Perspectives and Insights into the Competition for Aminoacyl-tRNAs between the Translational Machinery and for tRNA Dependent Non-Ribosomal Peptide Bond Formation
Abstract
:1. Introduction
2. L/F-Transferase and Its Biological Functions
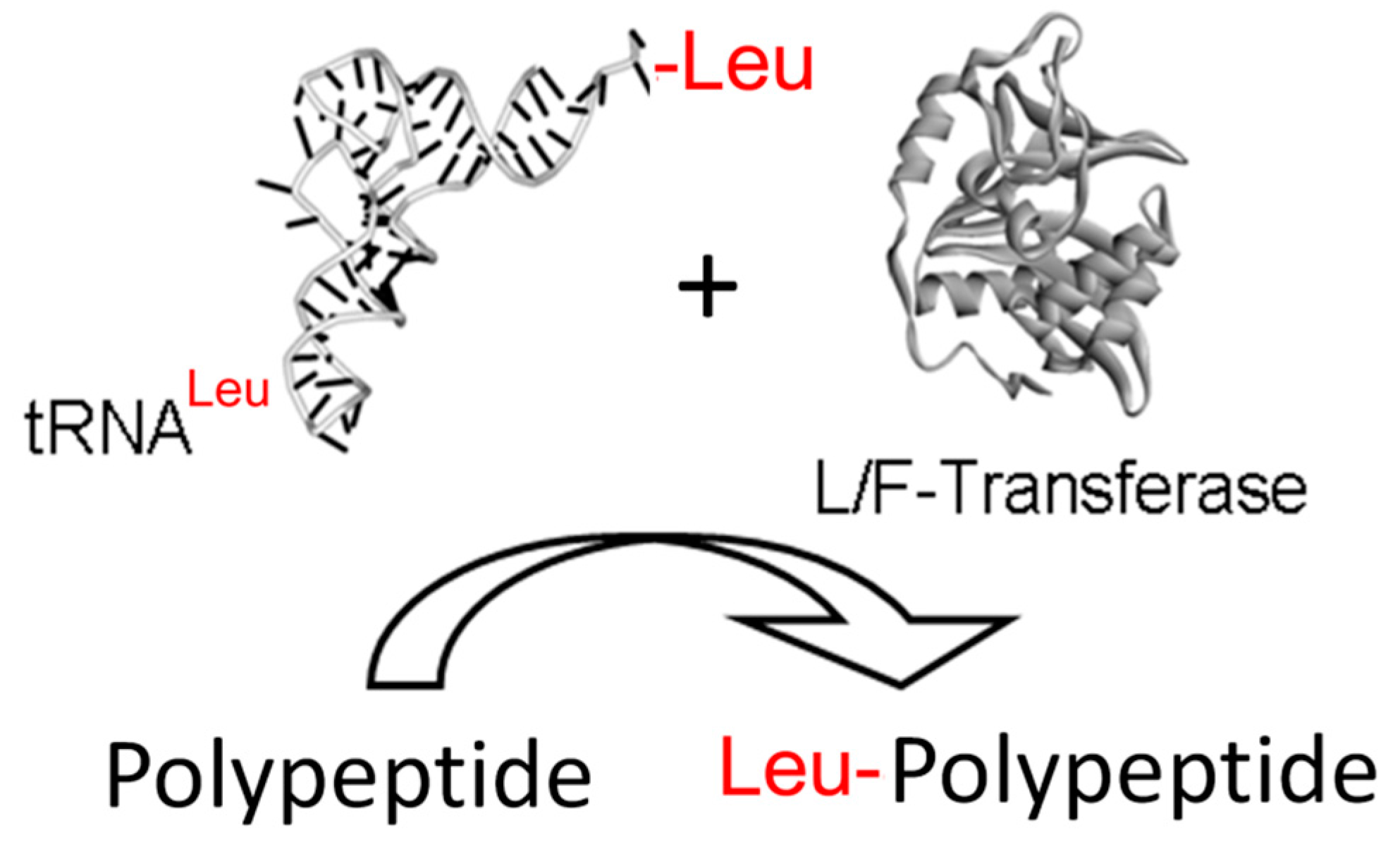
3. Substrate Recognition by L/F-Transferase
3.1. Sequence Recognition of Protein Substrates by L/F-Transferase
3.2. tRNA Recognition by L/F-Transferase
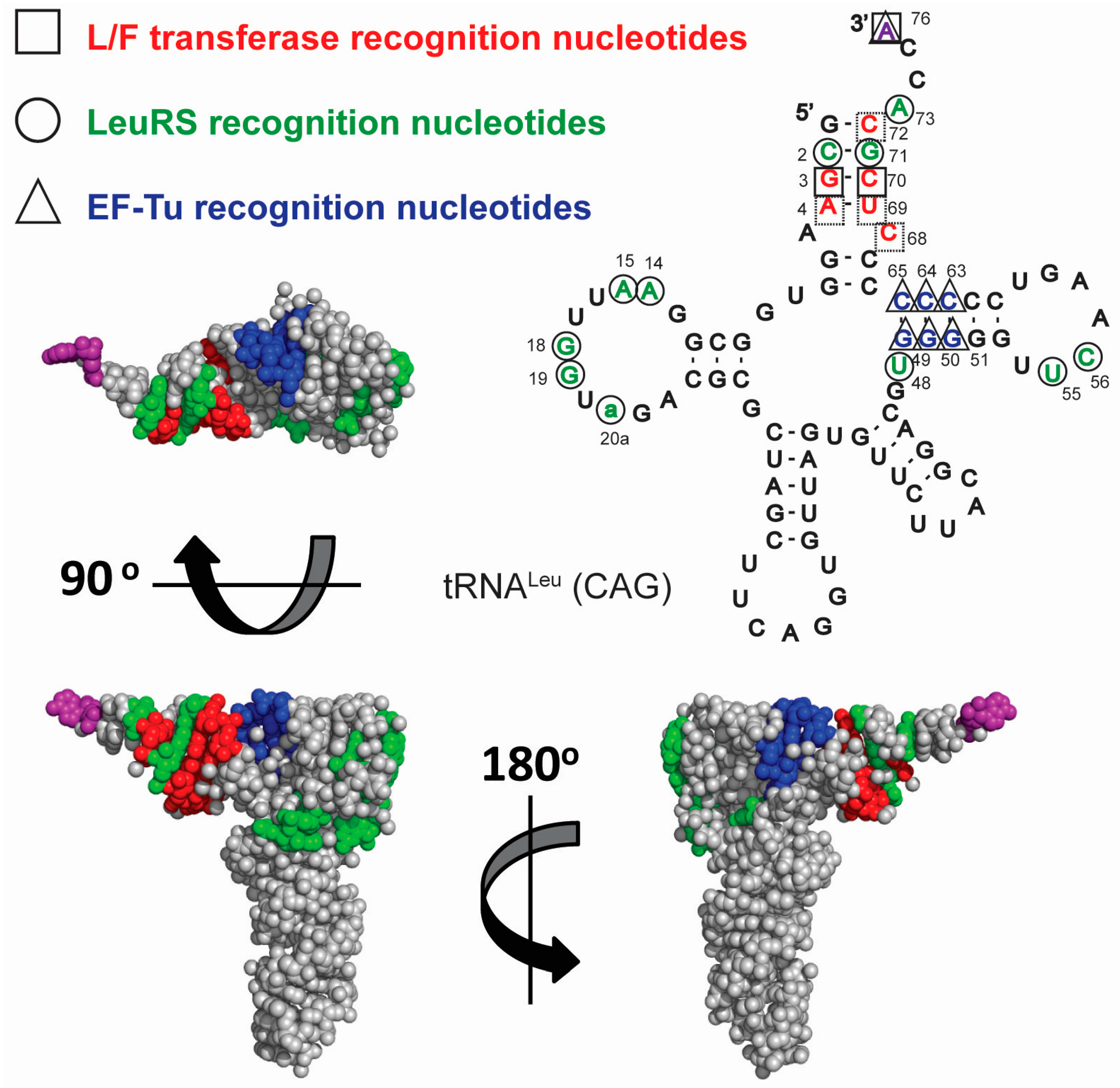
3.3. tRNA Availability
4. Bacterial Stringent Response
4.1. Stringent Response and the Inactivation of EF-Tu and Translation
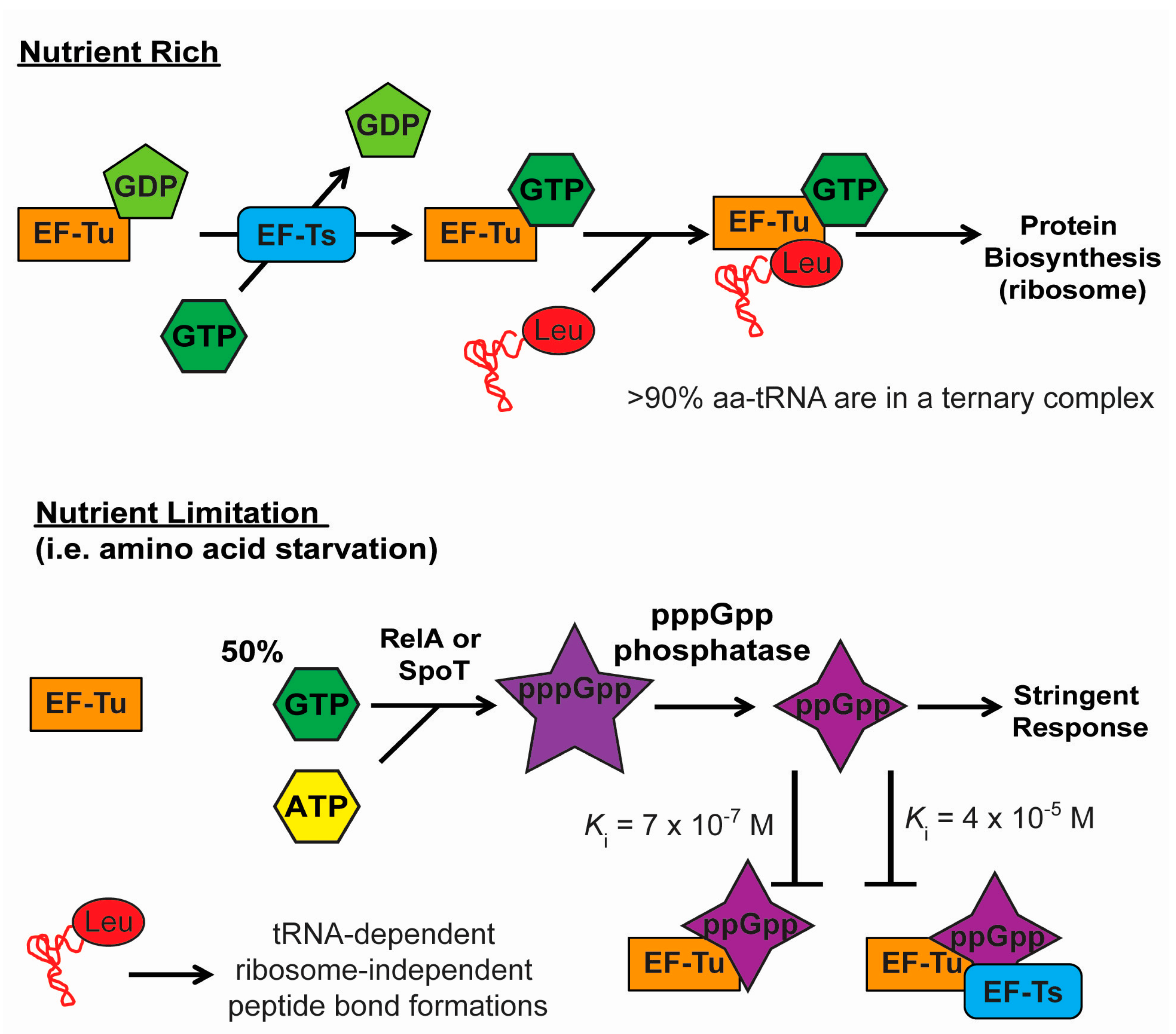
4.2. Stringent Response and Selective Aminoacylation of tRNA Isoacceptors
5. Hypothesis: Is the Stringent Response the Key to the Function of L/F-Transferase?
6. Eukaryotic Aminoacyl-tRNA Protein Transferase and tRNA Substrates
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raina, M.; Ibba, M. Trnas as regulators of biological processes. Front Genet. 2014, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Marquet, R.; Isel, C.; Ehresmann, C.; Ehresmann, B. Trnas as primer of reverse transcriptases. Biochimie 1995, 77, 113–124. [Google Scholar] [CrossRef]
- Biarrotte-Sorin, S.; Maillard, A.P.; Delettre, J.; Sougakoff, W.; Arthur, M.; Mayer, C. Crystal structures of weissella viridescens femx and its complex with udp-murnac-pentapeptide: Insights into femabx family substrates recognition. Structure 2004, 12, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.E.; Prince, D.B.; Mutchler, V.T.; Curry, K.A.; Ho, A.M.; Sarver, R.W.; Hagadorn, J.C.; Choi, G.H.; Garlick, R.L. X-ray crystal structure of staphylococcus aureus fema. Structure 2002, 10, 1107–1115. [Google Scholar] [CrossRef]
- Roy, H.; Ibba, M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl. Acad. Sci. USA 2008, 105, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Hebecker, S.; Arendt, W.; Heinemann, I.U.; Tiefenau, J.H.; Nimtz, M.; Rohde, M.; Soll, D.; Moser, J. Alanyl-phosphatidylglycerol synthase: Mechanism of substrate recognition during tRNA-dependent lipid modification in pseudomonas aeruginosa. Mol. Microbiol. 2011, 80, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Hebecker, S.; Krausze, J.; Hasenkampf, T.; Schneider, J.; Groenewold, M.; Reichelt, J.; Jahn, D.; Heinz, D.W.; Moser, J. