Sleep Position Detection with a Wireless Audio-Motion Sensor—A Validation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol and Devices
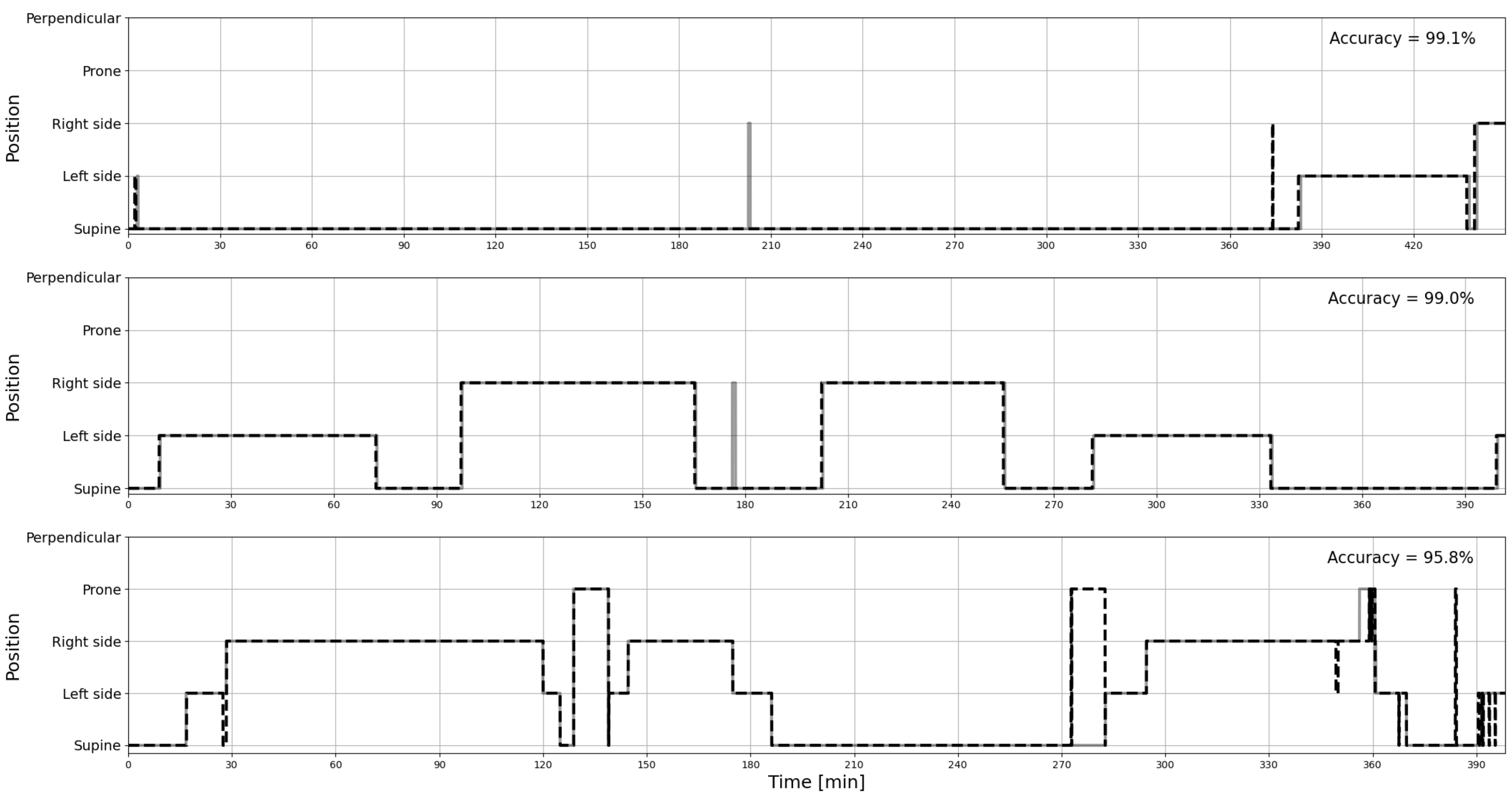
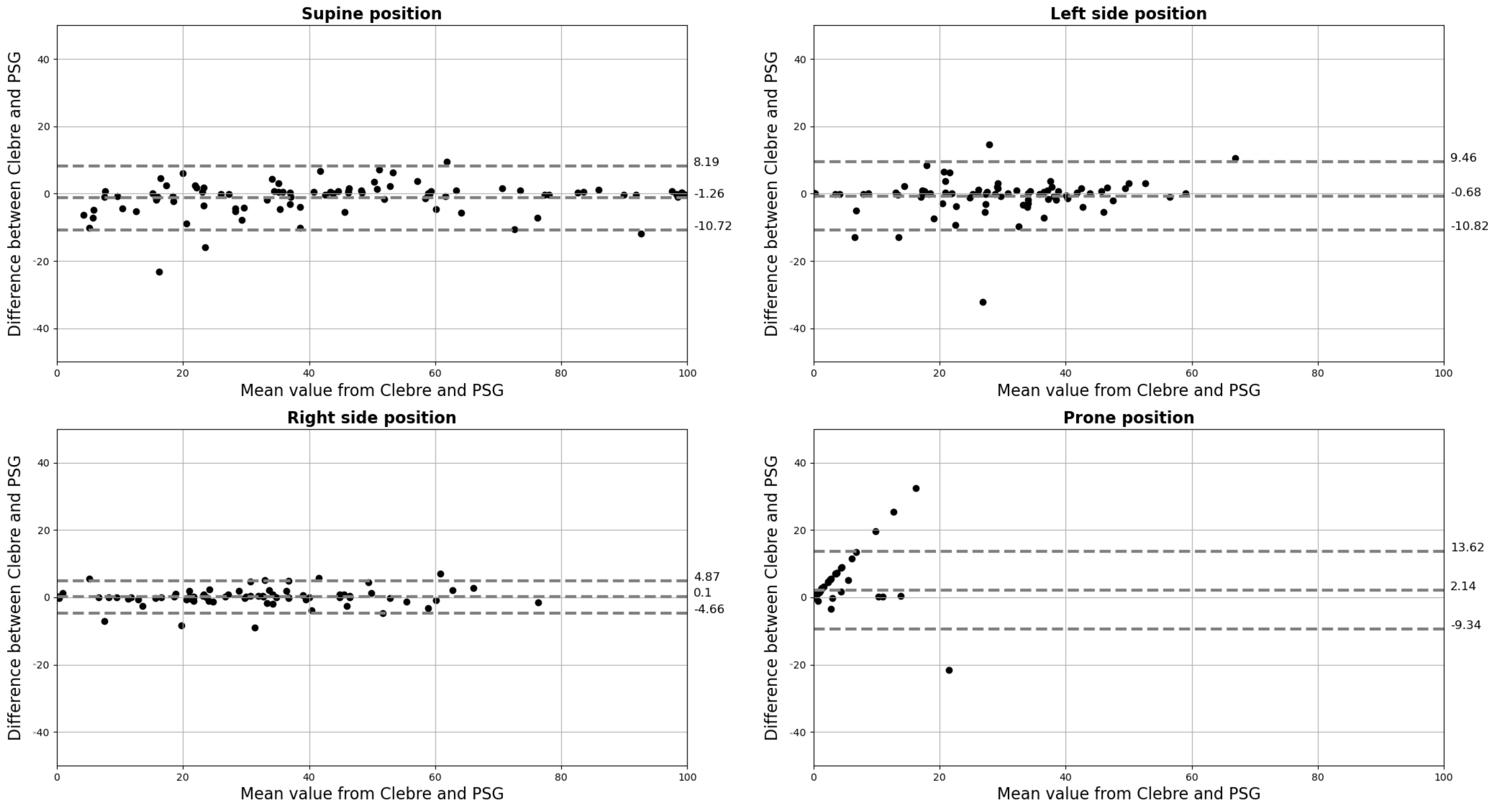
2.3. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strollo, P.J., Jr.; Rogers, R.M. Obstructive sleep apnea. N. Engl. J. Med. 1996, 334, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, R.D.; Diaz, F.; Lloyd, S. The effects of sleep posture and sleep stage on apnea frequency. Sleep 1991, 14, 351–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, S.; Gillin, J.C.; Littner, M.; Shepard, J. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999, 22, 662–689. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Paek, J.H.; Chung, Y.S.; Kim, W.S. Clinical features in patients with positional obstructive sleep apnea according to its subtypes. Sleep Breath. 2017, 21, 109–117. [Google Scholar] [CrossRef]
- Kim, K.T.; Cho, Y.W.; Kim, D.E.; Hwang, S.H.; Song, M.L.; Motamedi, G.K. Two subtypes of positional obstructive sleep apnea: Supine-predominant and supine-isolated. Clin. Neurophysiol. 2016, 127, 565–570. [Google Scholar] [CrossRef]
- Mador, M.J.; Kufel, T.J.; Magalang, U.J.; Rajesh, S.; Watwe, V.; Grant, B.J. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest 2005, 128, 2130–2137. [Google Scholar] [CrossRef] [Green Version]
- Oksenberg, A.; Gadoth, N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J. Sleep Res. 2014, 23, 204–210. [Google Scholar] [CrossRef]
- Ravesloot, M.; Van Maanen, J.; Dun, L.; De Vries, N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea—A review of the literature. Sleep Breath. 2013, 17, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Młyńczak, M.; Valdez, T.A.; Kukwa, W. Joint Apnea and Body Position Analysis for Home Sleep Studies Using a Wireless Audio and Motion Sensor. IEEE Access 2020, 8, 170579–170587. [Google Scholar] [CrossRef]
- New York Heart Association (NYHA). Functional Classification: The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels; NYHA: New York, NY, USA, 1994. [Google Scholar]
- Nox A1 Manual. Available online: https://noxmedical.com/wp-content/uploads/2021/01/Nox-A1-US-Manual.pdf (accessed on 4 May 2022).
