The Pathology of Type 2 Inflammation-Associated Itch in Atopic Dermatitis
Abstract
:1. Introduction
2. Disease Burden of AD
3. Type 2 Inflammation and AD
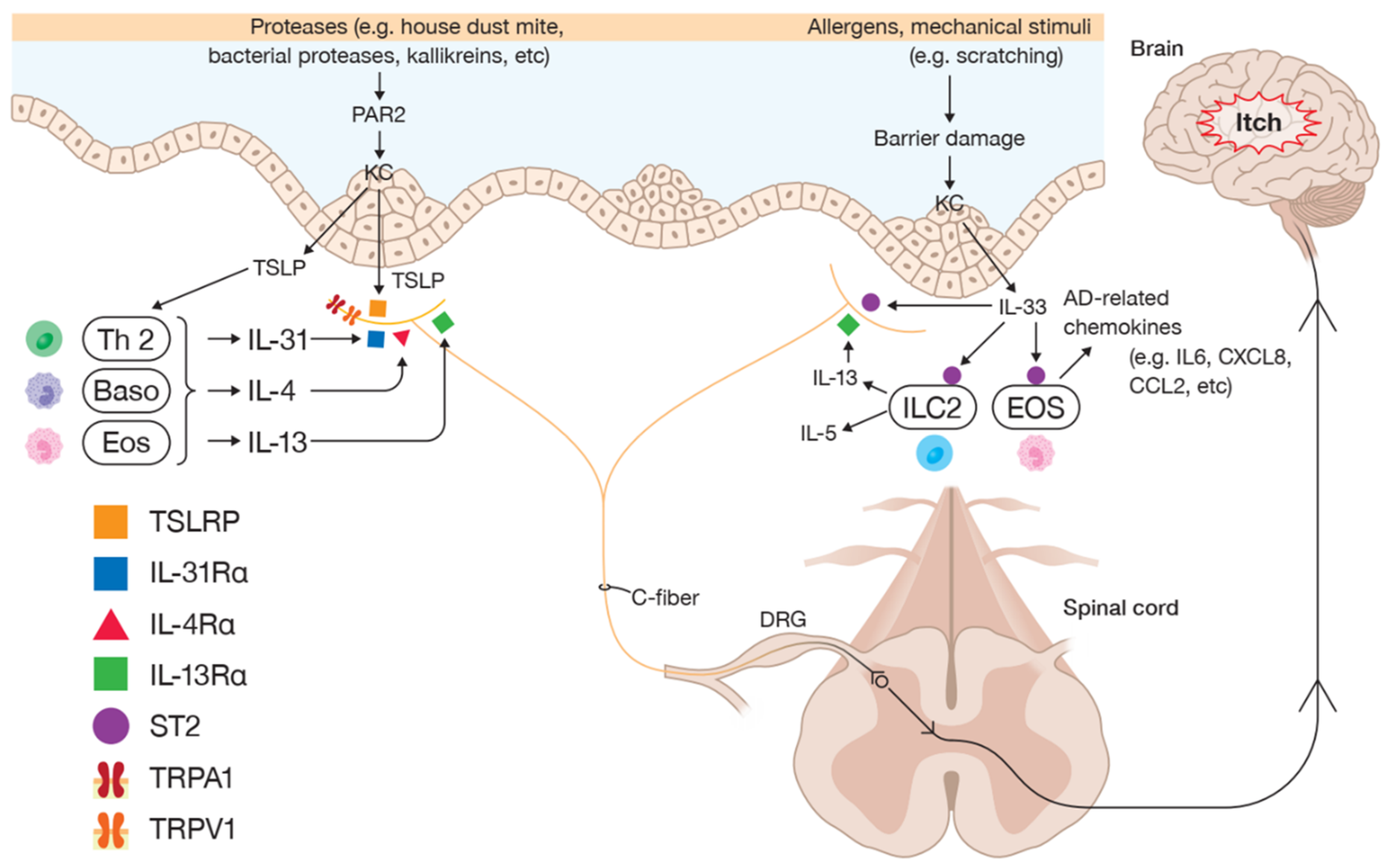
4. Neuroimmune Interactions Associated with Type 2 Inflammation in Pruritic AD
4.1. IL-4 and IL-13
4.2. IL-31
4.3. IL-33
4.4. TSLP
5. Treatment for Itch in AD
5.1. Anti-IL-4 Receptor Antibody
5.2. Anti-IL-13
5.3. Anti-IL-31 Signaling
5.3.1. Anti-IL-31
5.3.2. Anti-IL-31RA
5.4. JAK Inhibitors
5.5. A Phosphodiesterase 4 (PDE4) Inhibitor
5.6. Anti-TSLP
5.7. Anti-IL-33
6. Conclusions
Funding
Conflicts of Interest
References
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Guttman-Yassky, E. Assessing the current treatment of atopic dermatitis: Unmet needs. J. Allergy Clin. Immunol. 2017, 139, S47–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Wong, L.S.; Wu, T.; Lee, C.H. Inflammatory and Noninflammatory Itch: Implications in Pathophysiology-Directed Treatments. Int. J. Mol. Sci. 2017, 18, 1485. [Google Scholar] [CrossRef] [Green Version]
- Hawro, T.; Przybylowicz, K.; Spindler, M.; Hawro, M.; Stec, M.; Altrichter, S.; Weller, K.; Magerl, M.; Reidel, U.; Alarbeed, E.; et al. The characteristics and impact of pruritus in adult dermatology patients: A prospective, cross-sectional study. J. Am. Acad. Dermatol. 2021, 84, 691–700. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schappi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef] [Green Version]
- Drucker, A.M.; Wang, A.R.; Li, W.Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, J.A.; Lien, L.; Dalgard, F.; Bjertness, E.; Stern, R.S. Suicidal ideation, mental health problems, and social function in adolescents with eczema: A population-based study. J. Investig. Dermatol. 2014, 134, 1847–1854. [Google Scholar] [CrossRef] [Green Version]
- Xerfan, E.M.S.; Tomimori, J.; Andersen, M.L.; Tufik, S.; Facina, A.S. Sleep disturbance and atopic dermatitis: A bidirectional relationship? Med. Hypotheses 2020, 140, 109637. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chiang, B.L. Mechanism of Sleep Disturbance in Children with Atopic Dermatitis and the Role of the Circadian Rhythm and Melatonin. Int. J. Mol. Sci. 2016, 17, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariens, L.F.M.; van Nimwegen, K.J.M.; Shams, M.; de Bruin, D.T.; van der Schaft, J.; van Os-Medendorp, H.; De Bruin-Weller, M. Economic Burden of Adult Patients with Moderate to Severe Atopic Dermatitis Indicated for Systemic Treatment. Acta Derm.-Venereol. 2019, 99, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.F.; Rajagopalan, M.; Chu, C.Y.; Encarnacion, L.; Gerber, R.A.; Santos-Estrella, P.; Llamado, L.J.Q.; Tallman, A.M. Burden of atopic dermatitis in Asia. J. Dermatol. 2019, 46, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.M.; Shaw, F.M.; Zhang, C.; Culler, S.D.; Chen, S.C. The Annual Direct and Indirect Health Care Costs for Patients with Chronic Pruritus and their Determining Factors. J. Investig. Dermatol. 2020, 140, 699–701.e5. [Google Scholar] [CrossRef]
- Lee, B.W.; Detzel, P.R. Treatment of childhood atopic dermatitis and economic burden of illness in Asia Pacific countries. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 18–24. [Google Scholar] [CrossRef]
- Olsson, M.; Bajpai, R.; Wee, L.W.Y.; Yew, Y.W.; Koh, M.J.A.; Thng, S.; Car, J.; Jarbrink, K. The cost of childhood atopic dermatitis in a multi-ethnic Asian population: A cost-of-illness study. Br. J. Dermatol. 2020, 182, 1245–1252. [Google Scholar] [CrossRef]