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proc. Natl. Acad. Sci. USA 2015, 112, 10691–10696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ntai, I.; Kelleher, N.L.; Walsh, C.T. tRNA-dependent peptide bond formation by the transferase pacb in biosynthesis of the pacidamycin group of pentapeptidyl nucleoside antibiotics. Proc. Natl. Acad. Sci. USA 2011, 108, 12249–12253. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.P.; Qian, X.L.; Alemany, L.B.; Moran, S.; Parry, R.J. Investigations of valanimycin biosynthesis: Elucidation of the role of seryl-tRNA. Proc. Natl. Acad. Sci. USA 2008, 105, 6543–6547. [Google Scholar] [CrossRef] [PubMed]
- Bachmair, A.; Finley, D.; Varshavsky, A. In vivo half-life of a protein is a function of its amino-terminal residue. Science 1986, 234, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, S.; Kyritsis, A.; Malissovas, N.; Becker, H.D.; Stathopoulos, C. On the role of an unusual trnagly isoacceptor in staphylococcus aureus. Biochimie 2009, 91, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.W.; Shrader, T.E.; Rocap, G.; Varshavsky, A. The N-end rule in bacteria. Science 1991, 254, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Bachmair, A.; Varshavsky, A. The degradation signal in a short-lived protein. Cell 1989, 56, 1019–1032. [Google Scholar] [CrossRef]
- Graciet, E.; Wellmer, F. The plant N-end rule pathway: Structure and functions. Trends Plant Sci. 2010, 15, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gonda, D.K.; Bachmair, A.; Wunning, I.; Tobias, J.W.; Lane, W.S.; Varshavsky, A. Universality and structure of the N-end rule. J. Biol. Chem. 1989, 264, 16700–16712. [Google Scholar] [PubMed]
- Kwon, Y.T.; Kashina, A.S.; Davydov, I.V.; Hu, R.G.; An, J.Y.; Seo, J.W.; Du, F.; Varshavsky, A. An essential role of N-terminal arginylation in cardiovascular development. Science 2002, 297, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.G.; Sheng, J.; Qi, X.; Xu, Z.; Takahashi, T.T.; Varshavsky, A. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 2005, 437, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Tasaki, T.; Moroi, K.; An, J.Y.; Kimura, S.; Davydov, I.V.; Kwon, Y.T. Rgs4 and rgs5 are in vivo substrates of the N-end rule pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 15030–15035. [Google Scholar] [CrossRef] [PubMed]
