Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Trial Enrollment
2.2. CSF and Serum Collection and Processing
2.3. Biochemical Analysis
2.4. Quantitative Immunoblotting
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Quanterix Single Molecule Array (SIMOA) N4PB Digital ELISA
2.7. Neurological Evaluation
2.8. Statistical Analysis
3. Results
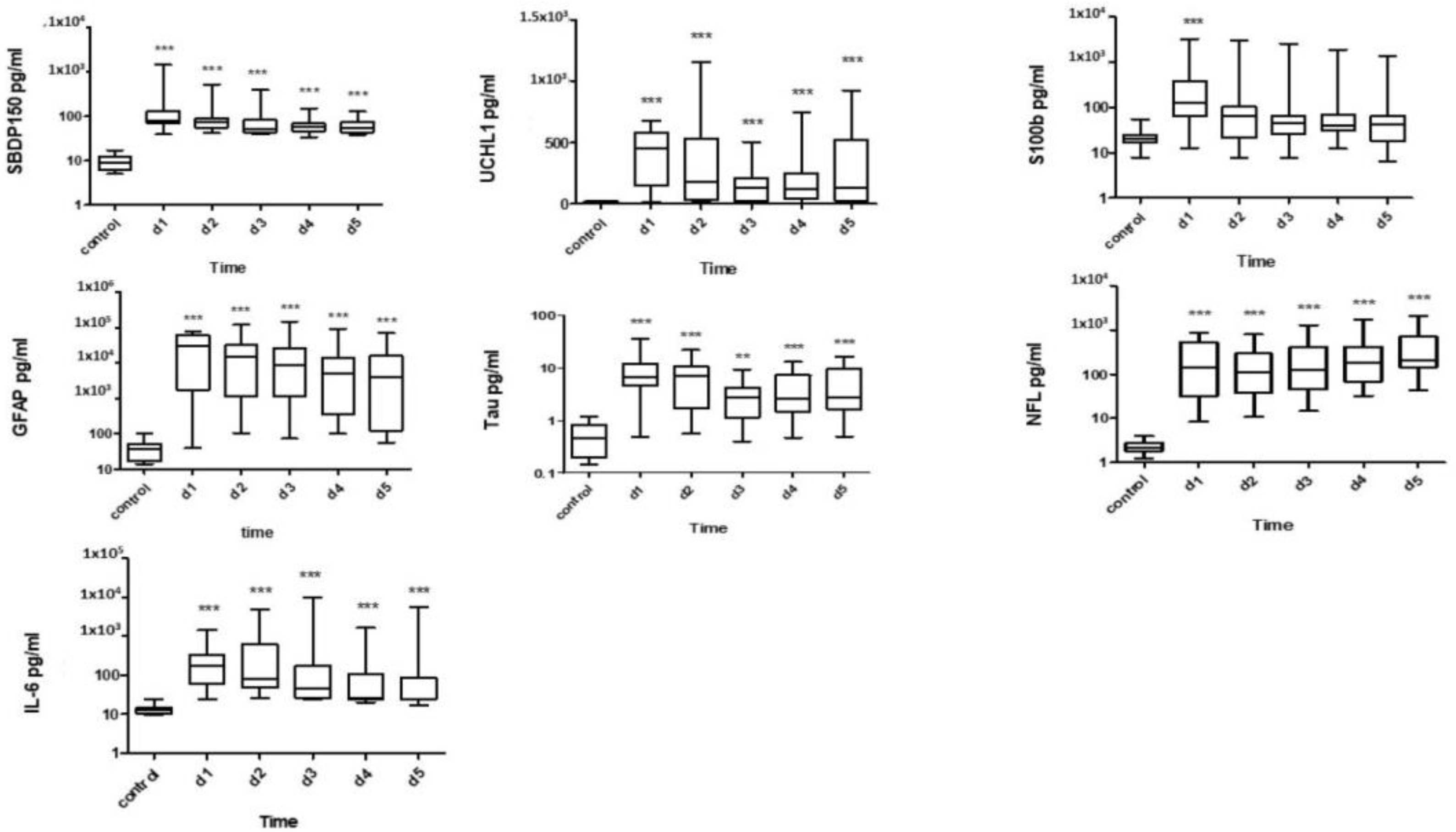
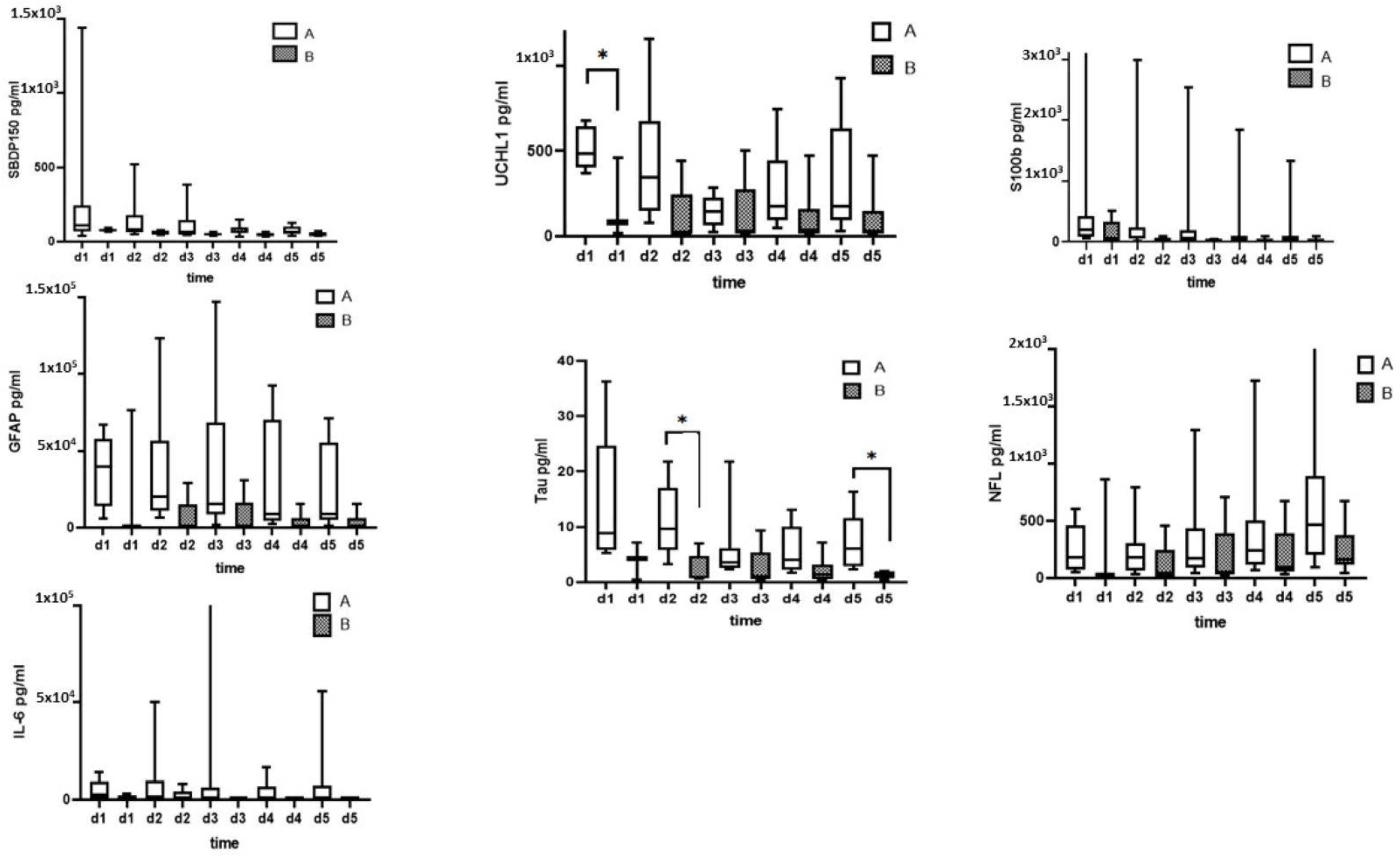
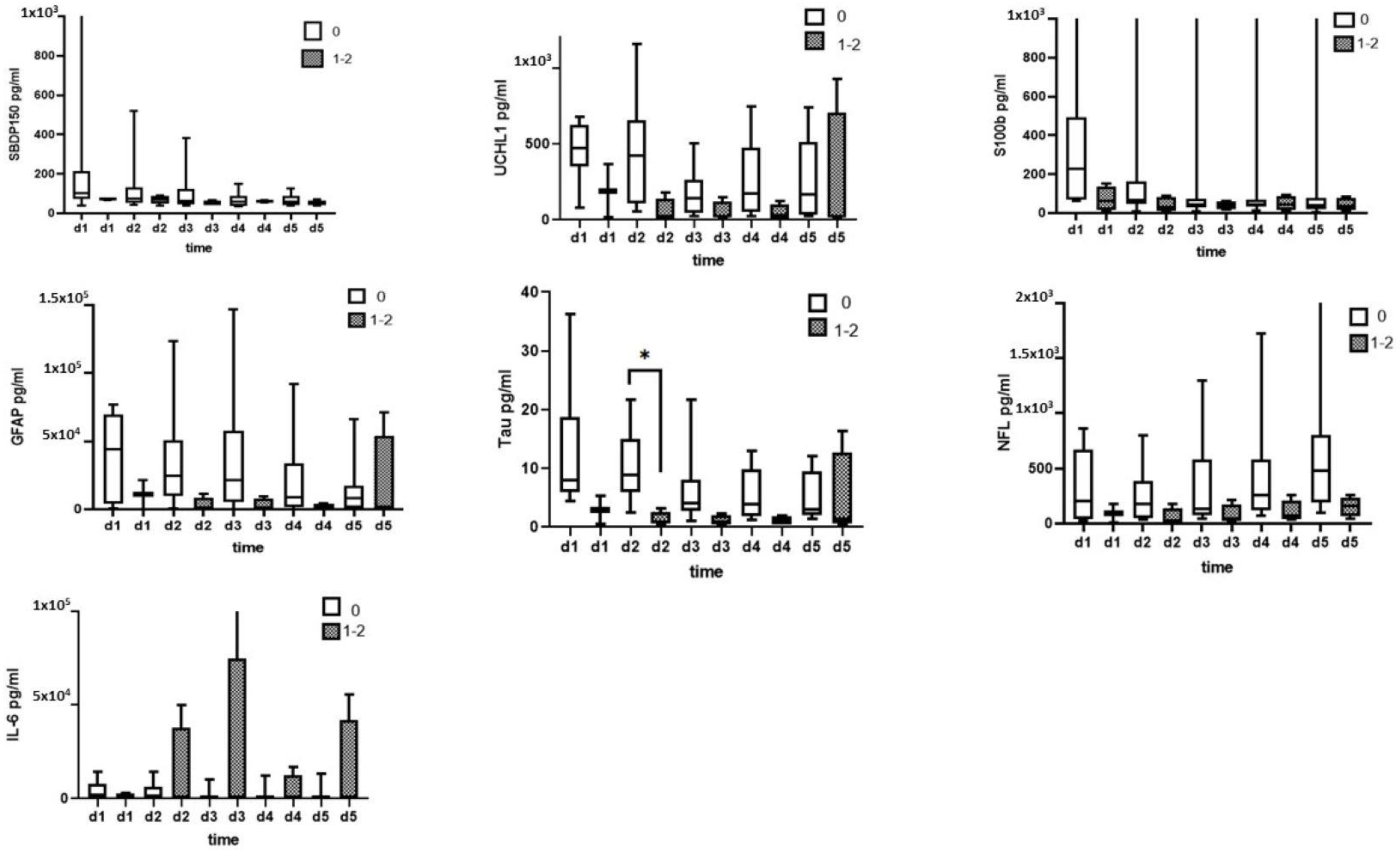
3.1. CSF Biomarkers Measured with Immunoblotting
3.2. CSF Biomarkers Measured with ELISA
3.3. Serum Biomarkers Measured with ELISA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCI | Spinal cord injury |
| TBI | Traumatic brain injury |
| CSF | Cerebrospinal fluid |
| SBDP | αII-spectrin breakdown product |
| UCH-L1 | Ubiquitin C-terminal hydrolase-L1 |
| S100B | S100 calcium-binding protein-B |
| GFAP | Glial fibrillary acidic protein |
| GBDP | GFAP breakdown product |
| NF-L | Neurofilament light chain |
| IL | Interleukin |
References
- Khadour, F.A.; Khadour, Y.A.; Meng, L.; Lixin, C.; Xu, T. Epidemiological features of traumatic spinal cord injury in Wuhan, China. J. Orthop. Surg. Res. 2023, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.X.; Riviello, J.J., Jr.; Davila-Williams, D.; Thomas, S.P.; Erklauer, J.C.; Bauer, D.F.; Cokley, J.A. Pharmacologic and acute management of spinal cord injury in adults and children. Curr. Treat. Options Neurol. 2022, 24, 285–304. [Google Scholar] [CrossRef]
- Alostaz, H.; Cai, L. Biomarkers from Secondary Complications in Spinal Cord Injury. Curr. Pharmacol. Rep. 2022, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tong, D.; Wang, M.; Xu, C.; Gong, X.; Wang, Z.; Li, C. Peptidomics Analysis Reveals Serum Biomarkers in Spinal Cord Injury Patients. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Gong, C.; Liu, L.; Shen, Y. Biomarkers mining for spinal cord injury based on integrated multi-transcriptome expression profile data. J. Orthop. Surg. Res. 2021, 16, 267. [Google Scholar] [CrossRef]
