Raman Spectroscopy Discloses Altered Molecular Profile in Thyroid Adenomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Thyroid Tissue
2.3. Morphological Assessment
2.4. Immunohistochemistry
2.5. Raman Analysis
2.6. Cluster Analysis of Raman Spectra
2.7. Molecular Analysis
3. Results
3.1. Morphological Evaluation
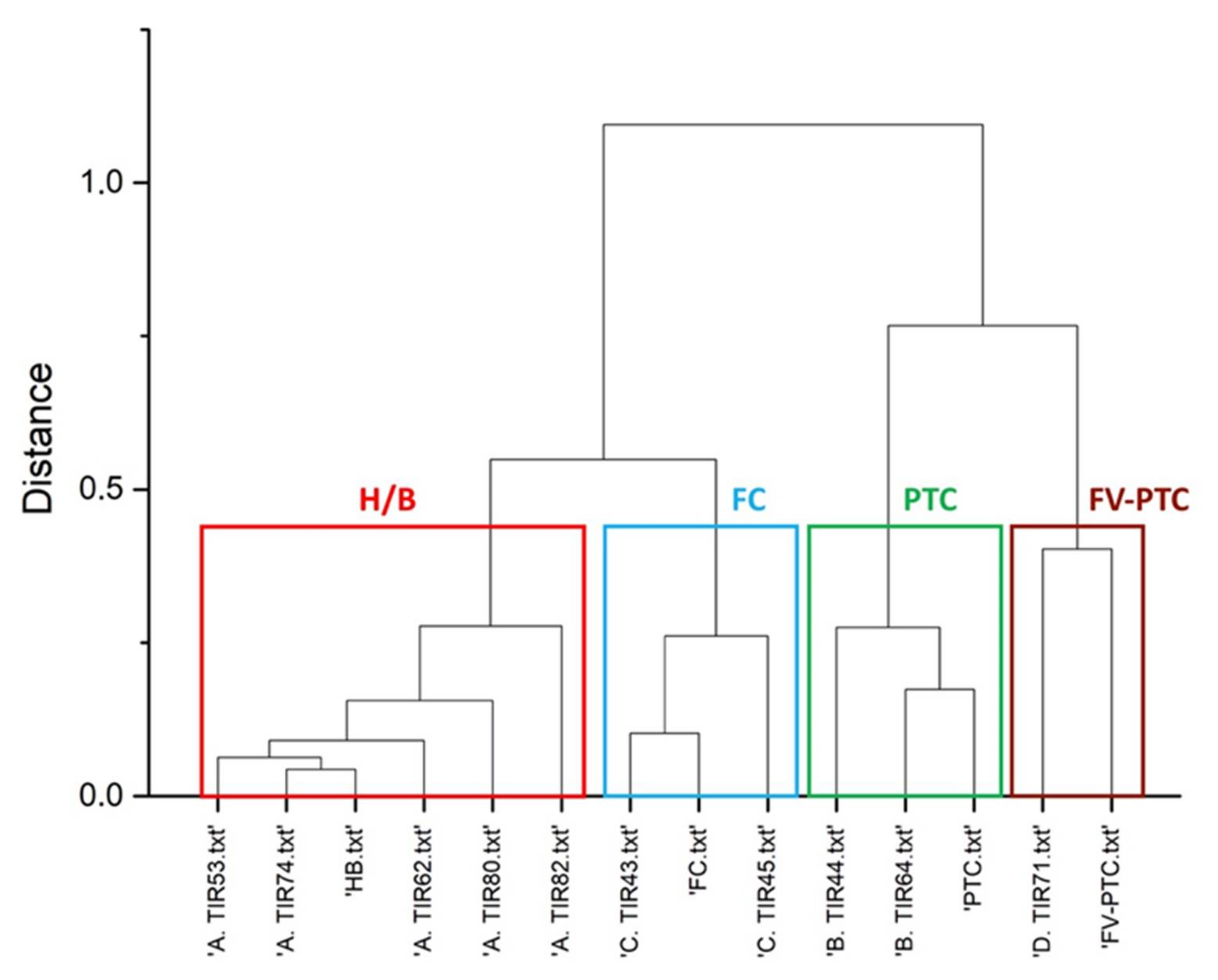
3.2. Raman Clustering
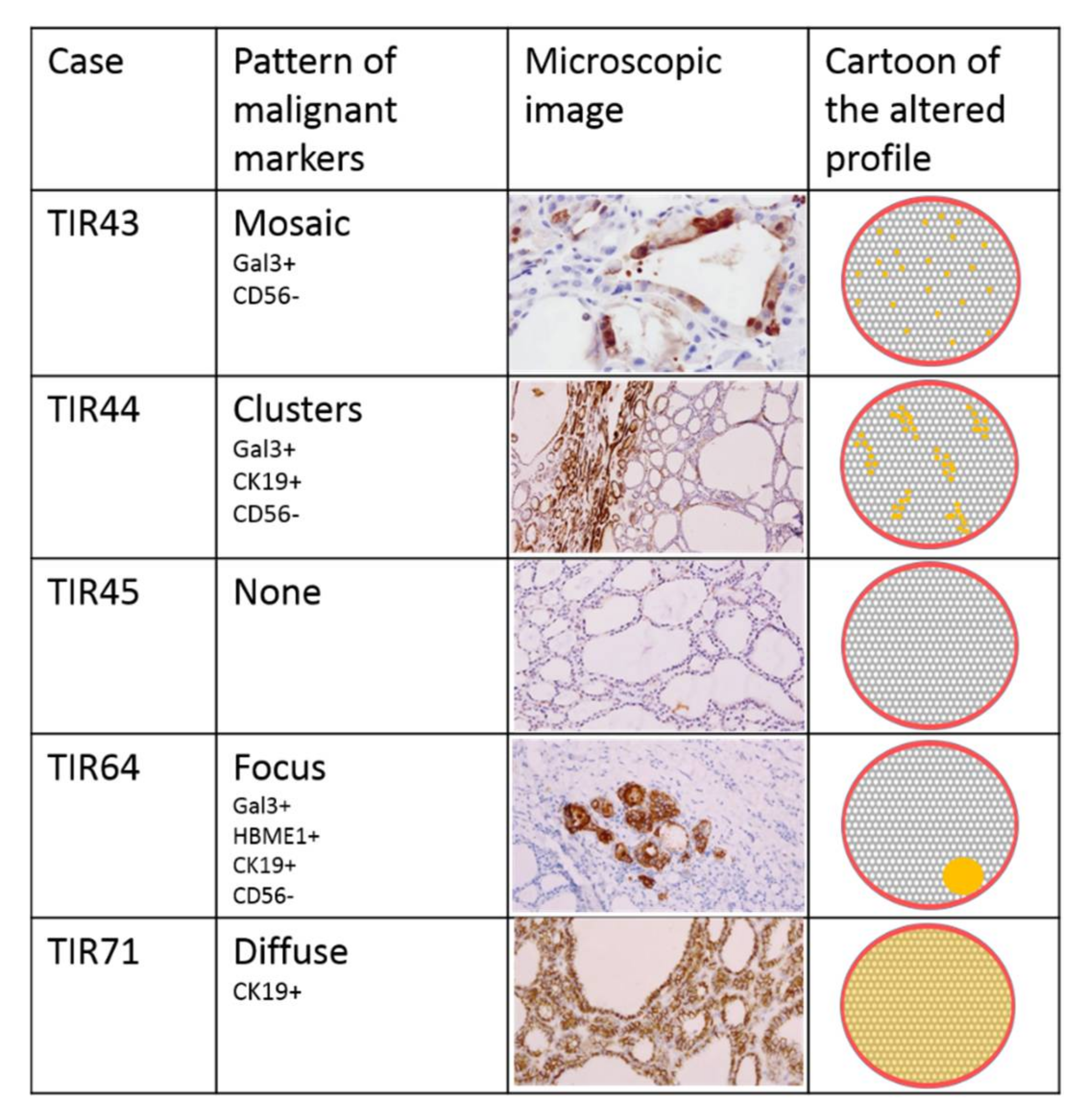
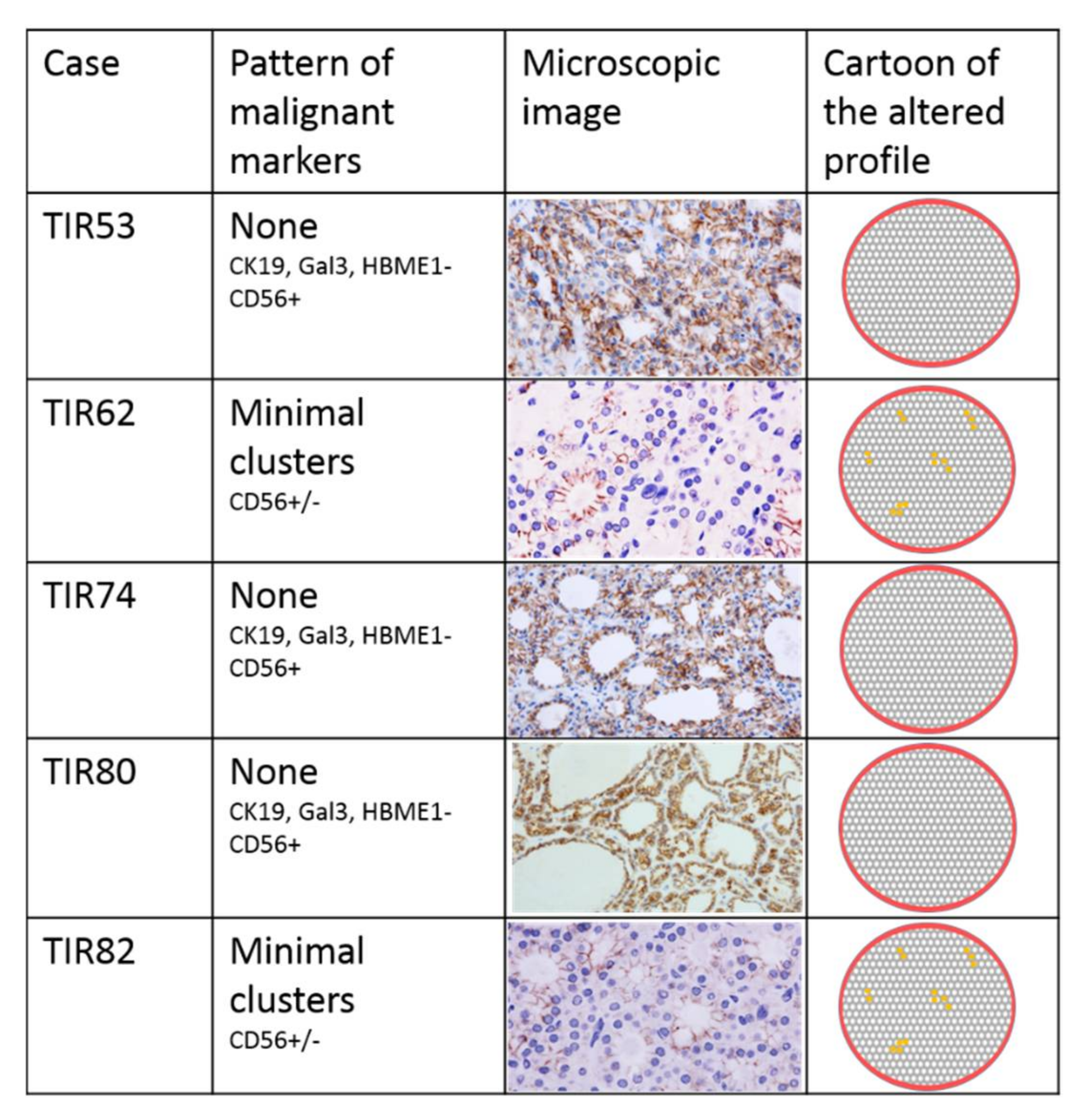
3.3. Immunohistochemical Assessment
3.4. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trimboli, P.; Treglia, G.; Guidobaldi, L.; Saggiorato, E.; Nigri, G.; Crescenzi, A.; Romanelli, F.; Orlandi, F.; Valabrega, S.; Sadeghi, R.; et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: A meta-analysis. Endocrine 2014, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. (Eds.) World Health Organization Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-4493-6. [Google Scholar]
- Arcolia, V.; Journe, F.; Renaud, F.; Leteurtre, E.; Gabius, H.J.; Remmelink, M.; Saussez, S. Combination of galectin-3, CK19 and HBME-1 immunostaining improves the diagnosis of thyroid cancer. Oncol. Lett. 2017, 14, 4183–4189. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017, 8, 49421–49442. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Paschke, R.; Cantara, S.; Crescenzi, A.; Jarzab, B.; Musholt, T.J.; Sobrinho Simoes, M. European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur. Thyroid J. 2017, 6, 115–129. [Google Scholar] [CrossRef]
- Ye, L.; Zhou, X.; Huang, F.; Wang, W.; Qi, Y.; Xu, H.; Yang, S.; Shen, L.; Fei, X.; Xie, J.; et al. The genetic landscape of benign thyroid nodules revealed by whole exome and transcriptome sequencing. Nat. Commun. 2017, 8, 15533. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, M.S.; Jung, C.K.; Park, H.C.; Kim, S.Y.; Liu, J.; Bae, J.S.; Lee, S.H.; Kim, T.M.; Lee, S.H.; et al. Mutational burdens and evolutionary ages of thyroid follicular adenoma are comparable to those of follicular carcinoma. Oncotarget 2016, 7, 69638–69648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Y.; Ye, B.; Hu, H.; Fan, C.; Wang, T.; Zheng, Y.; Lv, J.; Ma, Y.; Xiang, M. Gene expression differences between thyroid carcinoma, thyroid adenoma and normal thyroid tissue. Oncol. Rep. 2018, 40, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Campennì, A.; Giovinazzo, S.; Siracusa, M.; Ruggeri, R.M. Hepatocyte growth factor/C-met axis in thyroid cancer: From diagnostic biomarker to therapeutic target. Biomark. Insights 2017, 12. [Google Scholar] [CrossRef]
- Kallaway, C.; Almond, L.M.; Barr, H.; Wood, J.; Hutchings, J.; Kendall, C.; Stone, N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagn. Photodyn. Ther. 2013, 10, 207–219. [Google Scholar] [CrossRef]
- Krafft, C.; Popp, J. Raman4Clinics: The prospects of Raman-based methods for clinical application. Anal. Bioanal. Chem. 2015, 407, 8263–8264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palermo, A.; Fosca, M.; Tabacco, G.; Marini, F.; Graziani, V.; Santarsia, M.C.; Longo, F.; Lauria, A.; Cesareo, R.; Giovannoni, I.; et al. Raman Spectroscopy Applied to Parathyroid Tissues: A New Diagnostic Tool to Discriminate Normal Tissue from Adenoma. Anal. Chem. 2018, 90, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Graziani, V.; Fosca, M.; Taffon, C.; Rocchia, M.; Crucitti, P.; Pozzilli, P.; Onetti Muda, A.; Caricato, M.; Crescenzi, A. RAMAN spectroscopy imaging improves the diagnosis of papillary thyroid carcinoma. Sci. Rep. 2016, 6, 35117. [Google Scholar] [CrossRef] [PubMed]
- Sbroscia, M.; Di Gioacchino, M.; Ascenzi, P.; Crucitti, P.; di Masi, A.; Giovannoni, I.; Longo, F.; Mariotti, D.; Naciu, A.M.; Palermo, A.; et al. Thyroid cancer diagnosis by Raman spectroscopy. Sci. Rep. 2020, 10, 13342. [Google Scholar] [CrossRef]