- Leu, N.A.; Kurosaka, S.; Kashina, A. Conditional tek promoter-driven deletion of arginyltransferase in the germ line causes defects in gametogenesis and early embryonic lethality in mice. PLoS ONE 2009, 4, e7734. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, M.; Wilson, R.; Tenev, T.; Zachariou, A.; Paul, A.; Deas, E.; Meier, P. Degradation of diap1 by the N-end rule pathway is essential for regulating apoptosis. Nat. Cell Biol. 2003, 5, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Herman-Bachinsky, Y.; Ryoo, H.D.; Ciechanover, A.; Gonen, H. Regulation of the drosophila ubiquitin ligase diap1 is mediated via several distinct ubiquitin system pathways. Cell Death Differ. 2007, 14, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Wickliffe, K.E.; Leppla, S.H.; Moayeri, M. Killing of macrophages by anthrax lethal toxin: Involvement of the N-end rule pathway. Cell Microbiol. 2008, 10, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Piatkov, K.I.; Brower, C.S.; Varshavsky, A. The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc. Natl. Acad. Sci. USA 2012, 109, E1839–E1847. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Payoe, R.; Fahlman, R.P. The c-terminal proteolytic fragment of the breast cancer susceptibility type 1 protein (brca1) is degraded by the N-end rule pathway. J. Biol. Chem. 2012, 287, 7495–7502. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Kashina, A. Posttranslational arginylation as a global biological regulator. Dev. Biol 2011, 358, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.J.; Soffer, R.L. A soluble enzyme from Escherichia coli which catalyzes the transfer of leucine and phenylalanine from trna to acceptor proteins. Biochem. Biophys. Res. Commun. 1969, 36, 47–53. [Google Scholar] [CrossRef]
- Shrader, T.E.; Tobias, J.W.; Varshavsky, A. The N-end rule in Escherichia coli: Cloning and analysis of the leucyl, phenylalanyl-tRNA-protein transferase gene aat. J. Bacteriol. 1993, 175, 4364–4374. [Google Scholar] [PubMed]
- Fung, A.W.; Ebhardt, H.A.; Abeysundara, H.; Moore, J.; Xu, Z.; Fahlman, R.P. An alternative mechanism for the catalysis of peptide bond formation by L/F transferase: Substrate binding and orientation. J. Mol. Biol. 2011, 409, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Abramochkin, G.; Shrader, T.E. The leucyl/phenylalanyl-tRNA-protein transferase. Overexpression and characterization of substrate recognition, domain structure, and secondary structure. J. Biol. Chem. 1995, 270, 20621–20628. [Google Scholar] [CrossRef] [PubMed]
- Ninnis, R.L.; Spall, S.K.; Talbo, G.H.; Truscott, K.N.; Dougan, D.A. Modification of patase by L/F-transferase generates a clps-dependent N-end rule substrate in Escherichia coli. EMBO J. 2009, 28, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Zahn, R.; Bukau, B.; Mogk, A. Clps is the recognition component for Escherichia coli substrates of the N-end rule degradation pathway. Mol. Microbiol. 2009, 72, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 2003, 100, 2261–2265. [Google Scholar] [CrossRef] [PubMed]
