FIP1L1–PDGFRα-Positive Loeffler Endocarditis—A Distinct Cause of Heart Failure in a Young Male: The Role of Multimodal Diagnostic Tools
Abstract
:1. Introduction
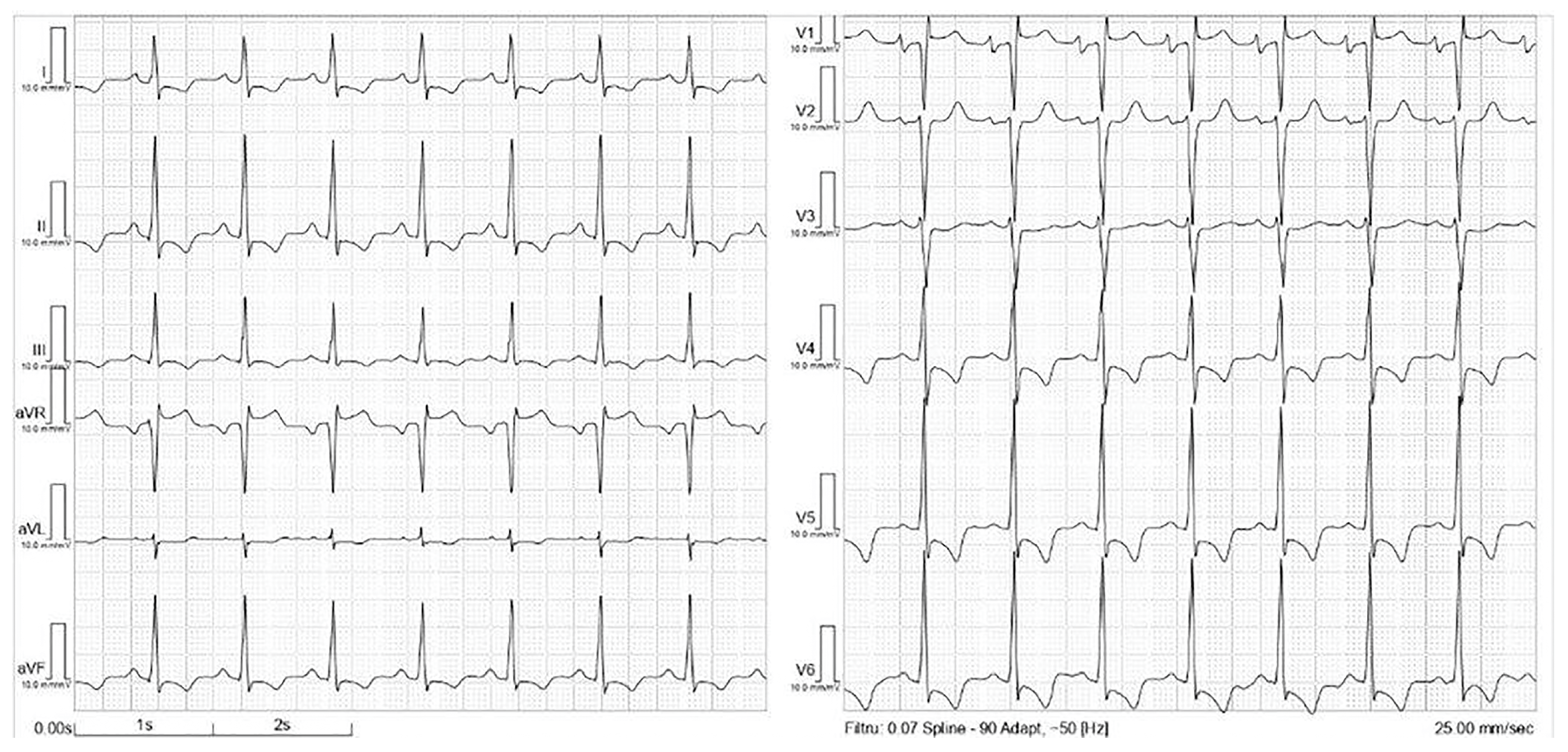
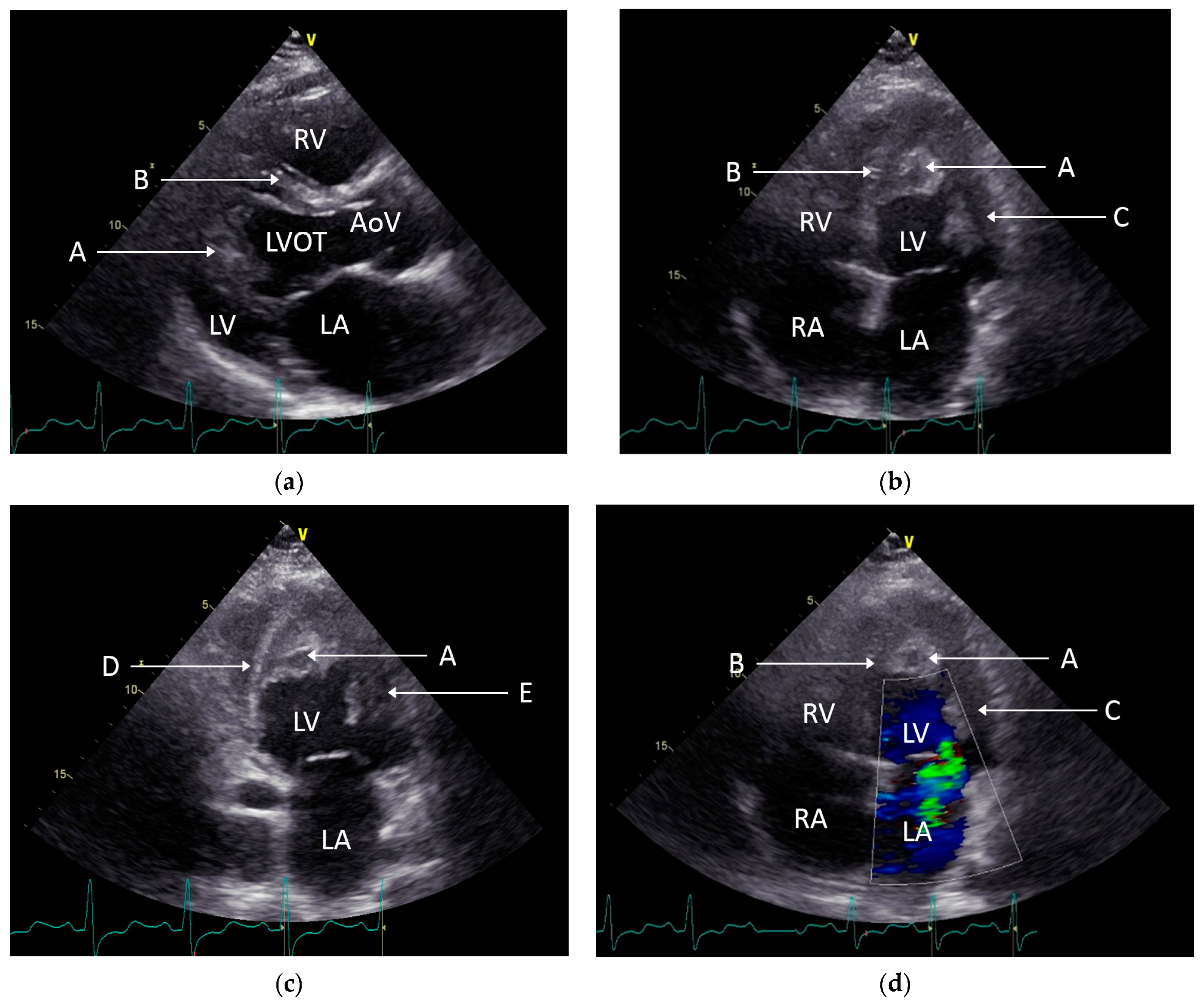
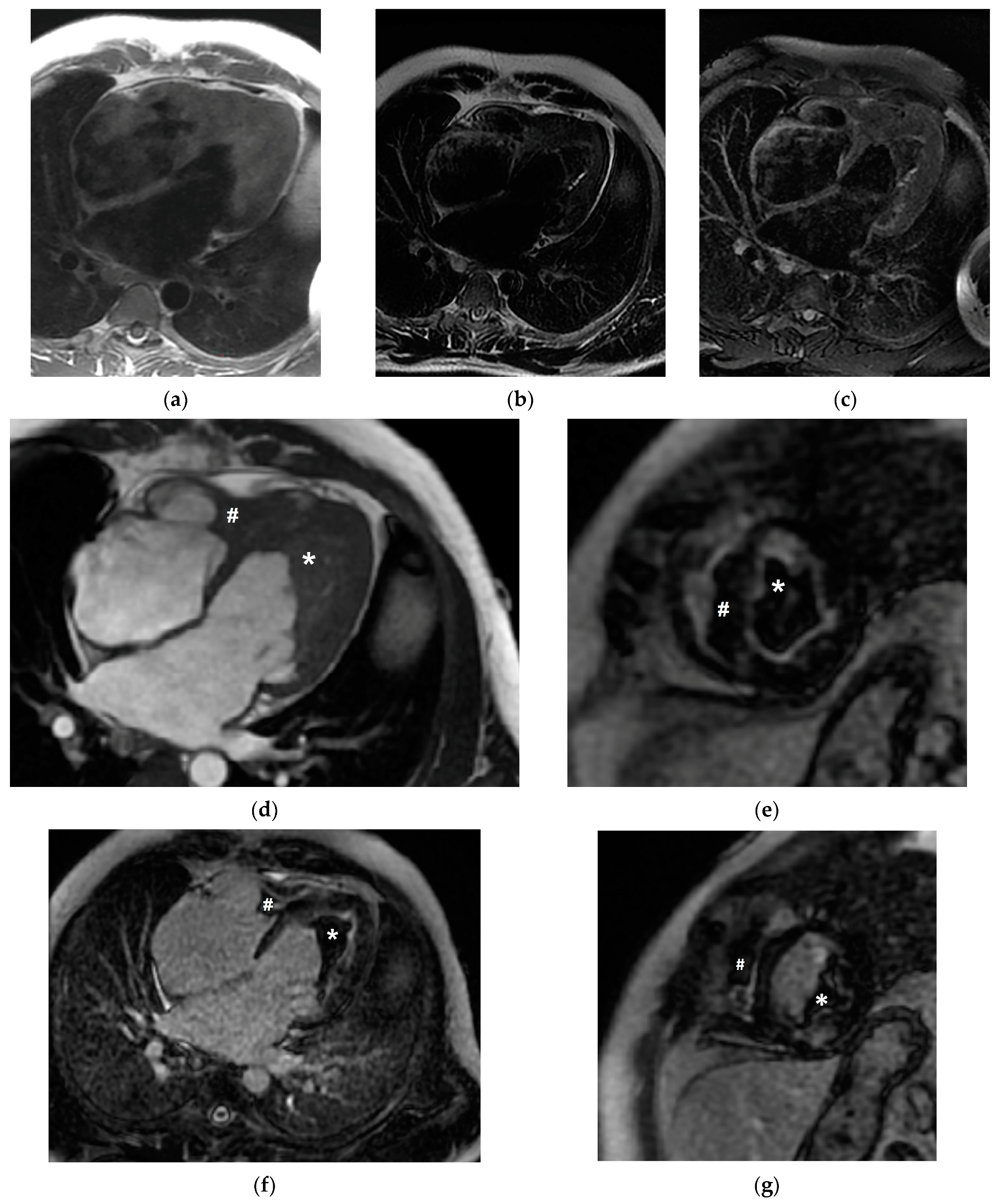
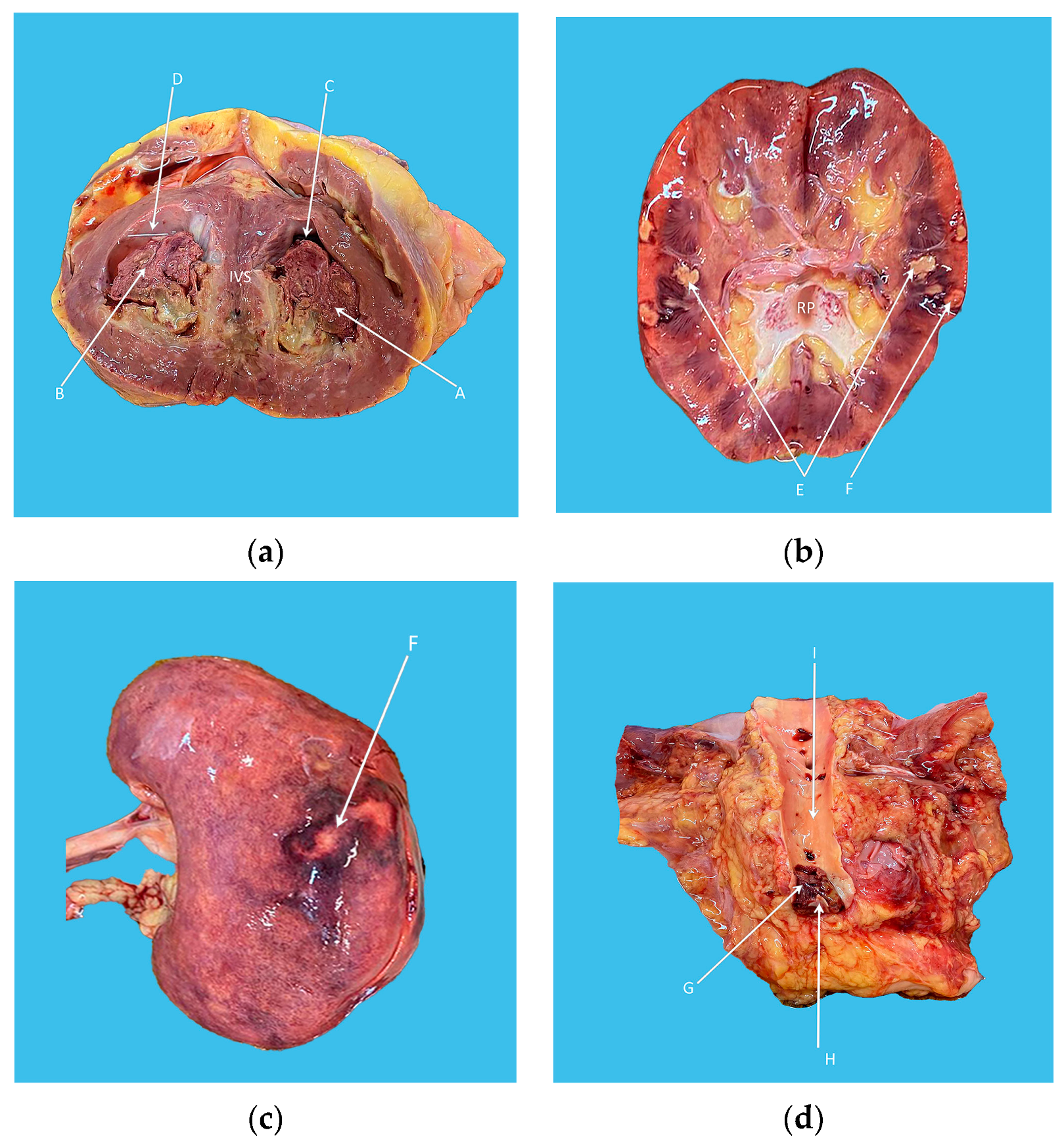
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valent, P.; Klion, A.D.; Horny, H.P.; Roufosse, F.; Gotlib, J.; Weller, P.F.; Hellmann, A.; Metzgeroth, G.; Leiferman, K.M.; Arock, M.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012, 130, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Degenfeld-Schonburg, L.; Sadovnik, I.; Horny, H.P.; Arock, M.; Simon, H.U.; Reiter, A.; Bochner, B.S. Eosinophils and eosinophil-associated disorders: Immunological, clinical, and molecular complexity. Semin. Immunopathol. 2021, 43, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Lupu, S.; Pop, M.; Mitre, A. Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway. Diagnostics 2022, 12, 2157. [Google Scholar] [CrossRef]
- Requena, G.; van den Bosch, J.; Akuthota, P.; Kovalszki, A.; Steinfeld, J.; Kwon, N.; Van Dyke, M.K. Clinical Profile and Treatment in Hypereosinophilic Syndrome Variants: A Pragmatic Review. J. Allergy Clin. Immunol. Pract. 2022, 10, 2125–2134. [Google Scholar] [CrossRef]
- Arustamyan, M.; Hoosain, J.; Mattson, J.; Hasni, S.F.; Cho, S.H.; Gorodin Kiliddar, P. Loeffler Endocarditis: A Manifestation of Hypereosinophilic Syndrome. CASE 2019, 4, 74–77. [Google Scholar] [CrossRef]
