Radioiodine in Differentiated Thyroid Carcinoma: Do We Need Diagnostic Pre-Ablation Iodine-123 Scintigraphy to Optimize Treatment?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Patient Management
2.3. TxWBS
2.4. TxWBS Qualitative Assessment
2.5. TxWBS Semi-Quantitative Assessment
2.6. Statistical Analysis
3. Results
3.1. Findings on TxWBS
3.2. Thyroid Remnant Size
3.3. Treatment Success
3.4. Lymph Node Metastasis
3.5. Consequences for Patient Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sciuto, R.; Romano, L.; Rea, S.; Marandino, F.; Sperduti, I.; Maini, C.L. Natural history and clinical outcome of differentiated thyroid carcinoma: A retrospective analysis of 1503 patients treated at a single institution. Ann. Oncol. 2009, 20, 1728–1735. [Google Scholar] [CrossRef]
- Verburg, F.A.; de Keizer, B.; Lips, C.J.; Zelissen, P.M.; de Klerk, J.M. Prognostic significance of successful ablation with radi-oiodine of differentiated thyroid cancer patients. Eur. J. Endocrinol. 2005, 152, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Puntoni, M.; Bottoni, G.; Treglia, G.; Foppiani, L.; Bertoli, M.; Catrambone, U.; Arlandini, A.; Dib, B.; Altrinetti, V.; et al. Differentiated Thyroid Cancer lymph-node relapse. Role of adjuvant radioactive iodine therapy after lymphadenectomy. Eur. J. Nucl. Med. Mol. Imaging 2016, 44, 926–934. [Google Scholar] [CrossRef]
- Clerc, J.; Verburg, F.A.; Avram, A.M.; Giovanella, L.; Hindié, E.; Taïeb, D. Radioiodine treatment after surgery for differentiated thyroid cancer: A reasonable option. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Oltmann, S.C.; Schneider, D.F.; Leverson, G.; Sivashanmugam, T.; Chen, H.; Sippel, R.S. Radioactive iodine remnant uptake after completion thyroidectomy: Not such a complete cancer operation. Ann. Surg. Oncol. 2013, 21, 1379–1383. [Google Scholar] [CrossRef] [Green Version]
- Frangos, S.; Iakovou, I.P.; Marlowe, R.J.; Eftychiou, N.; Patsali, L.; Vanezi, A.; Savva, A.; Mpalaris, V.; Giannoula, E.I. Difficulties in deciding whether to ablate patients with putatively “low–intermediate-risk” differentiated thyroid carcinoma: Do guidelines mainly apply in the centres that produce them? Results of a retrospective, two-centre quality assurance study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2045–2055. [Google Scholar] [CrossRef]
- Wu, D.; Ylli, D.; Heimlich, M.S.L.; Burman, K.D.; Wartofsky, L.; Van Nostrand, D. 124 I positron emission tomography/computed tomography versus conventional radioiodine imaging in differentiated thyroid cancer: A review. Thyroid 2019, 29, 1523–1535. [Google Scholar] [CrossRef]
- Chen, M.-K.; Yasrebi, M.; Samii, J.; Staib, L.H.; Doddamane, I.; Cheng, D.W. The Utility of I-123 pretherapy scan in I-131 Radioiodine Therapy for thyroid cancer. Thyroid 2012, 22, 304–309. [Google Scholar] [CrossRef]
- Avram, A.M.; Esfandiari, N.H.; Wong, K.K. Preablation 131-I Scans With SPECT/CT Contribute to thyroid cancer risk stratification and 131-I therapy planning. J. Clin. Endocrinol. Metab. 2015, 100, 1895–1902. [Google Scholar] [CrossRef]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I Scans With SPECT/CT in postoperative thyroid cancer patients: What Is the impact on staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar] [CrossRef] [Green Version]
- Yap, B.K.; Murby, B. No adverse affect in clinical outcome using low preablation diagnostic 131-I Activity in differentiated thyroid cancer: Refuting thyroid-stunning effect. J. Clin. Endocrinol. Metab. 2014, 99, 2433–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, L.F.; Waxman, A.D.; Braunstein, G.D. Thyroid Stunning. Thyroid 2003, 13, 333–340. [Google Scholar] [CrossRef]
- Lamartina, L.; Borget, I.; Mirghani, H.; Al Ghuzlan, A.; Berdelou, A.; Bidault, F.; Deandreis, D.; Baudin, E.; Travagli, J.-P.; Schlumberger, M.; et al. Surgery for neck recurrence of differentiated thyroid cancer: Outcomes and risk factors. J. Clin. Endocrinol. Metab. 2017, 102, 1020–1031. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid Nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.; Wiersinga, W. European conensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Evidence Based Nation-Wide Guideline Thyroid Carcinoma Version 2.0. Available online: https://richtlijnendatabase.nl/richtlijn/schildkliercarcinoom/algemeen.html (accessed on 1 March 2021).