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2020, 69, 187–196. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Tojima, I.; Matsumoto, K.; Kikuoka, H.; Hara, S.; Yamamoto, S.; Shimizu, S.; Kouzaki, H.; Shimizu, T. Evidence for the induction of Th2 inflammation by group 2 innate lymphoid cells in response to prostaglandin D2 and cysteinyl leukotrienes in allergic rhinitis. Allergy 2019, 74, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Morita, H.; Sokolowska, M.; Tan, G.; Boonpiyathad, T.; Opitz, L.; Orimo, K.; Archer, S.K.; Jansen, K.; Tang, M.L.K.; et al. Gene expression signatures of circulating human type 1, 2, and 3 innate lymphoid cells. J. Allergy Clin. Immunol. 2019, 143, 2321–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Cui, X.; Li, W.; Lv, J.; Du, L.; Mi, W.; Li, H.; Chen, Z.; Leng, Q.; Zhou, H.; et al. BLT1 signaling in epithelial cells mediates allergic sensitization via promotion of IL-33 production. Allergy 2019, 74, 495–506. [Google Scholar] [CrossRef]
- Ro, M.; Lee, A.J.; Kim, J.H. 5-/12-Lipoxygenase-linked cascade contributes to the IL-33-induced synthesis of IL-13 in mast cells, thus promoting asthma development. Allergy 2018, 73, 350–360. [Google Scholar] [CrossRef]
- Pasha, M.A.; Patel, G.; Hopp, R.; Yang, Q. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc. 2019, 40, 138–145. [Google Scholar] [CrossRef]
- von Moltke, J.; O’Leary, C.E.; Barrett, N.A.; Kanaoka, Y.; Austen, K.F.; Locksley, R.M. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J. Exp. Med. 2017, 214, 27–37. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra116. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Farinas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; He, H.; Canter, T.; Han, J.; Lefferdink, R.; Erickson, T.; Rangel, S.; Kameyama, N.; Kim, H.J.; Pavel, A.B.; et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J. Allergy Clin. Immunol. 2020, 145, 215–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Farinas, M.; Tintle, S.J.; Shemer, A.; Chiricozzi, A.; Nograles, K.; Cardinale, I.; Duan, S.; Bowcock, A.M.; Krueger, J.G.; Guttman-Yassky, E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy Clin. Immunol. 2011, 127, 954–964.e4. [Google Scholar] [CrossRef] [Green Version]
- Pacor, M.L.; Di Lorenzo, G.; Martinelli, N.; Mansueto, P.; Rini, G.B.; Corrocher, R. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: A randomized study. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2004, 34, 639–645. [Google Scholar] [CrossRef]
- Hijnen, D.; De Bruin-Weller, M.; Oosting, B.; Lebre, C.; De Jong, E.; Bruijnzeel-Koomen, C.; Knol, E. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J. Allergy Clin. Immunol. 2004, 113, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Jahnz-Rozyk, K.; Targowski, T.; Paluchowska, E.; Owczarek, W.; Kucharczyk, A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy 2005, 60, 685–688. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, C.; Zhang, L. Recent developments and highlights in allergic rhinitis. Allergy 2019, 74, 2320–2328. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Altunbulakli, C.; Morita, H.; Sugita, A.; Kubo, T.; Kimura, R.; Goto, H.; Yamamoto, O.; Ruckert, B.; Akdis, M.; et al. Human type 2 innate lymphoid cells disrupt skin keratinocyte tight junction barrier by IL-13. Allergy 2019, 74, 2534–2537. [Google Scholar] [CrossRef]