- Mattis, D.M.; Wang, S.A.; Lu, C.M. Contemporary Classification and Diagnostic Evaluation of Hypereosinophilia. Am. J. Clin. Pathol. 2020, 154, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Renneville, A.; MacIntyre, E.; Mastrilli, S.; Ackermann, F.; Cayuela, J.M.; Rousselot, P.; Schmidt-Tanguy, A.; Fain, O.; Michel, M.; et al. The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases. Medicine 2013, 92, e1–e9. [Google Scholar] [CrossRef]
- Qu, S.Q.; Qin, T.J.; Xu, Z.F.; Zhang, Y.; Ai, X.F.; Li, B.; Zhang, H.L.; Fang, L.W.; Pan, L.J.; Hu, N.B.; et al. Long-term outcomes of imatinib in patients with FIP1L1/PDGFRA associated chronic eosinophilic leukemia: Experience of a single center in China. Oncotarget 2016, 7, 33229–33236. [Google Scholar] [CrossRef]
- Butt, N.M.; Lambert, J.; Ali, S.; Beer, P.A.; Cross, N.C.; Duncombe, A.; Ewing, J.; Harrison, C.N.; Knapper, S.; McLornan, D.; et al. Guideline for the investigation and management of eosinophilia. Br. J. Haematol. 2017, 176, 553–572. [Google Scholar] [CrossRef]
- Gotlib, J.; Cools, J. Five years since the discovery of FIP1L1-PDGFRA: What we have learned about the fusion and other molecularly defined eosinophilias. Leukemia 2008, 22, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Buitenhuis, M.; Verhagen, L.P.; Cools, J.; Coffer, P.J. Molecular mechanisms underlying FIP1L1-PDGFRA-mediated myeloproliferation. Cancer Res. 2007, 67, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, J.; Couteau-Chardon, A.; Trichereau, J.; Panel, K.; Gesquiere, C.; Ben Abdelali, R.; Bidet, A.; Bladé, J.S.; Cayuela, J.M.; Cony-Makhoul, P.; et al. Epidemiology, clinical picture and long-term outcomes of FIP1L1-PDGFRA-positive myeloid neoplasm with eosinophilia: Data from 151 patients. Am. J. Hematol. 2020, 95, 1314–1323. [Google Scholar] [CrossRef]
- Tefferi, A.; Gotlib, J.; Pardanani, A. Hypereosinophilic syndrome and clonal eosinophilia: Point-of-care diagnostic algorithm and treatment update. Mayo Clin. Proc. 2010, 85, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A. The Diagnostic Work-Up of Hypereosinophilia. Pathobiology 2019, 86, 39–52. [Google Scholar] [CrossRef]
- Helbig, G.; Moskwa, A.; Hus, M.; Piszcz, J.; Swiderska, A.; Urbanowicz, A.; Całbecka, M.; Seferyńska, I.; Raźny, M.; Rodzaj, M.; et al. Durable remission after treatment with very low doses of imatinib for FIP1L1-PDGFRα-positive chronic eosinophilic leukaemia. Cancer Chemother. Pharmacol. 2011, 67, 967–969. [Google Scholar] [CrossRef]
- Zhong, Z.; Yang, Z.; Peng, Y.; Wang, L.; Yuan, X. Diagnosis and treatment of eosinophilic myocarditis. J. Transl. Autoimmun. 2021, 4, 100118. [Google Scholar] [CrossRef]
- Kim, N.K.; Kim, C.Y.; Kim, J.H.; Jang, S.Y.; Bae, M.H.; Lee, J.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Chae, S.C. A Hypereosinophilic Syndrome with Cardiac Involvement from Thrombotic Stage to Fibrotic Stage. J. Cardiovasc. Ultrasound. 2015, 23, 100–102. [Google Scholar] [CrossRef]