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with Low-dose radioiodine and thyrotropin alfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Castagna, M.G.; Cevenini, G.; Theodoropoulou, A.; Maino, F.; Memmo, S.; Claudia, C.; Belardini, V.; Brianzoni, E.; Pacini, F. Post-surgical thyroid ablation with low or high radioiodine activities results in similar outcomes in intermediate risk differentiated thyroid cancer patients. Eur. J. Endocrinol. 2013, 169, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosário, P.W.; Calsolari, M.R. Thyroid Ablation with 1.1 GBq (30 mCi) Iodine-131 in Patients with papillary thyroid carcinoma at intermediate risk for recurrence. Thyroid 2014, 24, 826–831. [Google Scholar] [CrossRef]
- Schmidt, M.; Görges, R.; Drzezga, A.; Dietlein, M. A Matter of controversy: Is radioiodine therapy favorable in differentiated thyroid carcinoma? J. Nucl. Med. 2018, 59, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Van Nostrand, D.; Aiken, M.; Atkins, F.; Moreau, S.; Garcia, C.; Acio, E.; Burman, K.; Wartofsky, L. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid 2009, 19, 849–855. [Google Scholar] [CrossRef]
- Schmidt, D.; Szikszai, A.; Linke, R.; Bautz, W.; Kuwert, T. Impact of 131 I SPECT/Spiral CT on Nodal staging of differentiated thyroid carcinoma at the first radioablation. J. Nucl. Med. 2008, 50, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Bravo, P.E.; Goudarzi, B.; Rana, U.; Filho, P.T.; Castillo, R.; Rababy, C.; Ewertz, M.; Ziessman, H.A.; Cooper, D.S.; Ladenson, P.W.; et al. Clinical significance of discordant findings between pre-therapy 123 I and post-therapy 131 I whole body scan in patients with thyroid cancer. Int. J. Clin. Exp. Med. 2013, 6, 320–333. [Google Scholar] [PubMed]
- De Geus-Oei, L.-F.; Oei, H.-Y.; Hennemann, G.; Krenning, E.P. Sensitivity of 123 I whole-body scan and thyroglobulin in the detection of metastases or recurrent differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 768–774. [Google Scholar] [CrossRef]
- Song, H.; Mosci, C.; Akatsu, H.; Basina, M.; Dosiou, C.; Iagaru, A. Diagnostic 123 I whole body scan prior to ablation of thyroid remnant in patients with papillary thyroid cancer. Clin. Nucl. Med. 2018, 43, 705–709. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017.