- Sugita, K.; Steer, C.A.; Martinez-Gonzalez, I.; Altunbulakli, C.; Morita, H.; Castro-Giner, F.; Kubo, T.; Wawrzyniak, P.; Ruckert, B.; Sudo, K.; et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J. Allergy Clin. Immunol. 2018, 141, 300–310.e11. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.R.; Flavell, R.A. Transgenic mice which overproduce Th2 cytokines develop spontaneous atopic dermatitis and asthma. Int. Immunol. 2004, 16, 1155–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyle, M.; Cevikbas, F.; Harden, J.L.; Guttman-Yassky, E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp. Dermatol. 2019, 28, 756–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; von Andrian, U.H. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015, 36, 578–604. [Google Scholar] [CrossRef] [Green Version]
- Veiga-Fernandes, H.; Mucida, D. Neuro-Immune Interactions at Barrier Surfaces. Cell 2016, 165, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, S.; Abdulnour, R.E.; Burkett, P.R.; Lee, S.; Cronin, S.J.; Pascal, M.A.; Laedermann, C.; Foster, S.L.; Tran, J.V.; Lai, N.; et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 2015, 87, 341–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015, 43, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, D.M.; Wilson, S.R.; Hoon, M.A. Why we scratch an itch: The molecules, cells and circuits of itch. Nat. Neurosci. 2014, 17, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.R.; Kim, B.S. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.B.; Kim, B.S. Pruritus in allergy and immunology. J. Allergy Clin. Immunol. 2019, 144, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e213. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.A.; Thaci, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef]
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic Dermatitis Is an IL-13-Dominant Disease with Greater Molecular Heterogeneity Compared to Psoriasis. J. Investig. Dermatol. 2019, 139, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Gonzalez, J.; Shemer, A.; Malajian, D.; Xu, H.; Zheng, X.; Khattri, S.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suarez-Farinas, M.; et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 2015, 136, 104–115.e7. [Google Scholar] [CrossRef]
- Campion, M.; Smith, L.; Gatault, S.; Metais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Kato, A.; Fujii, E.; Watanabe, T.; Takashima, Y.; Matsushita, H.; Furuhashi, T.; Morita, A. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J. Dermatol. Sci. 2014, 74, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lerner, E.A. Is the Nervous System More Important Than the Immune System in Itch and Atopic Dermatitis? J. Investig. Dermatol. Symp. Proc. 2018, 19, S94. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef]
- Cevikbas, F.; Steinhoff, A.; Homey, B.; Steinhoff, M. Neuroimmune interactions in allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 365–373. [Google Scholar] [CrossRef]
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Voisin, T.; Bouvier, A.; Chiu, I.M. Neuro-immune interactions in allergic diseases: Novel targets for therapeutics. Int. Immunol. 2017, 29, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Cherry, W.B.; Yoon, J.; Bartemes, K.R.; Iijima, K.; Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008, 121, 1484–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.D.; Salter, B.M.; Oliveria, J.P.; El-Gammal, A.; Tworek, D.; Smith, S.G.; Sehmi, R.; Gauvreau, G.M.; PM, O.A.B. IL-33 and Its Receptor ST2 after Inhaled Allergen Challenge in Allergic Asthmatics. Int. Arch. Allergy Immunol. 