- Chung, H.; Lee, D.H.; Kim, K.; Choi, Y.H.; Bae, S.J. Out-of-hospital cardiac arrest due to cervical spine injury by uncertain trauma; A study of two cases. Turk. J. Trauma Emerg. Surg. 2023, 29, 255–258. [Google Scholar] [CrossRef]
- Polinder, S.; Meerding, W.J.; Mulder, S.; Petridou, E.; van Beeck, E. Assessing the burden of injury in six European countries. Bull. World Health Organ. 2007, 85, 27–34. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury—Repair and regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Pedro, K.M.; Fehlings, M.G. Time is spine: What’s over the horizon. J. Clin. Orthop. Trauma 2022, 35, 102043. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Tay, J.; Hsu, S.-H.; Chen, W.-T.; Fang, Y.-D.; Liew, C.-Q.; Chou, E.H.; Wolfshohl, J.; d’Etienne, J.; Wang, C.-H. Early versus late surgical decompression for traumatic spinal cord injury on neurological recovery: A systematic review and meta-analysis. J. Neurotrauma 2021, 38, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; Fozzato, S.; Petrucci, Q.A.; Gallina, M.; Accetta, R.; Passeri, A.; Prevot, L.B.; Marinelli, E. Spinal cord injuries in the absence of post-traumatic radiographic anomalies (SCIWORA): The traumatic moment between patient anterior state and efficient/concurrent causes of injury. Acta Biomed. 2023, 94, 2. [Google Scholar]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. Wars 2011, 71, 281–299. [Google Scholar] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, T.; Che, M.; Wang, Y.; He, C.; Liu, L.; Lv, Z.; Xiao, C.; Wang, H.; Zhang, S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater. Today Bio 2022, 18, 100524. [Google Scholar] [CrossRef]
- Yokobori, S.; Zhang, Z.; Moghieb, A.; Mondello, S.; Gajavelli, S.; Dietrich, W.D.; Bramlett, H.; Hayes, R.L.; Wang, M.; Wang, K.K. Acute diagnostic biomarkers for spinal cord injury: Review of the literature and preliminary research report. World Neurosurg. 2015, 83, 867–878. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef]