- Abdulrezzak, U.; Tutus, A.; Isik, I.; Kurt, Y.; Kula, M. The quantitative comparison of low dose and standard dose radio iodine therapy effectiveness in patients with low risk differentiated thyroid cancer. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 314–322. [Google Scholar]
- Spencer, C.A. Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J. Clin. Endocrinol. Metab. 2011, 96, 3615–3627. [Google Scholar] [CrossRef] [Green Version]
- Ruf, J.; Lehmkuhl, L.; Bertram, H.; Sandrock, D.; Amthauer, H.; Humplik, B.; Munz, D.L.; Felix, R. Impact of SPECT and integrated low-dose CT after radioiodine therapy on the management of patients with thyroid carcinoma. Nucl. Med. Commun. 2004, 25, 1177–1182. [Google Scholar] [CrossRef]
- Tharp, K.; Israel, O.; Hausmann, J.; Bettman, L.; Martin, W.H.; Daitzchman, M.; Sandler, M.P.; Delbeke, D. Impact of 131 I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Zago, S.; Leboulleux, S.; Mirghani, H.; Deandreis, D.; Baudin, E.; Schlumberger, M. Resection margins and prognosis in locally invasive thyroid cancer. Head Neck 2013, 36, 1034–1038. [Google Scholar] [CrossRef]
- Zeuren, R.; Biagini, A.; Grewal, R.K.; Randolph, G.W.; Kamani, D.; Sabra, M.M.; Shaha, A.R.; Tuttle, R.M. RAI thyroid bed uptake after total thyroidectomy: A novel SPECT-CT anatomic classification system. Laryngoscope 2015, 125, 2417–2424. [Google Scholar] [CrossRef] [Green Version]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Mustafa, M.; Bartenstein, P.; Kuwert, T.; Schmidt, D.; Ilhan, H. Rate of elimination of radioiodineavid lymph node metastases of differentiated thyroid carcinoma by postsurgical radioiodine ablation. Nuklearmedizin 2016, 55, 221–227. [Google Scholar] [CrossRef]
- Baek, S.-K.; Jung, K.-Y.; Kang, S.-M.; Kwon, S.-Y.; Woo, J.-S.; Cho, S.-H.; Chung, E.-J. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2010, 20, 147–152. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Gerard, S.K.; Cavalieri, R.R. I-123 Diagnostic thyroid tumor whole-body scanning with imaging at 6, 24, and 48 hours. Clin. Nucl. Med. 2002, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S.; Fish, S.; Nakhoda, K.; Zhuang, H.; Alavi, A.; Mandel, S.J. Comparison of I-123 and I-131 for whole-body imaging after stimulation by recombinant human thyrotropin. Clin. Nucl. Med. 2003, 28, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Bakheet, S.; Al Mandil, M.; Al-Hajjaj, A.; Almahfouz, A.; Al Haj, A. 123 I Isotope as a diagnostic agent in the follow-up of patients with differentiated thyroid cancer: Comparison with Post 131 I therapy whole body scanning. J. Clin. Endocrinol. Metab. 