- Seethala, R.R.; Baloch, Z.W.; Barletta, J.A.; Khanafshar, E.; Mete, O.; Sadow, P.M.; LiVolsi, V.A.; Nikiforov, Y.E.; Tallini, G.; Thompson, L.D. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: A review for pathologists. Mod. Pathol. 2018, 31, 39–55. [Google Scholar] [CrossRef]
- Sponziello, M.; Brunelli, C.; Verrienti, A.; Grani, G.; Pecce, V.; Abballe, L.; Ramundo, V.; Damante, G.; Russo, D.; Lombardi, C.P.; et al. Performance of a dual-component molecular assay in cytologically indeterminate thyroid nodules. Endocrine 2020, 68, 458–465. [Google Scholar] [CrossRef]
- Sponziello, M.; Silvestri, G.; Verrienti, A.; Perna, A.; Rosignolo, F.; Brunelli, C.; Pecce, V.; Rossi, E.D.; Lombardi, C.P.; Durante, C.; et al. A novel nonsense EIF1AX mutation identified in a thyroid nodule histologically diagnosed as oncocytic carcinoma. Endocrine 2018, 62, 492–495. [Google Scholar] [CrossRef]
- Verrienti, A.; Pecce, V.; Abballe, L.; Ramundo, V.; Falcone, R.; Inanloo Nigi Jak, F.; Brunelli, C.; Fadda, G.; Bosco, D.; Ascoli, V.; et al. Analytical validation of a novel targeted next-generation sequencing assay for mutation detection in thyroid nodule aspirates and tissue. Endocrine 2020, 69, 451–455. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. TI-RADS. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Invest. 2014, 37, 593–599. [Google Scholar] [CrossRef]
- Stephenson, A.; Lau, L.; Eszlinger, M.; Paschke, R. The Thyrotropin Receptor Mutation Database Update. Thyroid 2020, 30, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Gordon, D.; Chiefari, E.; Yong, S.; DeJong, S.; Pitale, S.; Russo, D.; Filetti, S. A Phe 486 thyrotropin receptor mutation in an autonomously functioning follicular carcinoma that was causing