- Dalkilic, T.; Fallah, N.; Noonan, V.K.; Salimi Elizei, S.; Dong, K.; Belanger, L.; Ritchie, L.; Tsang, A.; Bourassa-Moreau, E.; Heran, M.K. Predicting injury severity and neurological recovery after acute cervical spinal cord injury: A comparison of cerebrospinal fluid and magnetic resonance imaging biomarkers. J. Neurotrauma 2018, 35, 435–445. [Google Scholar] [CrossRef]
- Sogut, O.; Guloglu, C.; Orak, M.; Sayhan, M.; Gokdemir, M.; Ustundag, M.; Akkus, Z. Trauma scores and neuron-specific enolase, cytokine and C-reactive protein levels as predictors of mortality in patients with blunt head trauma. J. Int. Med. Res. 2010, 38, 1708–1720. [Google Scholar] [CrossRef]
- Kwon, B.K.; Bloom, O.; Wanner, I.-B.; Curt, A.; Schwab, J.M.; Fawcett, J.; Wang, K.K. Neurochemical biomarkers in spinal cord injury. Spinal Cord 2019, 57, 819–831. [Google Scholar] [CrossRef]
- Rodrigues, L.F.; Moura-Neto, V.; de Sampaio e Spohr, T.C.L. Biomarkers in spinal cord injury: From prognosis to treatment. Mol. Neurobiol. 2018, 55, 6436–6448. [Google Scholar] [CrossRef]
- Onal, O.; Aslanlar, E.; Apiliogullari, S.; Erkocak, O.F.; Celik, J.B. Post-spinal hyperacute subdural hematoma. Rev. Bras. Anestesiol. 2017, 67, 110–112. [Google Scholar] [CrossRef]
- Yang, Z.; Bramlett, H.M.; Moghieb, A.; Yu, D.; Wang, P.; Lin, F.; Bauer, C.; Selig, T.M.; Jaalouk, E.; Weissman, A.S. Temporal profile and severity correlation of a panel of rat spinal cord injury protein biomarkers. Mol. Neurobiol. 2018, 55, 2174–2184. [Google Scholar] [CrossRef]
- Stukas, S.; Cooper, J.; Gill, J.; Fallah, N.; Skinnider, M.A.; Belanger, L.; Ritchie, L.; Tsang, A.; Dong, K.; Streijger, F. Association of CSF and Serum Neurofilament Light and Glial Fibrillary Acidic Protein, Injury Severity, and Outcome in Spinal Cord Injury. Neurology 2023, 100, e1221–e1233. [Google Scholar] [CrossRef]