2001, 86, 5294–5300. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.; Jentzen, W.; Sraieb, M.; Costa, P.F.; Conti, M.; Umutlu, L.; Antoch, G.; Nader, M.; Herrmann, K.; Fendler, W.P.; et al. Comparing lesion detection efficacy and image quality across different PET system generations to optimize the iodine-124 PET protocol for recurrent thyroid cancer. EJNMMI Phys. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Kist, J.W.; De Keizer, B.; Van Der Vlies, M.; Brouwers, A.H.; Huysmans, D.A.; Van Der Zant, F.M.; Hermsen, R.; Stokkel, M.P.; Hoekstra, O.S.; Vogel, W.V.; et al. 124 I PET/CT to Predict the Outcome of Blind 131 I treatment in patients with biochemical recurrence of differentiated thyroid cancer: Results of a multicenter diagnostic cohort study (THYROPET). J. Nucl. Med. 2015, 57, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Walrand, S.; Hesse, M.; Jamar, F. Statistical and radiobiological analysis of the so-called thyroid stunning. EJNMMI Res. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hilditch, T.E.; Dempsey, M.F.; Bolster, A.A.; McMenemin, R.M.; Reed, N.S. Self-stunning in thyroid ablation: Evidence from comparative studies of diagnostic 131 I and 123 I. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 783–788. [Google Scholar] [CrossRef]
- Verburg, F.A.; Mäder, U.; Reiners, C.; Hänscheid, H. Long-term survival in differentiated thyroid cancer is worse after low-activity initial post-surgical 131 I therapy in both high- and low-risk patients. J. Clin. Endocrinol. Metab. 2014, 99, 4487–4496. [Google Scholar] [CrossRef] [Green Version]
- Pryma, D.A. Controversies on the use of radioiodine in thyroid cancer: We need more and better data. J. Nucl. Med. 2018, 59, 1184–1186. [Google Scholar] [CrossRef]
- Tuttle, R.M. Controversial Issues in Thyroid Cancer Management. J. Nucl. Med. 2018, 59, 1187–1194. [Google Scholar] [CrossRef]
- Vardarli, I.; Weidemann, F.; Aboukoura, M.; Herrmann, K.; Binse, I.; Görges, R. Longer-term recurrence rate after low versus high dose radioiodine ablation for differentiated thyroid Cancer in low and intermediate risk patients: A meta-analysis. BMC Cancer 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, consensus, and collaboration in the use of 131 I therapy in differentiated thyroid cancer: A joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar] [CrossRef] [Green Version]
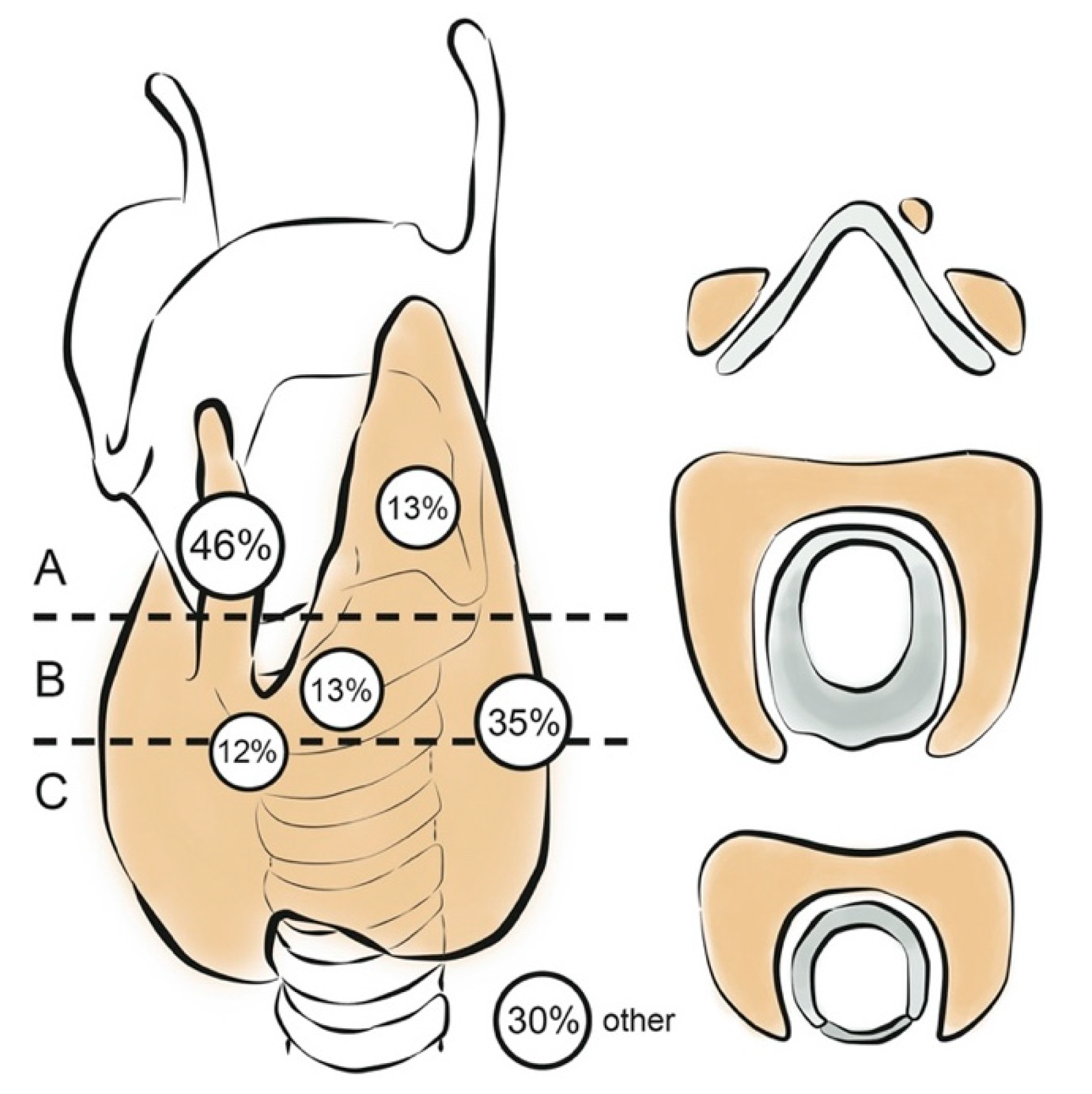
| n (%) | |||
|---|---|---|---|
| Total number of patients | 97 (100%) | ||