hyperthyroidism. Thyroid 2000, 10, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Führer, D.; Tannapfel, A.; Sabri, O.; Lamesch, P.; Paschke, R. Two somatic TSH receptor mutations in a patient with toxic metastasising follicular thyroid carcinoma and non-functional lung metastases. Endocr. Relat. Cancer 2003, 10, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
| Case | TIR43 | TIR44 | TIR45 | TIR53 | TIR62 | TIR64 | TIR71 | TIR74 | TIR80 | TIR82 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | F | M | F | F | M | F | M | M | F | F | |
| Age | 42 | 63 | 78 | 27 | 52 | 66 | 44 | 39 | 67 | 32 | |
| Max Diameter (mm) | 38 | 30 | 8 | 18 | 15 | 16 | 12 | 38 | 18 | 22 | |
| FNA | TIR3B | n.a. | TIR2 | TIR3A | TIR3B | TIR3A | TIR2 | TIR3A | n.a. | n.a. | |
| Clinical Features | TSH | 1.67 | 0.9 | 0.5 | 0.5 | 0.004 | 2.39 | 0.16 | 0.6 | 0.01 | 0.02 |
| TIRADS (1,2,3,4,5,6) | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 3 | 3 | |
| Morphological Features | Growth Pattern | micro/ normo follicular | micro/ macro follicular | micro/ normo follicular | micro/ normo follicular | micro/ macro follicular | micro/ macro follicular | micro follicular | micro/ macro follicular | micro/ macro follicular | micro/ normo follicular |
| Thyrocytes | Hurtle | Usual | Hurtle | Hurtle | Hurtle | Usual | Usual | Hurtle | Usual | Usual | |
| NIFTP score | 1 | 1 | 0 | 0 | 1 | 2 * | 1 | 0 | 0 | 1 | |
| Capsule | Irregular | Thin | Irregular | Thin | Thin | Thin | Thin | Thin | Thin | Irregular | |
| Immuno- Histochemical Markers | Gal3 | - mosaic | + cluster | - | - | - | + focus | - | - | - | - |
| HBME-1 | - | - | - | - | - | + focus | - | - | - | - | |
| CK19 | - | + cluster | - | - | - | + focus | + diffuse | - | - | - | |
| CD56 | - mosaic | - cluster | + | + | - cluster | - focus | - cluster | + | + | - cluster | |