- Baltazares-Lipp, M.E.; Soto-González, J.I.; Aboitiz-Rivera, C.M.; Carmona-Ruíz, H.A.; Ortega, B.S.; Blachman-Braun, R. Hypereosinophilic Syndrome: A Case of Fatal Löffler Endocarditis. Case Rep. Cardiol. 2016, 2016, 2359532. [Google Scholar] [CrossRef]
- Polito, M.V.; Hagendorff, A.; Citro, R.; Prota, C.; Silverio, A.; De Angelis, E.; Klingel, K.; Metze, M.; Stöbe, S.; Hoffmann, K.T.; et al. Loeffler’s Endocarditis: An Integrated Multimodality Approach. J. Am. Soc. Echocardiogr. 2020, 33, 1427–1441. [Google Scholar] [CrossRef]
- Guglielmo, M.; Pontone, G. Clinical implications of cardiac magnetic resonance imaging fibrosis. Eur. Heart J. Suppl. 2022, 24 (Suppl. I), I123–I126. [Google Scholar] [CrossRef] [PubMed]
- Mankad, R.; Bonnichsen, C.; Mankad, S. Hypereosinophilic syndrome: Cardiac diagnosis and management. Heart 2016, 102, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Thampi, S.; Saba, S.G.; Jermyn, R. Loeffler Endocarditis: A Unique Presentation of Right-Sided Heart Failure Due to Eosinophil-Induced Endomyocardial Fibrosis. Clin. Med. Insights Case Rep. 2017, 10, 1179547617723643. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Haran, M.; Shetty, V.; Sadiq, A.; Hollander, G.; Shani, J. Hypereosinophilic syndrome presenting with biventricular cardiac thrombi. Echocardiography 2010, 27, E57–E59. [Google Scholar] [CrossRef]
- Afzal, S.; Ahmed, T.; Saleem, T.; Chan, A. Loeffler’s Endocarditis and the Diagnostic Utility of Multimodality Imaging. Cureus 2020, 12, e10061. [Google Scholar] [CrossRef]