| Age (years) (median, range) | 45 (14–88) | ||
| Female | 68 (70%) | ||
| Body mass index (kg/m2, IQR) | 25.4 (22.3–30.6) | ||
| Ultrasound suspicious lymph nodes upon presentation | 42 (43%) | ||
| Cytology confirmed lymph node metastases upon presentation | 25 (25%) | ||
| Surgical intent | (suspicion of) malignancy | 81 (84%) | |
| benign | goiter | 10 (10%) | |
| parathyroidectomy | 2 (2%) | ||
| other | 4 (4%) | ||
| Initial total thyroidectomy | 68 (70%) | ||
| Two-stage surgery | 29 (30%) | ||
| Lymph node dissection | formal neck dissection of ≥1 level | 39 (40%) | |
| other | 6 (6%) | ||
| Hospital of surgery | all surgeries at community hospital | 6 (6%) | |
| all surgeries at tertiary care center | 83 (86%) | ||
| HT community, TT tertiary care | 8 (8%) | ||
| Histopathology | Tumor size (mm, IQR) | 23.0 (13.5–36.5) | |
| PTC | classic type | 52 (54%) | |
| tall cell variant | 2 (2%) | ||
| diffuse sclerosing variant | 7 (7%) | ||
| oncocytic variant | 1 (1%) | ||
| cribriform variant | 1 (1%) | ||
| FVPTC | 15 (15%) | ||
| FTC | 13 (13%) | ||
| Hürthle cell carcinoma | 3 (3%) | ||
| poorly differentiated carcinoma | 3 (3%) | ||
| lymph node metastasis | 43 (44%) | ||
| ATA risk | low | 18 (19%) | |
| intermediate | 38 (39%) | ||
| high | 41 (42%) | ||
| I-131 preparation | thyroid hormone withdrawal | 91 (94%) | |
| rhTSH | 6 (6%) | ||
| TRB Ratio | |||
|---|---|---|---|
| N | Median (IQR) | p-Value | |
| Overall thyroid remnant size | 87 | 11.6 (7.07–28.7) | |
| Clinical factors | |||
| Age (years) | 87 | R2 = 0.142 a | 0.190 a |
| Female sex | 58 | 10.85 (5.67–27.3) | 0.331 b |
| Body mass index (kg/m2) | 87 | R2 = 0.012 a | 0.914 a |
| History of neck surgery | 7 | 6.20 (1.86–57.56) | 0.318 b |
| Peri-operative factors | |||
| Surgery with oncological intent | 73 | 10.78 (5.13–24.44) | 0.009 b |
| Two-stage thyroidectomy | 25 | 16.05 (9.52–76.70) | 0.013 b |
| Lymph node dissection | |||
| no | 51 | 16.05 (9.02–34.70) | 0.003 c |
| central compartment (level 6/7) | 7 | 1.86 (0.46–8.13) | |
| central + lateral compartment (level 2–7) | 29 | 9.55 (4.76–22.48) | |
| Leading surgeon | |||
| surgeon A | 44 | 9.60 (4.67–21.22) | 0.136 c |
| surgeon B | 25 | 16.22 (9.88–47.37) | |
| surgeon C | 7 | 19.37 (6.20–144.17) | |
| other | 11 | 12.36 (7.07–18.75) | |
| Duo of dedicated thyroid surgeons | 33 | 9.65 (4.51–23.53) | 0.046 b |
| ≥1 surgery at referring hospital | 12 | 10.29 (8.51–54.46) | 0.410 b |
| Difficult surgical procedure * | 36 | 13.14 (7.80–27.92) | 0.263 b |
| Recurrent laryngeal nerve injury | 9 | 10.92 (8.52–50.72) | 0.540 b |