- Wortham, B.W.; Patel, C.N.; Oliveira, M.A. Polyamines in bacteria: Pleiotropic effects yet specific mechanisms. Adv. Exp. Med. Biol. 2007, 603, 106–115. [Google Scholar] [PubMed]
- Wolf, S.G.; Frenkiel, D.; Arad, T.; Finkel, S.E.; Kolter, R.; Minsky, A. DNA protection by stress-induced biocrystallization. Nature 1999, 400, 83–85. [Google Scholar] [PubMed]
- Azam, T.A.; Ishihama, A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J. Biol. Chem. 1999, 274, 33105–33113. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Neher, S.B.; Kim, Y.I.; Sauer, R.T.; Baker, T.A. Proteomic discovery of cellular substrates of the clpxp protease reveals five classes of clpx-recognition signals. Mol. Cell 2003, 11, 671–683. [Google Scholar] [CrossRef]
- Stephani, K.; Weichart, D.; Hengge, R. Dynamic control of dps protein levels by clpxp and clpap proteases in Escherichia coli. Mol. Microbiol. 2003, 49, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Humbard, M.A.; Surkov, S.; de Donatis, G.M.; Jenkins, L.M.; Maurizi, M.R. The N-degradome of Escherichia coli.: Limited proteolysis in vivo generates a large pool of proteins bearing N-degrons. J. Biol. Chem. 2013, 288, 28913–28924. [Google Scholar] [CrossRef] [PubMed]
- Soffer, R.L. Peptide acceptors in the leucine, phenylalanine transfer reaction. J. Biol. Chem. 1973, 248, 8424–8428. [Google Scholar] [PubMed]
- Kawaguchi, J.; Maejima, K.; Kuroiwa, H.; Taki, M. Kinetic analysis of the leucyl/phenylalanyl-tRNA-protein transferase with acceptor peptides possessing different N-terminal penultimate residues. FEBS Open Bio. 2013, 3, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W.; Fahlman, R.P. The molecular basis for the post-translational addition of amino acids by L/F transferase in the N-end rule pathway. Curr. Protein Pept. Sci. 2015, 16, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W.; Ebhardt, H.A.; Krishnakumar, K.S.; Moore, J.; Xu, Z.; Strazewski, P.; Fahlman, R.P. Probing the leucyl/phenylalanyl tRNA protein transferase active site with tRNA substrate analogues. Protein Pept. Lett. 2014, 21, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Kuroiwa, H.; Sisido, M. Chemoenzymatic transfer of fluorescent non-natural amino acids to the N terminus of a protein/peptide. Chembiochem 2008, 9, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Kuroiwa, H.; Sisido, M. The next-a (N-terminal EXtension with Transferase and Ars) reaction. Nucleic Acids Symp. Ser. (Oxf.) 2009, 53, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ebhardt, H.A.; Xu, Z.; Fung, A.W.; Fahlman, R.P. Quantification of the post-translational addition of amino acids to proteins by maldi-tof mass spectrometry. Anal. Chem. 2009, 81, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Fegley, M.W.; Warner, J.B.; Grindley, C.L.; Marotta, N.P.; Petersson, E.J. N-terminal protein modification using simple aminoacyl transferase substrates. J. Am. Chem. Soc. 2011, 133, 15139–15147. [Google Scholar] [CrossRef] [PubMed]
- Abramochkin, G.; Shrader, T.E. Aminoacyl-tRNA recognition by the leucyl/phenylalanyl-tRNA-protein transferase. J. Biol. Chem. 1996, 271, 22901–22907. [Google Scholar] [PubMed]
- Taki, M.; Kuroiwa, H. Unexpectedly fast transfer of positron-emittable artificial substrate into N-terminus of peptide/protein mediated by wild-type L/F-tRNA-protein transferase. Amino Acids 2015, 47, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W.; Leung, C.C.; Fahlman, R.P. The determination of trnaleu recognition nucleotides for Escherichia coli. L/F transferase. RNA 2014, 20, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Kaji, H. Utilization of isoaccepting leucyl-tRNA in the soluble incorporation system and protein synthesizing systems from E. coli. FEBS Lett. 1974, 43, 199–202. [Google Scholar] [CrossRef]
- Sprinzl, M.; Vassilenko, K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005, 33, D139–D140. [Google Scholar] [CrossRef] [PubMed]
- Holmes, W.M.; Goldman, E.; Miner, T.A.; Hatfield, G.W. Differential utilization of leucyl-tRNAs by Escherichia coli. Proc. Natl. Acad. Sci. USA 1977, 74, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Nilsson, L.; Kurland, C.G. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 1996, 260, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, V.; Kurland, C.G. Growth rate dependence of transfer RNA abundance in Escherichia coli. EMBO J. 1990, 9, 4359–4366. [Google Scholar] [PubMed]
- Scarpulla, R.C.; Deutch, C.E.; Soffer, R.L. Transfer of methionyl residues by leucyl, phenylalanyl-tRNA-protein transferase. Biochem. Biophys. Res. Commun. 1976, 71, 584–589. [Google Scholar] [CrossRef]