2018, 176, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallrapp, A.; Burkett, P.R.; Riesenfeld, S.J.; Kim, S.J.; Christian, E.; Abdulnour, R.E.; Thakore, P.I.; Schnell, A.; Lambden, C.; Herbst, R.H.; et al. Calcitonin Gene-Related Peptide Negatively Regulates Alarmin-Driven Type 2 Innate Lymphoid Cell Responses. Immunity 2019, 51, 709–723.e6. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kawakita, A.; Okazaki, S.; Murai, H.; Yasutomi, M.; Ohshima, Y. IL-33 enhanced the proliferation and constitutive production of IL-13 and IL-5 by fibrocytes. BioMed Res. Int. 2014, 2014, 738625. [Google Scholar] [CrossRef] [Green Version]
- Petra, A.I.; Tsilioni, I.; Taracanova, A.; Katsarou-Katsari, A.; Theoharides, T.C. Interleukin 33 and interleukin 4 regulate interleukin 31 gene expression and secretion from human laboratory of allergic diseases 2 mast cells stimulated by substance P and/or immunoglobulin E. Allergy Asthma Proc. 2018, 39, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar] [CrossRef] [Green Version]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Snast, I.; Reiter, O.; Hodak, E.; Friedland, R.; Mimouni, D.; Leshem, Y.A. Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2018, 19, 145–165. [Google Scholar] [CrossRef]
- Paller, A.S.; Bansal, A.; Simpson, E.L.; Boguniewicz, M.; Blauvelt, A.; Siegfried, E.C.; Guttman-Yassky, E.; Hultsch, T.; Chen, Z.; Mina-Osorio, P.; et al. Clinically Meaningful Responses to Dupilumab in Adolescents with Uncontrolled Moderate-to-Severe Atopic Dermatitis: Post-hoc Analyses from a Randomized Clinical Trial. Am. J. Clin. Dermatol. 2020, 21, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Deleuran, M.; Thaci, D.; Beck, L.A.; de Bruin-Weller, M.; Blauvelt, A.; Forman, S.; Bissonnette, R.; Reich, K.; Soong, W.; Hussain, I.; et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J. Am. Acad. Dermatol. 2020, 82, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults with Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Dermatol. 2021, 184, 450–463. [Google Scholar] [CrossRef]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; Nemolizumab, J.P.S.G. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, O.; Furue, M.; Nakagawa, H.; Shiramoto, M.; Hanada, R.; Matsuki, S.; Imayama, S.; Kato, M.; Hasebe, I.; Taira, K.; et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2016, 174, 296–304. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Lacour, J.P.; Spelman, L.; Galimberti, R.; Eichenfield, L.F.; Bissonnette, R.; King, B.A.; Thyssen, J.P.; Silverberg, J.I.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M.; et al. Efficacy and Safety of Baricitinib Combined with Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 1333–1343. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020, 47, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaci, D.; Pangan, A.L.; Hong, H.C.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverberg, J.I.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Costanzo, A.; Rosmarin, D.; Lynde, C.; Liu, J.; Gamelli, A.; et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week-52 AD Up study results. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e6. [Google Scholar] [CrossRef] [Green Version]
