Optical Density Optimization of Malaria Pan Rapid Diagnostic Test Strips for Improved Test Zone Band Intensity
Abstract
:1. Introduction
1.1. Components of Immuno-Chromatographic Test Kits
1.2. The Protein
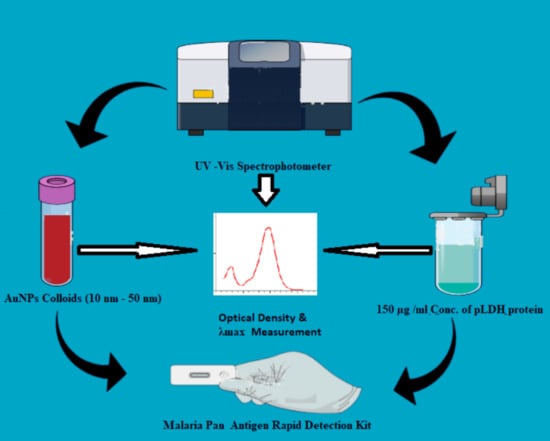
2. Materials and Methods
2.1. Reagents, Instruments and Other Support Materials
2.2. Experimental
2.2.1. Preparation of Gold Nanoparticles (AuNPs)
2.2.2. Protein Conjugation with Gold NPs
2.2.3. Centrifugation
2.2.4. Conjugate Pad Prepration
2.2.5. Membrane Coating
2.2.6. Test Line Reagents Concentration
2.2.7. Control Line Reagents Concentration
3. Results and Discussion
3.1. Gold NP Characterization
3.2. Monitoring the Protein Loss
3.3. Test Line Intensity Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, M.N.; Misra, R.N. Immunochromatographic methods in malaria diagnosis. Med. J. Armed Forces India 2007, 63, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Manta, P.; Agarwal, S.; Singh, G.; Bhamrah, S.S. Formulation, development and sensitivity, specificity comparison of gold, platinum and silver nano particle based HIV ½ and hCG IVD rapid test kit (Immune chromatoghraphic test device). World J. Pharm. Sci. 2015, 4, 1870–1905. [Google Scholar]
- Maltha, J.; Gillet, P.; Jacobs, J. Malaria rapid diagnostic tests in endemic settings. Clin. Microbes Infect. 2013, 19, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gronowski, A.M. Handbook of Clinical Laboratory Testing during Pregnancy; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Benjamin, G.Y.; Bartholomew, B.; Abdullahi, J.; Labaran, L.M. Prevalence of Plasmodium falciparum and Haemoglobin Genotype Distribution among Malaria Patients in Zaria, Kaduna State, Nigeria. South Asian J. Parasitol. 2019, 23, 1–7. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singhal, M.; McKenzie, K.G.; Osborn, J.L.; Arjyal, A.; Dongol, S.; Baker, S.G.; Basnyat, B.; Farrar, J.; Dolecek, C.; et al. A rapid, multiplexed, high-throughput flow-through membrane immunoassay: A convenient alternative to ELISA. Diagnosis 2013, 3, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Huang, T.H.; Chen, C.A.; Ho, N.Y.; Chou, Y.J.; Chen, C.F. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.; Tse, H. Lateral Flow Immunoassay; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar] [CrossRef]
- Lou, S.; Ye, J.Y.; Li, K.Q.; Wu, A. A gold nanoparticle-based immunochromatographic assay: The influence of nanoparticulate size. Analyst 2012, 137, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, Y.T.; Hong, S.B.; Kim, J.; Heo, N.S.; Lee, M.K.; Lee, S.J.; Kim, B.I.; Kim, I.S.; Huh, Y.S.; et al. Development of lateral flow assay based on size-controlled gold nanoparticles for detection of hepatitis B surface antigen. Sensors 2016, 16, 2154. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Chen, Z.; Li, L.; Xia, J. Barcode lateral flow immunochromatographic strip for prostate acid phosphatase determination. J. Pharm. Biomed. Anal. 2011, 56, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Safenkova, I.; Zherdev, A.; Dzantiev, B. Factors influencing the detection limit of the lateral-flow sandwich immunoassay: A case study with potato virus X. Anal. Bioanal. Chem. 2012, 403, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Gautam, R.K.; Jaiswal, A.; Chattopadhyaya, M.C.; Sharma, Y.C. Rapid scavenging of methylene blue dye from a liquid phase by adsorption on alumina nanoparticles. RSC Adv. 2015, 5, 14425–14440. [Google Scholar] [CrossRef]