- Wang, H.-D.; Wei, Z.-J.; Li, J.-J.; Feng, S.-Q. Application value of biofluid-based biomarkers for the diagnosis and treatment of spinal cord injury. Neural Regen. Res. 2022, 17, 963. [Google Scholar]
- Leister, I.; Haider, T.; Mattiassich, G.; Kramer, J.L.K.; Linde, L.D.; Pajalic, A.; Grassner, L.; Altendorfer, B.; Resch, H.; Aschauer-Wallner, S.; et al. Biomarkers in Traumatic Spinal Cord Injury-Technical and Clinical Considerations: A Systematic Review. Neurorehabilit. Neural Repair 2020, 34, 95–110. [Google Scholar] [CrossRef]
- Loy, D.N.; Sroufe, A.E.; Pelt, J.L.; Burke, D.A.; Cao, Q.L.; Talbott, J.F.; Whittemore, S.R. Serum biomarkers for experimental acute spinal cord injury: Rapid elevation of neuron-specific enolase and S-100beta. Neurosurgery 2005, 56, 391–397; discussion 391–397. [Google Scholar] [CrossRef]
- Lubieniecka, J.M.; Streijger, F.; Lee, J.H.; Stoynov, N.; Liu, J.; Mottus, R.; Pfeifer, T.; Kwon, B.K.; Coorssen, J.R.; Foster, L.J.; et al. Biomarkers for severity of spinal cord injury in the cerebrospinal fluid of rats. PLoS ONE 2011, 6, e19247. [Google Scholar] [CrossRef]
- Marquardt, G.; Setzer, M.; Seifert, V. Serum biomarkers for experimental acute spinal cord injury: Rapid elevation of neuron-specific enolase and S-100 beta. Neurosurgery 2006, 58, E590. [Google Scholar] [CrossRef]
- Ottens, A.K.; Kobeissy, F.H.; Fuller, B.F.; Liu, M.C.; Oli, M.W.; Hayes, R.L.; Wang, K.K. Novel neuroproteomic approaches to studying traumatic brain injury. Prog. Brain Res. 2007, 161, 401–418. [Google Scholar] [CrossRef]
- Mallah, K.; Quanico, J.; Trede, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I. Lipid Changes Associated with Traumatic Brain Injury Revealed by 3D MALDI-MSI. Anal. Chem. 2018, 90, 10568–10576. [Google Scholar] [CrossRef]