| Molecular Analysis (NGS + dPCR) | SNVs/ Indels | Negative | Negative | TSHR p.I486F; AF:28% | TSHR p.D633Y; AF:39% | Negative | Negative | Negative | Negative | TSHR p.S505N AF:25%; EIF1AX p.R13L; AF:24% | TSHR p.D633E; AF:40% |
| Gene Fusions | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | |
| miRNA | n.a. | n.a. | Negative | n.a. | Negative | Negative | Negative | Negative | Negative | Negative | |
| Raman Cluster | Malignant FC | Malignant PTC | Malignant FC | Benign | Benign | Malignant PTC | Malignant FV-PTC | Benign | Benign | Benign | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodo, A.; Verri, M.; Palermo, A.; Naciu, A.M.; Sponziello, M.; Durante, C.; Di Gioacchino, M.; Paolucci, A.; di Masi, A.; Longo, F.; et al. Raman Spectroscopy Discloses Altered Molecular Profile in Thyroid Adenomas. Diagnostics 2021, 11, 43. https://doi.org/10.3390/diagnostics11010043
Sodo A, Verri M, Palermo A, Naciu AM, Sponziello M, Durante C, Di Gioacchino M, Paolucci A, di Masi A, Longo F, et al. Raman Spectroscopy Discloses Altered Molecular Profile in Thyroid Adenomas. Diagnostics. 2021; 11(1):43. https://doi.org/10.3390/diagnostics11010043
Chicago/Turabian StyleSodo, Armida, Martina Verri, Andrea Palermo, Anda Mihaela Naciu, Marialuisa Sponziello, Cosimo Durante, Michael Di Gioacchino, Alessio Paolucci, Alessandra di Masi, Filippo Longo, and et al. 2021. "Raman Spectroscopy Discloses Altered Molecular Profile in Thyroid Adenomas" Diagnostics 11, no. 1: 43. https://doi.org/10.3390/diagnostics11010043
APA StyleSodo, A., Verri, M., Palermo, A., Naciu, A. M., Sponziello, M., Durante, C., Di Gioacchino, M., Paolucci, A., di Masi, A., Longo, F., Crucitti, P., Taffon, C., Ricci, M. A., & Crescenzi, A. (2021). Raman Spectroscopy Discloses Altered Molecular Profile in Thyroid Adenomas. Diagnostics, 11(1), 43. https://doi.org/10.3390/diagnostics11010043