- Stent, G.S.; Brenner, S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc. Natl. Acad. Sci. USA 1961, 47, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Cashel, M. Regulation of bacterial ppGpp and pppGpp. Annu. Rev. Microbiol. 1975, 29, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Cashel, M.; Gallant, J. Two compounds implicated in the function of the rc gene of Escherichia coli. Nature 1969, 221, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The rela/spot homolog (rsh) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef] [PubMed]
- Haseltine, W.A.; Block, R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. USA 1973, 70, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Fernandez, I.S.; Kelley, A.C.; Carton, D.G.; Ramakrishnan, V.; Valle, M. The ribosome triggers the stringent response by rela via a highly distorted tRNA. EMBO Rep. 2013, 14, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Shyp, V.; Tankov, S.; Ermakov, A.; Kudrin, P.; English, B.P.; Ehrenberg, M.; Tenson, T.; Elf, J.; Hauryliuk, V. Positive allosteric feedback regulation of the stringent response enzyme rela by its product. EMBO Rep. 2012, 13, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Faron, M.; Fletcher, J.R.; Rasmussen, J.A.; Long, M.E.; Allen, L.A.; Jones, B.D. The Francisella tularensis migR, trmE, and cphA Genes Contribute to F. Tularensis Pathogenicity Island Gene Regulation and Intracellular Growth by Modulation of the Stress Alarmone ppGpp. Infect. Immun. 2013, 81, 2800–2811. [Google Scholar] [CrossRef] [PubMed]
- Payoe, R.; Fahlman, R.P. Dependence of rela-mediated (p)ppGpp formation on tRNA identity. Biochemistry 2011, 50, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- English, B.P.; Hauryliuk, V.; Sanamrad, A.; Tankov, S.; Dekker, N.H.; Elf, J. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc. Natl. Acad. Sci. USA 2011, 108, E365–E373. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, T.M.; Blaha, G.; Wilson, D.N.; Marahiel, M.A.; Nierhaus, K.H. Dissection of the mechanism for the stringent factor rela. Mol. Cell 2002, 10, 779–788. [Google Scholar] [CrossRef]
- Artsimovitch, I.; Patlan, V.; Sekine, S.; Vassylyeva, M.N.; Hosaka, T.; Ochi, K.; Yokoyama, S.; Vassylyev, D.G. Structural basis for transcription regulation by alarmone ppGpp. Cell 2004, 117, 299–310. [Google Scholar] [CrossRef]