- Devaux, S.; Cizkova, D.; Quanico, J.; Franck, J.; Nataf, S.; Pays, L.; Hauberg-Lotte, L.; Maass, P.; Kobarg, J.H.; Kobeissy, F.; et al. Proteomic Analysis of the Spatio-temporal Based Molecular Kinetics of Acute Spinal Cord Injury Identifies a Time- and Segment-specific Window for Effective Tissue Repair. Mol. Cell. Proteom. 2016, 15, 2641–2670. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Mondello, S.; Robicsek, S.A.; Gabrielli, A.; Brophy, G.M.; Papa, L.; Tepas, J., III; Robertson, C.; Buki, A.; Scharf, D.; Jixiang, M. αII-spectrin breakdown products (SBDPs): Diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma 2010, 27, 1203–1213. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Sarkis, G.; Torres, I.; Raghavan, V. Ubiquitin C-terminal hydrolase-L1 (UCH-L1) as a therapeutic and diagnostic target in neurodegeneration, neurotrauma and neuro-injuries. Expert Opin. Ther. Targets 2017, 21, 627–638. [Google Scholar] [CrossRef]
- Khetani, S.; Kollath, V.O.; Eastick, E.; Debert, C.; Sen, A.; Karan, K.; Sanati-Nezhad, A. Single-step functionalization of poly-catecholamine nanofilms for ultra-sensitive immunosensing of ubiquitin carboxyl terminal hydrolase-L1 (UCHL-1) in spinal cord injury. Biosens. Bioelectron. 2019, 145, 111715. [Google Scholar] [CrossRef]
- Stukas, S.; Gill, J.; Cooper, J.; Belanger, L.; Ritchie, L.; Tsang, A.; Dong, K.; Streijger, F.; Street, J.; Paquette, S. Characterization of cerebrospinal fluid ubiquitin C-terminal hydrolase L1 as a biomarker of human acute traumatic spinal cord injury. J. Neurotrauma 2021, 38, 2055–2064. [Google Scholar] [CrossRef]
- Cruz, C.D.; Coelho, A.; Antunes-Lopes, T.; Cruz, F. Biomarkers of spinal cord injury and ensuing bladder dysfunction. Adv. Drug Deliv. Rev. 2015, 82, 153–159. [Google Scholar] [CrossRef]
- Khan, M.; Dhammu, T.S.; Singh, I.; Singh, A.K. Amelioration of spinal cord injury in rats by blocking peroxynitrite/calpain activity. BMC Neurosci. 2018, 19, 50. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Saleem, T.H.; Elsadek, B.E.; Ashraf, O.; Hamdan, A.R.; El-Khateeb, E.E.S.; Abd Elwahab, S.M.; Hassan, M.H. Circulating ubiquitin carboxyl terminal hydrolase L1 and neuroglobin levels in traumatic spinal cord injuries: Relation to severity and outcomes. Int. J. Gen. Med. 2022, 15, 5795–5805. [Google Scholar] [CrossRef]