- Ogbogu, P.U.; Bochner, B.S.; Butterfield, J.H.; Gleich, G.J.; Huss-Marp, J.; Kahn, J.E.; Leiferman, K.M.; Nutman, T.B.; Pfab, F.; Ring, J.; et al. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 2009, 124, 1319–1325.e3. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, W.; Zhao, W.; Qin, L.; Pei, F.; Zheng, Y. Loeffler endocarditis as a rare cause of heart failure with preserved ejection fraction: A case report and review of literature. Medicine 2018, 97, e0079. [Google Scholar] [CrossRef]
- Helbig, G.; Lewandowski, K.; Świderska, A.; Rodzaj, M.; Seferyńska, I.; Gajkowska-Kulik, J. Exquisite response to imatinib mesylate in FIP1L1-PDGFRA-mutated hypereosinophilic syndrome: A 12-year experience of the Polish Hypereosinophilic Syndrome Study Group. Pol. Arch. Intern. Med. 2020, 130, 255–257. [Google Scholar] [CrossRef]
- Klion, A.D.; Robyn, J.; Maric, I.; Fu, W.; Schmid, L.; Lemery, S.; Noel, P.; Law, M.A.; Hartsell, M.; Talar-Williams, C.; et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia: Implications for optimal dosing. Blood 2007, 110, 3552–3556. [Google Scholar] [CrossRef]
- Cadenas, F.L.; Bua, B.R.; Campelo, M.D.; Rieu, J.B.; Bain, B.J. A myeloid neoplasm with FIP1L1-PDGFRA presenting as acute myeloid leukemia. Am. J. Hematol. 2020, 94, 1214–1215. [Google Scholar] [CrossRef]