- Roberts, J.W. Promoter-specific control of E. coli RNA polymerase by ppGpp and a general transcription factor. Genes Dev. 2009, 23, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gardiol, N.; Burr, T.; Salmond, G.P.; Welch, M. Rela-dependent (p)ppGpp production controls exoenzyme synthesis in erwinia carotovora subsp. Atroseptica. J. Bacteriol. 2007, 189, 7643–7652. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Iida, K.; Shiota, S.; Nakayama, H.; Yoshida, S. Elevated guanosine 5'-diphosphate 3'-diphosphate level inhibits bacterial growth and interferes with ftsz assembly. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Milon, P.; Tischenko, E.; Tomsic, J.; Caserta, E.; Folkers, G.; la Teana, A.; Rodnina, M.V.; Pon, C.L.; Boelens, R.; Gualerzi, C.O. The nucleotide-binding site of bacterial translation initiation factor 2 (if2) as a metabolic sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13962–13967. [Google Scholar] [CrossRef] [PubMed]
- Mitkevich, V.A.; Ermakov, A.; Kulikova, A.A.; Tankov, S.; Shyp, V.; Soosaar, A.; Tenson, T.; Makarov, A.A.; Ehrenberg, M.; Hauryliuk, V. Thermodynamic characterization of ppGpp binding to ef-g or if2 and of initiator tRNA binding to free if2 in the presence of gdp, gtp, or ppGpp. J. Mol. Biol. 2010, 402, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; Ehrenberg, M.; Andersson, S.G.; Kurland, C.G. Ppgpp inhibition of elongation factors tu, g and ts during polypeptide synthesis. Mol. Gen Genet. 1984, 197, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M.; Johanson, U.; Ehrenberg, M.; Hughes, D. Fusidic acid-resistant ef-g perturbs the accumulation of ppGpp. Mol. Microbiol. 2000, 37, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, J. Magic spot: (p) ppGpp. J. Cell Physiol. 2009, 220, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Pingoud, A.; Block, W. The elongation factor tu. Guanosine tetraphosphate complex. Eur. J. Biochem. 1981, 116, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.C.; Spat, P.; Krug, K.; Macek, B. Global dynamics of the Escherichia coli proteome and phosphoproteome during growth in minimal medium. J. Proteome Res. 2013, 12, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Bilgin, N.; Lindschau, C.; Mesters, J.R.; Kraal, B.; Hilgenfeld, R.; Erdmann, V.A.; Lippmann, C. Phosphorylation of elongation factor tu prevents ternary complex formation. J. Biol. Chem. 1995, 270, 14541–14547. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.F.; Gonzalez, R.L., Jr.; Dworkin, J. Protein synthesis during cellular quiescence is inhibited by phosphorylation of a translational elongation factor. Proc. Natl. Acad. Sci. USA 2015, 112, E3274–E3281. [Google Scholar] [CrossRef] [PubMed]