- Kwon, B.K.; Streijger, F.; Fallah, N.; Noonan, V.K.; Bélanger, L.M.; Ritchie, L.; Paquette, S.J.; Ailon, T.; Boyd, M.C.; Street, J. Cerebrospinal fluid biomarkers to stratify injury severity and predict outcome in human traumatic spinal cord injury. J. Neurotrauma 2017, 34, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, H.; Sun, J.; Xia, Y.; Zhu, R.; Zhang, X.; Tian, R. The prognostic value of serum neuron specific enolase (NSE) and S100B level in patients of acute spinal cord injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4510. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, C.W.; Lee, K.J.; Choe, J.W.; Kim, S.E.; Oh, J.H.; Park, Y.S. Elevated serum S100B levels in acute spinal fracture without head injury. Emerg. Med. J. 2010, 27, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Capirossi, R.; Piunti, B.; Fernández, M.; Maietti, E.; Rucci, P.; Negrini, S.; Giovannini, T.; Kiekens, C.; Calzà, L. Early CSF biomarkers and late functional outcomes in spinal cord injury. A Pilot Study. Int. J. Mol. Sci. 2020, 21, 9037. [Google Scholar] [CrossRef]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef]
- Guéz, M.; Hildingsson, C.; Rosengren, L.; Karlsson, K.; Toolanen, G. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J. Neurotrauma 2003, 20, 853–858. [Google Scholar] [CrossRef]
- Wang, K.K.; Kobeissy, F.H.; Shakkour, Z.; Tyndall, J.A. Thorough overview of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein as tandem biomarkers recently cleared by US Food and Drug Administration for the evaluation of intracranial injuries among patients with traumatic brain injury. Acute Med. Surg. 2021, 8, e622. [Google Scholar] [CrossRef]