| Factors related to I-131 | |||
| Dose I-131 | |||
| 1.1 GBq | 4 | 121.47 (36.73–366.66) | 0.061 c |
| 3.7 GBq | 31 | 12.36 (7.28–29.82) | |
| 5.55 GBq | 46 | 9.89 (5.31–27.36) | |
| 7.4 GBq | 6 | 14.64 (3.59–19.42) | |
| Preparation with rhTSH | 6 | 17.87 (10.12–126.84) | 0.150 b |
| Successful Treatment (n = 41) | Unsuccessful Treatment (n = 56) | p-Value | |
|---|---|---|---|
| Clinical factors | |||
| Age (years, median (IQR)) | 43.8 (38.8–52.9) | 49.0 (35.1–65.6) | 0.179 d |
| Female sex | 27 (65.9%) | 41 (73.2%) | 0.503 e |
| Body mass index (kg/m2) | 25.4 (22.9–28.9) | 25.5 (22.1–31.4) | 0.826 d |
| History of neck surgery | 3 (7.3%) | 4 (7.1%) | 1.000 f |
| Peri-operative factors | |||
| Surgery with oncological intent | 32 (78.0%) | 49 (87.5%) | 0.215 e |
| Two-stage surgery | 15 (36.6%) | 14 (25.0%) | 0.264 e |
| Lymph node dissection | |||
| no | 31 (75.6%) | 27 (48.2%) | 0.022 e |
| central compartment (level 6/7) | 2 (4.9%) | 5 (8.9%) | |
| central + lateral compartment (level 2–7) | 8 (19.5%) | 24 (42.9%) | |
| Leading surgeon | |||
| surgeon A | 19 (46.3%) | 27 (48.2%) | 0.594 e |
| surgeon B | 12 (29.3%) | 16 (28.6%) | |
| surgeon C | 6 (14.6%) | 4 (7.1%) | |
| other | 4 (9.8%) | 9 (16.1%) | |
| Duo of dedicated thyroid surgeons | 9 (34.6%) | 16 (38.1%) | 0.802 e |
| ≥1 surgery at referring hospital | 2 (4.9%) | 12 (21.4%) | 0.038 e |
| Difficult surgical procedure * | 10 (24.4%) | 28% (50.0%) | 0.012 e |
| Histopathological factors | |||
| Histopathology | |||
| PTC | 25 (61.0%) | 38 (67.9%) | 0.458 e |
| FVPTC | 7 (17.1%) | 8 (14.3%) | |
| FTC | 7 (17.1%) | 6 (10.7%) | |
| Hürthle cell carcinoma | 2 (4.9%) | 1 (1.8%) | |
| poorly differentiated carcinoma | 0 (0.0%) | 3 (5.4%) | |
| Tumour characteristics | |||
| tumour size (mm, median (IQR)) | 25.0 (14.5–30.0) | 22.0 (13.0–41.0) | 0.544 b |
| aggressive tumour type ** | 3 (7.3%) | 13 (23.2%) | 0.052 f |
| tumour multifocality | 16 (39.0%) | 23 (41.1%) | 1.000 e |
| capsular invasion | |||
| Minimally invasive | 5 (12.2%) | 4 (7.1%) | 0.415 f |
| Invasive | 8 (19.5%) | 7 (12.5%) | |
| vascular invasion | 13 (31.7%) | 19 (33.9%) | 0.831 e |
| extrathyroidal extension | 12 (29.3%) | 26 (46.4%) | 0.097 e |
| irradical resection (R1) *** | 10 (24.4%) | 27 (48.2%) | 0.021 e |
| ATA risk classification | |||
| low risk | 11 (26.8%) | 7 (12.5%) | 0.157 e |
| intermediate risk | 16 (39.0%) | 22 (39.3%) | |
| high risk | 14 (34.1%) | 27 (48.2%) | |
| pTNM classification | |||
| pT1a | 5 (12.2%) | 4 (7.1%) | 0.079 e |
| pT1b | 7 (17.1%) | 11 (19.6%) | |