- Arefi, M.; Garcia, J.L.; Briz, M.M.; de Arriba, F.; Rodriguez, J.N.; Martin-Nunez, G.; Martinez, J.; Lopez, J.; Suarez, J.G.; Moreno, M.J.; et al. Response to imatinib mesylate in patients with hypereosinophilic syndrome. Int. J. Hematol. 2012, 96, 320–326. [Google Scholar] [CrossRef]
- Gotlib, J.; Cools, J.; Malone, J.M., 3rd; Schrier, S.L.; Gilliland, D.G.; Coutré, S.E. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: Implications for diagnosis, classification, and management. Blood 2004, 103, 2879–2891. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Cortes, J.; Quintás-Cardama, A.; Manshouri, T.; Luthra, R.; Garcia-Manero, G.; Kantarjian, H.; Verstovsek, S. Imatinib has limited therapeutic activity for hypereosinophilic syndrome patients with unknown or negative PDGFRalpha mutation status. Leuk. Res. 2009, 33, 837–839. [Google Scholar] [CrossRef]
- Kumar, A.N.; Sathyanarayanan, V.; Devi, V.L.; Rajkumar, N.N.; Das, U.; Dutt, S.; Chinnagiriyappa, L.K. FIP1L1-PDGFRA-Positive Chronic Eosinophilic Leukemia: A Low-Burden Disease with Dramatic Response to Imatinib—A Report of 5 Cases from South India. Turk. J. Haematol. 2014, 31, 56–60. [Google Scholar] [CrossRef]
- Bonou, M.; Kapelios, C.J.; Benetos, G.; Moyssakis, I.; Giannakopoulou, N.; Diamantopoulos, P.; Korkolopoulou, P.; Variami, E.; Barbetseas, J. Hypereosinophilic Syndrome as a Rare Cause of Reversible Biventricular Heart Failure. Can. J. Cardiol. 2017, 33, 688.e5–688.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Si, D.; Zhang, Z.; Zhang, W. Loeffler endocarditis with intracardiac thrombus: Case report and literature review. BMC Cardiovasc. Disord. 