- Pouw, M.; Kwon, B.; Verbeek, M.; Vos, P.; Van Kampen, A.; Fisher, C.; Street, J.; Paquette, S.; Dvorak, M.; Boyd, M. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: A descriptive analysis of 16 subjects. Spinal Cord 2014, 52, 428–433. [Google Scholar] [CrossRef]
- Ahadi, R.; Khodagholi, F.; Daneshi, A.; Vafaei, A.; Mafi, A.A.; Jorjani, M. Diagnostic value of serum levels of GFAP, pNF-H, and NSE compared with clinical findings in severity assessment of human traumatic spinal cord injury. Spine 2015, 40, E823–E830. [Google Scholar] [CrossRef]
- Kuhle, J.; Gaiottino, J.; Leppert, D.; Petzold, A.; Bestwick, J.P.; Malaspina, A.; Lu, C.-H.; Dobson, R.; Disanto, G.; Norgren, N. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J. Neurol. Neurosurg. Psychiatry 2015, 86, 273–279. [Google Scholar] [CrossRef]
- Kosa, P.; Masvekar, R.; Komori, M.; Phillips, J.; Ramesh, V.; Varosanec, M.; Sandford, M.; Bielekova, B. Enhancing the clinical value of serum neurofilament light chain measurement. JCI Insight 2022, 7, e161415. [Google Scholar] [CrossRef] [PubMed]
- Chunyk, A.G.; Joyce, A.; Fischer, S.K.; Dysinger, M.; Mikulskis, A.; Jeromin, A.; Lawrence-Henderson, R.; Baker, D.; Yeung, D. A Multi-site In-depth Evaluation of the Quanterix Simoa from a User’s Perspective. AAPS J. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabilit. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Plantier, V.; Sanchez-Brualla, I.; Dingu, N.; Brocard, C.; Liabeuf, S.; Gackière, F.; Brocard, F. Calpain fosters the hyperexcitability of motoneurons after spinal cord injury and leads to spasticity. Elife 2019, 8, e51404. [Google Scholar] [CrossRef]
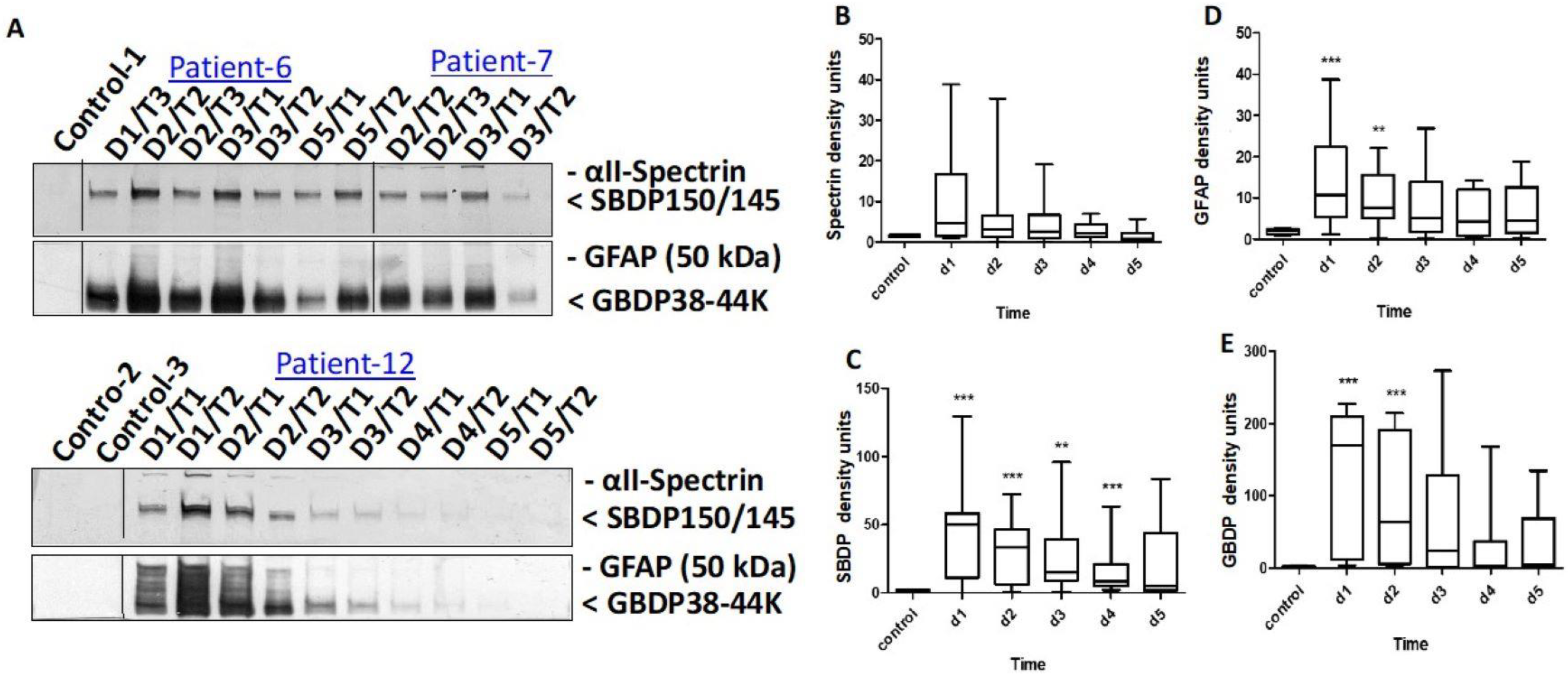
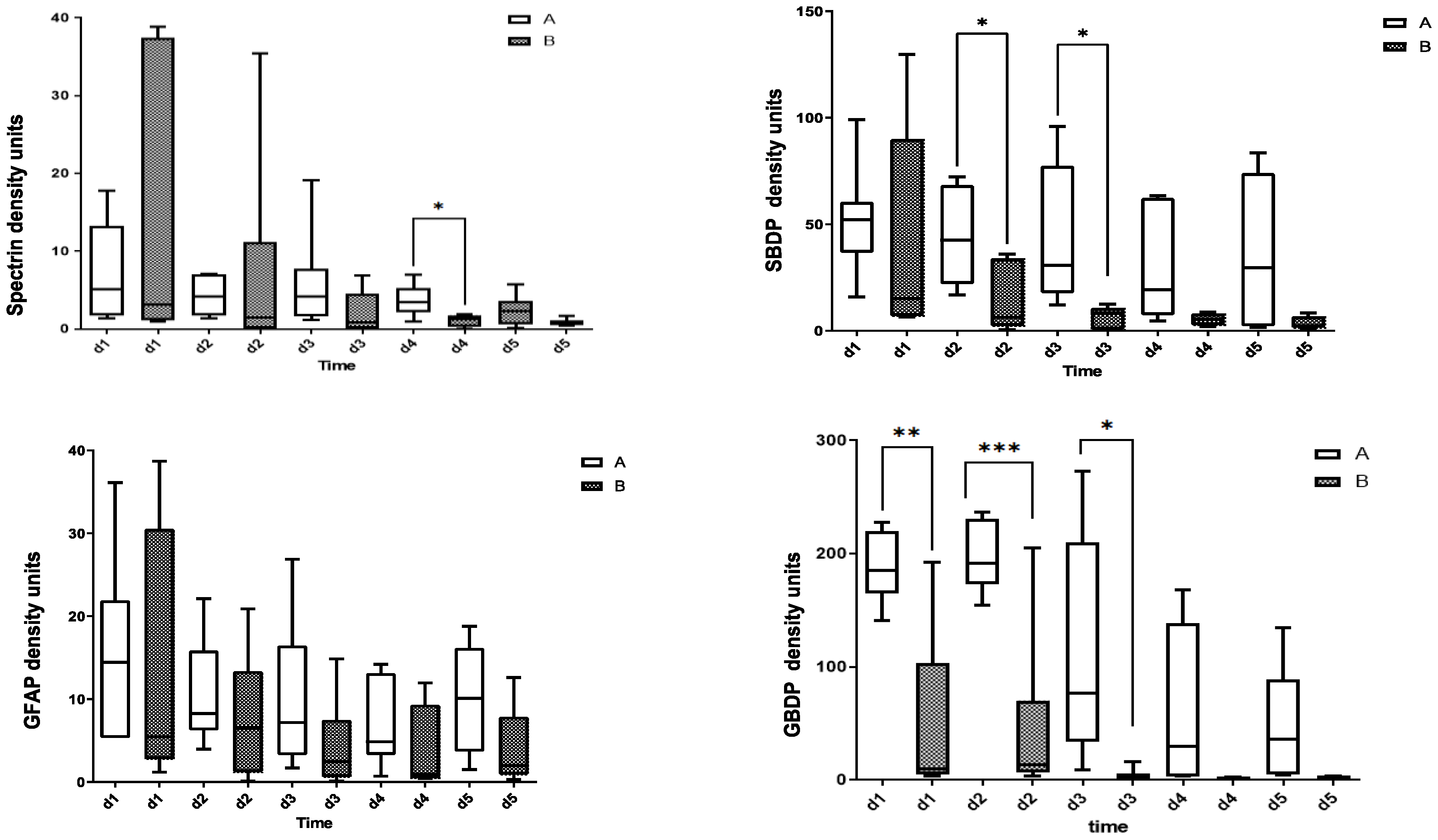
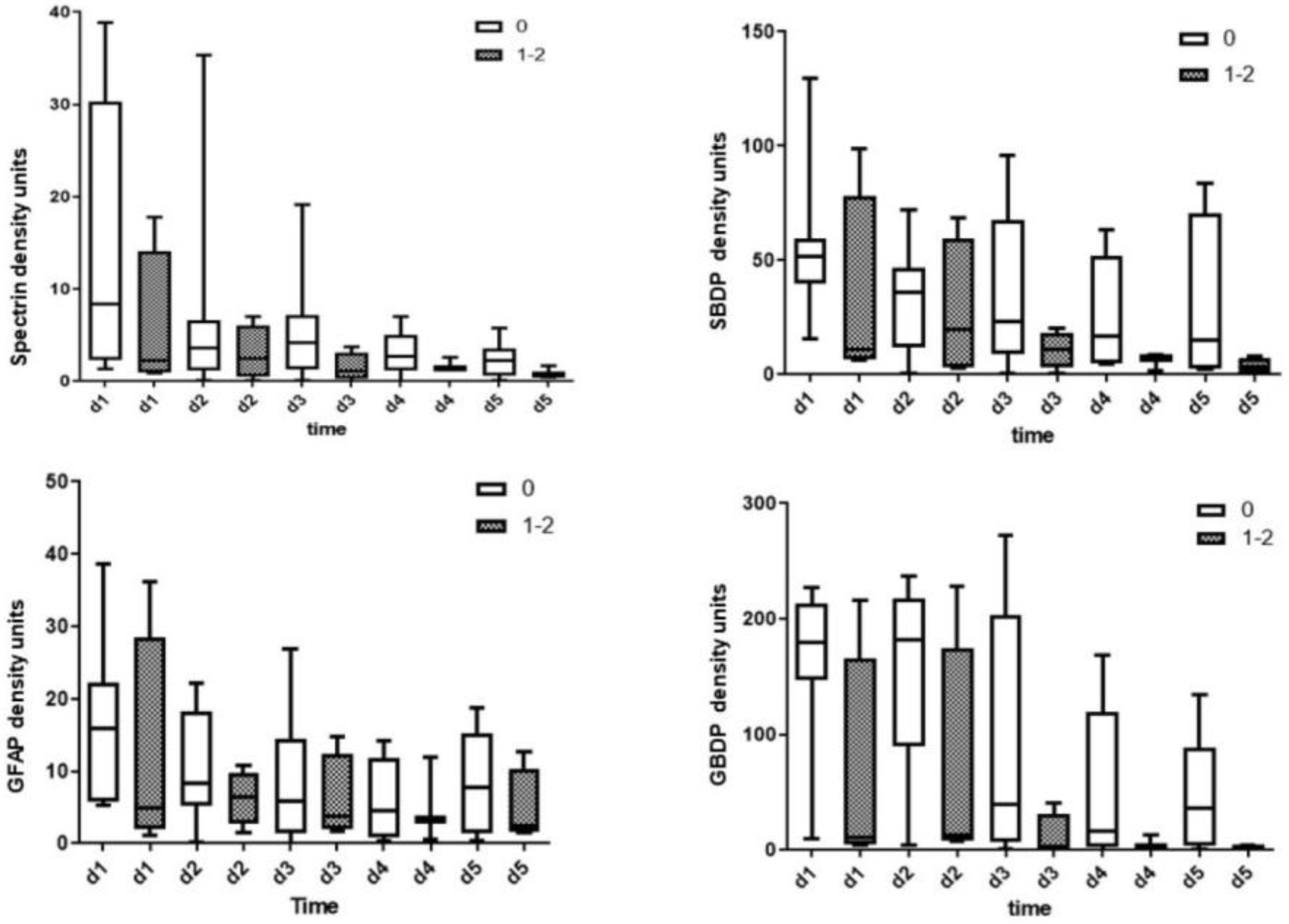


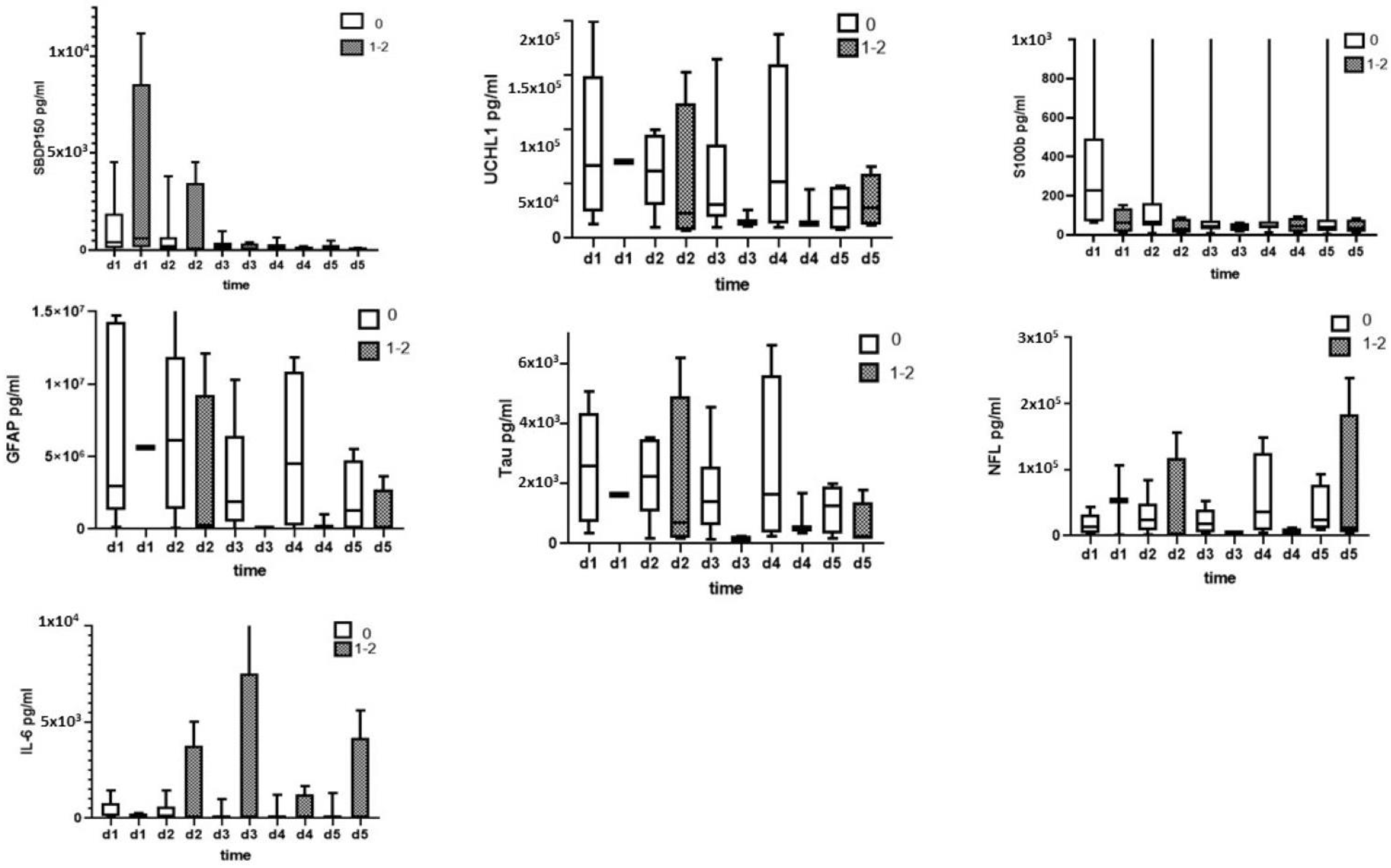
| Patient | Age | Gender | Cause and Level of Injury | I-AIS | D-AIS | ΔAIS (I-AIS-D-AIS) |
|---|---|---|---|---|---|---|
| P1 | 43 | Male | Pedestrian MVA, C7-T1 | A | A | 0 |