| pT2 | 14 (34.1%) | 9 (16.1%) | |
| pT3 | 15 (36.6%) | 27 (48.2%) | |
| pT4a | 0 (0.0%) | 5 (8.9%) | |
| pT4b | n.a. | n.a. | |
| Lymph node metastases | |||
| Nx | 10 (24.4%) | 5 (8.9%) | 0.030 e |
| N0 | 19 (46.3%) | 19 (33.9%) | |
| N1a | 4 (9.8%) | 8 (14.3%) | |
| N1b | 8 (19.5%) | 24 (42.9%) | |
| Factors related to I-131 therapy | |||
| Administered Dose I-131 | |||
| 1.1 GBq | 3 (7.3%) | 1 (1.8%) | 0.005 e |
| 3.7 GBq | 21 (51.2%) | 15 (26.8%) | |
| 5.55 GBq | 17 (41.5%) | 34 (60.7%) | |
| 7.4 GBq | 0 (0%) | 6 (10.7%) | |
| Thyroid remnant size (median TRB ratio, IQR) (n = 87) | 15.92 (8.48–44.37) | 9.89 (5.31–22.10) | 0.039 b |
| Clinically relevant findings on TxWBS | |||
| large remnant (TRB ratio ≥24.4) | 16 (40.5%) | 11 (20.0%) | 0.042 e |
| lymph node metastasis (n = 26) | 7 (17.1%) | 19 (33.9%) | 0.103 f |
| lymph node metastasis >10mm (n = 4) | 0 (0%) | 4 (7.1%) | 0.135 f |
| distant metastasis (n = 6) | 0 (0%) | 6 (10.7%) | 0.037 f |
| sTg at time of I-131 administration | |||
| <0.5 ng/mL | 15 (36.6%) | 11 (20.4%) | 0.033 e |
| 0.5–1.0 ng/mL | 5 (12.2%) | 2 (3.7%) | |
| >1.0 ng/mL | 21 (51.2%) | 41 (75.9%) | |
| Significance | Odds Ratio (95% CI) | |
|---|---|---|
| Thyroid surgery at tertiary care center | 0.022 | 7.094 (1.321–38.108) |
| Aggressive histopathological tumor type | 0.036 | 0.168 (0.032–0.892) |
| TNM N1b stage | 0.013 | 0.261 (0.091–0.751) |
| New lymph node metastasis on TxWBS | 0.046 | 0.327 (0.109–0.981) |
| Constant | 0.188 | 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Koster, E.J.; Sulaiman, T.; Hamming, J.F.; Schepers, A.; Snel, M.; van Velden, F.H.P.; de Geus-Oei, L.-F.; Vriens, D. Radioiodine in Differentiated Thyroid Carcinoma: Do We Need Diagnostic Pre-Ablation Iodine-123 Scintigraphy to Optimize Treatment? Diagnostics 2021, 11, 553. https://doi.org/10.3390/diagnostics11030553
de Koster EJ, Sulaiman T, Hamming JF, Schepers A, Snel M, van Velden FHP, de Geus-Oei L-F, Vriens D. Radioiodine in Differentiated Thyroid Carcinoma: Do We Need Diagnostic Pre-Ablation Iodine-123 Scintigraphy to Optimize Treatment? Diagnostics. 2021; 11(3):553. https://doi.org/10.3390/diagnostics11030553
Chicago/Turabian Stylede Koster, Elizabeth J., Taban Sulaiman, Jaap F. Hamming, Abbey Schepers, Marieke Snel, Floris H. P. van Velden, Lioe-Fee de Geus-Oei, and Dennis Vriens. 2021. "Radioiodine in Differentiated Thyroid Carcinoma: Do We Need Diagnostic Pre-Ablation Iodine-123 Scintigraphy to Optimize Treatment?" Diagnostics 11, no. 3: 553. https://doi.org/10.3390/diagnostics11030553