- Nox A1 Brochure. Available online: https://noxmedical.com/wp-content/uploads/2021/11/A1_brochure_US_adress.pdf (accessed on 4 May 2022).
- Nonin 3150 WristOx2 Operator’s Manual. Available online: https://www.nonin.com/wp-content/uploads/2018/10/3150-BLE-USB-Operators-Manual.pdf (accessed on 4 May 2022).
- AXIS M3106-LVE Datasheet. Available online: https://www.axis.com/dam/public/ed/2b/8a/datasheet-axis-m3106-lve-network-camera-en-US-278038.pdf (accessed on 4 May 2022).
- AXIS M3116-LVE Datasheet. Available online: https://www.axis.com/dam/public/d2/ca/50/datasheet-axis-m3116–lve-network-camera-en-US-353775.pdf (accessed on 4 May 2022).
- AASM Scoring Manual—American Academy of Sleep Medicine. Available online: http://www.aasmnet.org/scoringmanual/ (accessed on 4 May 2022).
- Kukwa, W.; Łaba, J.; Lis, T.; Sobczyk, K.; Mitchell, R.B.; Młyńczak, M. Supine sleep patterns as a part of phenotyping patients with sleep apnea—A pilot study. Sleep Breath. 2022. [Google Scholar] [CrossRef]
- Lin’s Concordance Correlation Coefficient. Available online: https://ncss-wpengine.netdna-ssl.com/wp-content/themes/ncss/pdf/Procedures/PASS/Lins_Concordance_Correlation_Coefficient.pdf (accessed on 4 May 2022).
- Giavarina, D. Understanding bland altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Ferrer-Lluis, I.; Castillo-Escario, Y.; Montserrat, J.M.; Jané, R. Analysis of Smartphone Triaxial Accelerometry for Monitoring Sleep-Disordered Breathing and Sleep Position at Home. IEEE Access 2020, 8, 71231–71244. [Google Scholar] [CrossRef]
- Ferrer-Lluis, I.; Castillo-Escario, Y.; Montserrat, J.M.; Jané, R. Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography. Sensors 2021, 21, 3689. [Google Scholar] [CrossRef]
- Bignold, J.J.; Mercer, J.D.; Antic, N.A.; McEvoy, R.D.; Catcheside, P.G. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J. Clin. Sleep Med. 2011, 7, 376–383. [Google Scholar] [CrossRef]
- Ravesloot, M.; Vonk, P.; Maurer, J.; Oksenberg, A.; De Vries, N. Standardized framework to report on the role of sleeping position in sleep apnea patients. Sleep Breath. 2021, 25, 1717–1728. [Google Scholar] [CrossRef]
- Standards of Practice Committee of the American Sleep Disorders Association. Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Sleep 1994, 17, 372–377. [Google Scholar]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Hudgel, D.; Sateia, M.; Schwab, R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012; Volume 176. [Google Scholar]
- Ferber, R.; Millman, R.; Coppola, M.; Fleetham, J.; Murray, C.F.; Iber, C.; McCall, W.V.; Nino-Murcia, G.; Pressman, M.; Sanders, M.; et al. Portable recording in the assessment of obstructive sleep apnea. Sleep 1994, 17, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Littner, M.R. Portable monitoring in the diagnosis of the obstructive sleep apnea syndrome. Semin. Respir. Crit. Care Med. 2005, 26, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, A.; Arons, E.; Radwan, H.; Silverberg, D.S. Positional vs. nonpositional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic and multiple sleep latency test data. Chest 1997, 112, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksenberg, A.; Khamaysi, I.; Silverberg, D.S.; Tarasiuk, A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest 2000, 118, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Oksenberg, A.; Arons, E.; Greenberg-Dotan, S.; Nasser, K.; Radwan, H. The significance of body posture on breathing abnormalities during sleep: Data analysis of 2077 obstructive sleep apnea patients. Harefuah 2009, 148, 304–309. [Google Scholar]
- Richard, W.; Kox, D.; den Herder, C.; Laman, M.; van Tinteren, H.; de Vries, N. The role of sleep position in obstructive sleep apnea syndrome. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2006, 263, 946–950. [Google Scholar] [CrossRef]