- Yosipovitch, G.; Gold, L.F.; Lebwohl, M.G.; Silverberg, J.I.; Tallman, A.M.; Zane, L.T. Early Relief of Pruritus in Atopic Dermatitis with Crisaborole Ointment, A Non-steroidal, Phosphodiesterase 4 Inhibitor. Acta Derm.-Venereol. 2018, 98, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Bissonnette, R.; Pavel, A.B.; Diaz, A.; Werth, J.L.; Zang, C.; Vranic, I.; Purohit, V.S.; Zielinski, M.A.; Vlahos, B.; Estrada, Y.D.; et al. Crisaborole and atopic dermatitis skin biomarkers: An intrapatient randomized trial. J. Allergy Clin. Immunol. 2019, 144, 1274–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, eaax2945. [Google Scholar] [CrossRef]
- Cabanillas, B.; Brehler, A.C.; Novak, N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.D.; Suarez-Farinas, M.; Dhingra, N.; Cardinale, I.; Li, X.; Kostic, A.; Ming, J.E.; Radin, A.R.; Krueger, J.G.; Graham, N.; et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Iannone, M.; Tonini, G.; Janowska, A.; Dini, V.; Romanelli, M. Definition of treatment goals in terms of clinician-reported disease severity and patient-reported outcomes in moderate-to-severe adult atopic dermatitis: A systematic review. Curr. Med. Res. Opin. 2021, 37, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Ariens, L.F.M.; van der Schaft, J.; Bakker, D.S.; Balak, D.; Romeijn, M.L.E.; Kouwenhoven, T.; Kamsteeg, M.; Giovannone, B.; Drylewicz, J.; van Amerongen, C.C.A.; et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: First clinical and biomarker results from the BioDay registry. Allergy 2020, 75, 116–126. [Google Scholar] [CrossRef]
- Abraham, S.; Haufe, E.; Harder, I.; Heratizadeh, A.; Kleinheinz, A.; Wollenberg, A.; Weisshaar, E.; Augustin, M.; Wiemers, F.; Zink, A.; et al. Implementation of dupilumab in routine care of atopic eczema: Results from the German national registry TREATgermany. Br. J. Dermatol. 2020, 183, 382–384. [Google Scholar] [CrossRef]
- Bansal, A.; Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Blauvelt, A.; de Bruin-Weller, M.; Corren, J.; Sher, L.; Guttman-Yassky, E.; Chen, Z.; et al. Conjunctivitis in Dupilumab Clinical Trials for Adolescents with Atopic Dermatitis or Asthma. Am. J. Clin. Dermatol. 2021, 22, 101–115. [Google Scholar] [CrossRef]
- Wollenberg, A.; Beck, L.A.; Blauvelt, A.; Simpson, E.L.; Chen, Z.; Chen, Q.; Shumel, B.; Khokhar, F.A.; Hultsch, T.; Rizova, E.; et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: Results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br. J. Dermatol. 2020, 182, 1120–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, M.R.; Brestoff, J.R.; Berrien-Elliott, M.M.; Trier, A.M.; Yang, T.B.; McCullen, M.; Collins, P.L.; Niu, H.; Bodet, N.D.; Wagner, J.A.; et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci. Transl. Med. 2020, 12, eaay1005. [Google Scholar] [CrossRef]
- Erickson, S.; Heul, A.V.; Kim, B.S. New and emerging treatments for inflammatory itch. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2021, 126, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Irie, H. Interleukin-31 as a Clinical Target for Pruritus Treatment. Front. Med. 2021, 8, 638325. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; King, B.A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar] [CrossRef] [Green Version]
