcinoma cell numbers. Eur. J. Clin. Investig. 2006, 36, 810–819. [Google Scholar] [CrossRef]
- Vesely, D.L. Family of peptides synthesized in the human body have anticancer effects. Anticancer Res. 2014, 34, 1459–1466. [Google Scholar]
- Vesely, D.L. Cardiac and renal hormones: Anticancer effects in vitro and in vivo. J. Investig. Med. 2009, 57, 22–28. [Google Scholar] [CrossRef]
- Mezzasoma, L.; Talesa, V.N.; Costanzi, E.; Bellezza, I. Natriuretic Peptides Regulate Prostate Cells Inflammatory Behavior: Potential Novel Anticancer Agents for Prostate Cancer. Biomolecules 2021, 11, 794. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G. Predictive Biomarkers and Novel Targets in the Treatment of Metastatic Renal Cell Carcinoma. Curr. Med. Chem. 2021, 28, 5213–5227. [Google Scholar] [CrossRef]
- Serafino, A.; Pierimarchi, P. Atrial natriuretic peptide: A magic bullet for cancer therapy targeting Wnt signaling and cellular pH regulators. Curr. Med. Chem. 2014, 21, 2401–2409. [Google Scholar] [CrossRef]
- Skelton, W.P.; Skelton, M.; Vesely, D.L. Central role of β-catenin in anticancer effects of cardiac hormones. Anticancer Res. 2013, 33, 2409–2414. [Google Scholar]
- Kook, H.; Itoh, H.; Choi, B.S.; Sawada, N.; Doi, K.; Hwang, T.J.; Kim, K.K.; Arai, H.; Baik, Y.H.; Nakao, K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1388–H1397. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Frost, C.D.; Lane, M.L.; Skelton IV, W.P.; Skelton, M.; Vesely, D.L. Novel dual inhibitors of vascular endothelial growth factor and VEGFR2 receptor. Eur. J. Clin. Investig. 2012, 42, 1061–1067. [Google Scholar] [CrossRef]
- Skelton, W.P.; Skelton, M.; Vesely, D.L. Inhibition of AKT in human pancreatic, renal and colorectal cancer cells by four cardiac hormones. Anticancer Res. 2013, 33, 785–790. [Google Scholar]
- Manimala, N.J.; Frost, C.D.; Lane, M.L.; Higuera, M.; Beg, R.; Vesely, D.L. Cardiac hormones target nuclear oncogenes c-Fos and c-Jun in carcinoma cells. Eur. J. Clin. Investig. 2013, 43, 1156–1162. [Google Scholar] [CrossRef]
- Medina, A.J.; Pinilla, O.A.; Portiansky, E.L.; Caldiz, C.I.; Ennis, I.L. Silencing of the Na+/H+ exchanger 1(NHE-1) prevents cardiac structural and functional remodeling induced by angiotensin II. Exp. Mol. Pathol. 2019, 107, 1–9. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, J.; Cai, Q.; He, Y.; Yang, D. Pleiotropic Roles of Atrial Natriuretic Peptide in Anti-Inflammation and Anti-Cancer Activity. Cancers 2022, 14, 3981. [Google Scholar] [CrossRef]
- Nojiri, T.; Yamamoto, K.; Maeda, H.; Takeuchi, Y.; Funakoshi, Y.; Inoue, M.; Okumura, M. Effect of low-dose human atrial natriuretic peptide on postoperative atrial fibrillation in patients undergoing pulmonary resection for lung cancer: A double-blind, placebo-controlled study. J. Thorac. Cardiovasc. Surg. 2012, 143, 488–494. [Google Scholar] [CrossRef]
| Asymptomatic CTRCD | LVEF | GLS and Serum Biomarkers |
| mild |
| |
| medium |
| |
| ||
| severe |
| HFA-ICOS Risk Stratification | Baseline | Surveillance |
|---|---|---|
| Low risk |
|
|
| Moderate risk |
|
|
| High and very high risk |
|
|
| Before Starting Therapy | Surveillance | |
|---|---|---|
| Low-risk patients |
|
|
| High-risk patients 2 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drăgan, A.; Sinescu, I. The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management. Diagnostics 2023, 13, 1912. https://doi.org/10.3390/diagnostics13111912
Drăgan A, Sinescu I. The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management. Diagnostics. 2023; 13(11):1912. https://doi.org/10.3390/diagnostics13111912
Chicago/Turabian StyleDrăgan, Anca, and Ioanel Sinescu. 2023. "The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management" Diagnostics 13, no. 11: 1912. https://doi.org/10.3390/diagnostics13111912