2021, 21, 615. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, A.; Moldovan, D.A.; Pop, M.; Benedek, I.; Kövecsi, A.; Dumbrava, R.A.; Iancu, D.G.; Cristescu, L.; Huma, L.; Tilea, I. FIP1L1–PDGFRα-Positive Loeffler Endocarditis—A Distinct Cause of Heart Failure in a Young Male: The Role of Multimodal Diagnostic Tools. Diagnostics 2023, 13, 1795. https://doi.org/10.3390/diagnostics13101795
Varga A, Moldovan DA, Pop M, Benedek I, Kövecsi A, Dumbrava RA, Iancu DG, Cristescu L, Huma L, Tilea I. FIP1L1–PDGFRα-Positive Loeffler Endocarditis—A Distinct Cause of Heart Failure in a Young Male: The Role of Multimodal Diagnostic Tools. Diagnostics. 2023; 13(10):1795. https://doi.org/10.3390/diagnostics13101795
Chicago/Turabian StyleVarga, Andreea, Diana Andreea Moldovan, Marian Pop, Istvan Benedek, Attila Kövecsi, Robert Adrian Dumbrava, Dragos Gabriel Iancu, Liviu Cristescu, Laurentiu Huma, and Ioan Tilea. 2023. "FIP1L1–PDGFRα-Positive Loeffler Endocarditis—A Distinct Cause of Heart Failure in a Young Male: The Role of Multimodal Diagnostic Tools" Diagnostics 13, no. 10: 1795. https://doi.org/10.3390/diagnostics13101795
APA StyleVarga, A., Moldovan, D. A., Pop, M., Benedek, I., Kövecsi, A., Dumbrava, R. A., Iancu, D. G., Cristescu, L., Huma, L., & Tilea, I. (2023). FIP1L1–PDGFRα-Positive Loeffler Endocarditis—A Distinct Cause of Heart Failure in a Young Male: The Role of Multimodal Diagnostic Tools. Diagnostics, 13(10), 1795. https://doi.org/10.3390/diagnostics13101795