- Kaur, K.; Forrest, J.A. Influence of particle size on the binding activity of proteins adsorbed onto gold nanoparticles. Langmuir 2012, 28, 2736–2744. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Byzova, N.A.; Safenkova, I.V.; Slutskaya, E.S.; Zherdev, A.V.; Dzantiev, B.B. Less is more: A comparison of antibody–gold nanoparticle conjugates of different ratios. Bioconjugate Chem. 2017, 28, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Larm, N.E.; Essner, J.B.; Pokpas, K.; Canon, J.A.; Jahed, N.; Iwuoha, E.I.; Baker, G.A. Room-temperature turkevich method: Formation of gold nanoparticles at the speed of mixing using cyclic oxocarbon reducing agents. J. Phys. Chem. C 2018, 122, 5105–5118. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef]







| S. No. | Gold Nanoparticles (AuNP) Size | Optical Density (OD) Observation |
|---|---|---|
| 1 |  10 nm 10 nm | 0.301 |
| 2 |  20 nm 20 nm | 0.354 |
| 3 |  30 nm 30 nm | 0.368 |
| 4 |  40 nm 40 nm | 0.426 |
| 5 |  50 nm 50 nm | 0.385 |
| Test Zone Intensity of Kit Developed by 10 nm of AuNPs | Test Zone Intensity of Kit Developed by 20 nm of AuNPs | Test Zone Intensity of Kit Developed by 30 nm of AuNPs | Test Zone Intensity of Kit Developed by 40 nm of AuNPs | Test Zone Intensity of Kit Developed by 50 nm of AuNPs |
|---|---|---|---|---|
 |  |  |  |  |
| +3 (High) Intensity | +3 (High) Intensity | +3 (High) Intensity | +3 (High) Intensity | +3 (High) Intensity |
| Specificity of Kit Developed by 10 nm of AuNPs | Specificity of Kit Developed by 20 nm of AuNPs | Specificity of Kit Developed by 30 nm of AuNPs | Specificity of Kit Developed by 40 nm of AuNPs | Specificity of Kit Developed by 50 nm of AuNPs |
|---|---|---|---|---|
 |  |  |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manta, P.; Nagraik, R.; Sharma, A.; Kumar, A.; Verma, P.; Paswan, S.K.; Bokov, D.O.; Shaikh, J.D.; Kaur, R.; Leite, A.F.V.; et al. Optical Density Optimization of Malaria Pan Rapid Diagnostic Test Strips for Improved Test Zone Band Intensity. Diagnostics 2020, 10, 880. https://doi.org/10.3390/diagnostics10110880
Manta P, Nagraik R, Sharma A, Kumar A, Verma P, Paswan SK, Bokov DO, Shaikh JD, Kaur R, Leite AFV, et al. Optical Density Optimization of Malaria Pan Rapid Diagnostic Test Strips for Improved Test Zone Band Intensity. Diagnostics. 2020; 10(11):880. https://doi.org/10.3390/diagnostics10110880
Chicago/Turabian StyleManta, Prince, Rupak Nagraik, Avinash Sharma, Akshay Kumar, Pritt Verma, Shravan Kumar Paswan, Dmitry O. Bokov, Juber Dastagir Shaikh, Roopvir Kaur, Ana Francesca Vommaro Leite, and et al. 2020. "Optical Density Optimization of Malaria Pan Rapid Diagnostic Test Strips for Improved Test Zone Band Intensity" Diagnostics 10, no. 11: 880. https://doi.org/10.3390/diagnostics10110880