- Deleanu, D.; Nedelea, I. Biological therapies for atopic dermatitis: An update. Exp. Ther. Med. 2019, 17, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, A.; Ogawa, Y.; Oki, C.; Kimoto, Y.; Nozawa, K.; Amano, W.; Noji, S.; Shiozaki, M.; Matsuo, A.; Shinozaki, Y.; et al. Pharmacological properties of JTE-052: A novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2015, 64, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Boinapally, V.; Zoltowska, A.; Adner, M.; Hellman, L.; Nilsson, G. Vaccination against IL-33 Inhibits Airway Hyperresponsiveness and Inflammation in a House Dust Mite Model of Asthma. PLoS ONE 2015, 10, e0133774. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, S.; Cao, S.; Liu, C.; Lyu, S.C.; Sindher, S.B.; Long, A.; Sampath, V.; Petroni, D.; Londei, M.; Nadeau, K.C. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019, 4, e131347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mediator | Mechanism | Drug | Status | Clinical Effects | References |
|---|---|---|---|---|---|
| IL-4, IL-13 | Anti-IL-4Rα | Dupilumab | Approved for moderate-to-severe AD (FDA) | Improvement in pruritus NRS by ≥4 points; IGA, EASI, SCORAD, DLQI | [53,84,85,86] |
| IL-13 | Anti-IL-13 | Lebrikizumab | Phase 2b | Improvement in pruritus NRS by ≥4 points; EASI, IGA, BSA, POEM | [87] |
| Tralokinumab | Phase 3 | Improvement in pruritus NRS by ≥4 points; IGA, BSA, EASI, SCORAD, POEM | [88,89] | ||
| IL-31 | Anti-IL-31 | BMS-981164 | Phase 1 | Data not yet released | https://clinicaltrials.gov/ct2/show/NCT01614756 (accessed on 15 September 2021) |
| Anti-IL-31Rα | Nemolizumab | Phase 3 | Improvement in pruritus VAS by 40–60% | [69,90,91,92] | |
| JAK | JAK1/JAK2 inhibitor | Baricitinib | Approved for AD in Japan and the EU; undergoing phase 3 trials in other countries | Improvement in pruritus NRS by ≥4 points; IGA, EASI, SCORAD, skin pain, POEM, DLQI | [93,94] https://clinicaltrials.gov/ct2/results?cond=Atopic+Dermatitis&term=baricitinib&cntry=&state=&city=&dist= (accessed on 15 September 2021) |
| JAK1, JAK2, JAK3, and a tyrosine kinase 2 inhibitor | Delgocitinib 0.5% (topical) | Approved for AD in Japan; undergoing phase 3 trials in other countries | Improvement in pruritus NRS points; IGA, EASI, BSA | [95,96] https://clinicaltrials.gov/ct2/show/NCT04949841?term=delgocitinib&cond=Atopic+Dermatitis&draw=2&rank=6 (accessed on 15 September 2021) | |
| JAK1/3 inhibitor | Tofacitinib 2% (topical) | Phase 2a | Improvement in ISI; EASI, PGA, BSA | [54] | |
| JAK1 inhibitor | Abrocitinib (oral) | Phase 3 | Improvement in pruritus NRS by ≥4 points; IGA, EASI | [97,98] | |
| Upadacitinib (oral) | Phase 3 | Improvement in pruritus NRS by ≥4 points; IGA, EASI | [99,100,101] | ||
| PDE4 | PDE4 inhibitor | Crisaborole 2% (topical) | Approved for mild-to-moderate AD (FDA) | Improvement in the severity pruritus scale & NRS points; IGA, AD signs, DLQI | [102,103,104] |
| TSLP | Anti-TSLPR | Tezepelumab | Phase 2a | Improvement in pruritus NRS points & the 5-D itch scale; EASI, IGA, SCORAD (Numerical improvement *) | [105] |
| #IL-33 | Anti-IL-33 | Etokimab | Phase 2a proof-of-concept study | Improvement in 5D itch scores; EASI, SCORAD, IGA, DLQI | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniaga, C.S.; Tominaga, M.; Takamori, K. The Pathology of Type 2 Inflammation-Associated Itch in Atopic Dermatitis. Diagnostics 2021, 11, 2090. https://doi.org/10.3390/diagnostics11112090
Moniaga CS, Tominaga M, Takamori K. The Pathology of Type 2 Inflammation-Associated Itch in Atopic Dermatitis. Diagnostics. 2021; 11(11):2090. https://doi.org/10.3390/diagnostics11112090
Chicago/Turabian StyleMoniaga, Catharina Sagita, Mitsutoshi Tominaga, and Kenji Takamori. 2021. "The Pathology of Type 2 Inflammation-Associated Itch in Atopic Dermatitis" Diagnostics 11, no. 11: 2090. https://doi.org/10.3390/diagnostics11112090