- Norman, M.B.; Middleton, S.; Erskine, O.; Middleton, P.G.; Wheatley, J.R.; Sullivan, C.E. Validation of the Sonomat: A contactless monitoring system used for the diagnosis of sleep disordered breathing. Sleep 2014, 37, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.B.; Pithers, S.M.; Teng, A.Y.; Waters, K.A.; Sullivan, C.E. Validation of the Sonomat Against PSG and Quantitative Measurement of Partial Upper Airway Obstruction in Children with Sleep-Disordered Breathing. Sleep 2017, 40, zsx017. [Google Scholar] [CrossRef]
- Liu, M.; Qin, L.; Ye, S. A Mattress System of Recognizing Sleep Postures Based on BCG Signal. Zhongguo Liao Xie Zhi Chin. J. Med. Instrum. 2019, 43, 243–247. [Google Scholar]
- Zhou, Y.; Shu, D.; Xu, H.; Qiu, Y.; Zhou, P.; Ruan, W.; Qin, G.; Jin, J.; Zhu, H.; Ying, K.; et al. Validation of novel automatic ultra-wideband radar for sleep apnea detection. J. Thorac. Dis. 2020, 12, 1286. [Google Scholar] [CrossRef]
- Kang, S.; Kim, D.K.; Lee, Y.; Lim, Y.H.; Park, H.K.; Cho, S.H.; Cho, S.H. Non-contact diagnosis of obstructive sleep apnea using impulse-radio ultra-wideband radar. Sci. Rep. 2020, 10, 5261. [Google Scholar] [CrossRef]
- Zhao, R.; Xue, J.; Dong, X.S.; Zhi, H.; Chen, J.; Zhao, L.; Zhang, X.; Li, J.; Penzel, T.; Han, F. Screening for obstructive sleep apnea using a contact-free system compared with polysomnography. J. Clin. Sleep Med. 2021, 17, 1075–1082. [Google Scholar] [CrossRef]
- Weinreich, G.; Terjung, S.; Wang, Y.; Werther, S.; Zaffaroni, A.; Teschler, H. Validation of a non-contact screening device for the combination of sleep-disordered breathing and periodic limb movements in sleep. Sleep Breath. 2018, 22, 131–138. [Google Scholar] [CrossRef]
- Jeng, P.Y.; Wang, L.C.; Hu, C.J.; Wu, D. A wrist sensor sleep posture monitoring system: An automatic labeling approach. Sensors 2021, 21, 258. [Google Scholar] [CrossRef]

| Characteristic | Males | Females | Total Patients |
|---|---|---|---|
| N | 68 | 21 | 89 |
| Age (years) | |||
| BMI (kg/m2) | |||
| TST (min) | |||
| Sleep efficiency * (%) | |||
| AHI (events/h) | |||
| AHI supine (events/h) | |||
| AHI non-supine (events/h) | |||
| Supine position in TST (min) | |||
| Supine position in TST (%) | |||
| Non-supine position in TST (min) | |||
| Non-supine position in TST (%) |
| Characteristic | Males | Females | Total Patients |
|---|---|---|---|
| OSA patients *; n (% from specific group) | 67 (98.5) | 18 (85.7) | 85 (95.5) |
| Supine OSA patients **; n (%) | 34 (38.2) | 13 (14.6) | 47 (52.8) |
| Supine-isolated OSA patients ***; n (%) | 5 (5.6) | 6 (6.7) | 11 (12.4) |
| Supine-predominant OSA patients ****; n (%) | 29 (32.6) | 7 (7.9) | 36 (40.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukwa, W.; Lis, T.; Łaba, J.; Mitchell, R.B.; Młyńczak, M. Sleep Position Detection with a Wireless Audio-Motion Sensor—A Validation Study. Diagnostics 2022, 12, 1195. https://doi.org/10.3390/diagnostics12051195
Kukwa W, Lis T, Łaba J, Mitchell RB, Młyńczak M. Sleep Position Detection with a Wireless Audio-Motion Sensor—A Validation Study. Diagnostics. 2022; 12(5):1195. https://doi.org/10.3390/diagnostics12051195
Chicago/Turabian StyleKukwa, Wojciech, Tomasz Lis, Jonasz Łaba, Ron B. Mitchell, and Marcel Młyńczak. 2022. "Sleep Position Detection with a Wireless Audio-Motion Sensor—A Validation Study" Diagnostics 12, no. 5: 1195. https://doi.org/10.3390/diagnostics12051195
APA StyleKukwa, W., Lis, T., Łaba, J., Mitchell, R. B., & Młyńczak, M. (2022). Sleep Position Detection with a Wireless Audio-Motion Sensor—A Validation Study. Diagnostics, 12(5), 1195. https://doi.org/10.3390/diagnostics12051195