| P2 | 38 | Male | Fall, T3-T4 | B | D | 2 |
| P3 | 21 | Male | Diving accident C5-C6 | B | D | 2 |
| P4 | 28 | Male | Diving accident C5-C6 | B | B | 0 |
| P5 | 19 | Male | MVA, C5-C6 | B | C | 1 |
| P6 | 22 | Male | Sport Injury C5 | A | A | 0 |
| P7 | 48 | Male | MVA, T3 | A | A | 0 |
| P8 | 38 | Female | MVA, T1/2 | A | A | 0 |
| P9 | 29 | Female | MVA, T12 | A | A | 0 |
| P10 | 57 | Male | Fall, T11-12 | A | A | 0 |
| P11 | 67 | Male | Fall, C3-C4 | A | A | 0 |
| P12 | 22 | Male | Sport Injury C5-C6 | A | B | 1 |
| P13 | 66 | Male | Fall T12-L1 | B | B | 0 |
| P14 | 22 | Male | Gunshot T-12 | A | A | 0 |
| P15 | 58 | Male | Fall, T4-T5 | B | B | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Apiliogullari, S.; Fu, Y.; Istanbouli, A.; Kaur, S.; Jabbal, I.S.; Moghieb, A.; Irfan, Z.; Patterson, R.L.; Kurup, M.; et al. Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury. Diagnostics 2023, 13, 1814. https://doi.org/10.3390/diagnostics13101814
Yang Z, Apiliogullari S, Fu Y, Istanbouli A, Kaur S, Jabbal IS, Moghieb A, Irfan Z, Patterson RL, Kurup M, et al. Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury. Diagnostics. 2023; 13(10):1814. https://doi.org/10.3390/diagnostics13101814
Chicago/Turabian StyleYang, Zhihui, Seza Apiliogullari, Yueqiang Fu, Ayah Istanbouli, Sehajpreet Kaur, Iktej Singh Jabbal, Ahmed Moghieb, Zoha Irfan, Robert Logan Patterson, Milin Kurup, and et al. 2023. "Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury" Diagnostics 13, no. 10: 1814. https://doi.org/10.3390/diagnostics13101814
APA StyleYang, Z., Apiliogullari, S., Fu, Y., Istanbouli, A., Kaur, S., Jabbal, I. S., Moghieb, A., Irfan, Z., Patterson, R. L., Kurup, M., Morrow, L., Cohn, M., Zhang, Z., Zhu, J., Hayes, R. L., Bramlett, H. M., Bullock, M. R., Dietrich, W. D., Wang, M. Y., ... Wang, K. W. (2023). Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury. Diagnostics, 13(10), 1814. https://doi.org/10.3390/diagnostics13101814