- Absalon, C.; Obuchowski, M.; Madec, E.; Delattre, D.; Holland, I.B.; Seror, S.J. Cpga, ef-tu and the stressosome protein yezb are substrates of the ser/thr kinase/phosphatase couple, prkc/prpc, in bacillus subtilis. Microbiology 2009, 155, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Gooley, A.A.; Herbert, B.R.; Molloy, M.P.; Binz, P.A.; Ou, K.; Sanchez, J.C.; Bairoch, A.; Williams, K.L.; et al. High-throughput mass spectrometric discovery of protein post-translational modifications. J. Mol. Biol. 1999, 289, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, J.M.; Kraal, B.; Sinjorgo, K.M.; Persoon, N.L.; Johanns, E.S.; Bosch, L. Methylation in vivo of elongation factor ef-tu at lysine-56 decreases the rate of tRNA-dependent gtp hydrolysis. Eur. J. Biochem. 1986, 160, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.A. Charging levels of four tRNA species in Escherichia coli rel(+) and rel(−) strains during amino acid starvation: A simple model for the effect of ppGpp on translational accuracy. J. Mol. Biol. 2001, 307, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.A.; Sorensen, M.A.; Elf, J.; Ehrenberg, M.; Pan, T. Selective charging of tRNA isoacceptors induced by amino-acid starvation. EMBO Rep. 2005, 6, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Elf, J.; Nilsson, D.; Tenson, T.; Ehrenberg, M. Selective charging of tRNA isoacceptors explains patterns of codon usage. Science 2003, 300, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Zaslaver, A.; Mayo, A.E.; Rosenberg, R.; Bashkin, P.; Sberro, H.; Tsalyuk, M.; Surette, M.G.; Alon, U. Just-in-time transcription program in metabolic pathways. Nat. Genet. 2004, 36, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Sriram, S.M.; Park, K.S.; Kwon, Y.T. The N-end rule pathway. Annu. Rev. Biochem. 2012, 81, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Cha-Molstad, H.; Sung, K.S.; Hwang, J.; Kim, K.A.; Yu, J.E.; Yoo, Y.D.; Jang, J.M.; Han, D.H.; Molstad, M.; Kim, J.G.; et al. Amino-terminal arginylation targets endoplasmic reticulum chaperone bip for autophagy through p62 binding. Nat. Cell Biol. 2015, 17, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Goitea, V.E.; Hallak, M.E. Calreticulin and arginylated calreticulin have different susceptibilities to proteasomal degradation. J. Biol. Chem. 2015, 290, 16403–16414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Patel, D.M.; Colavita, K.; Rodionova, I.; Buckley, B.; Scott, D.A.; Kumar, A.; Shabalina, S.A.; Saha, S.; Chernov, M.; et al. Arginylation regulates purine nucleotide biosynthesis by enhancing the activity of phosphoribosyl pyrophosphate synthase. Nat. Commun. 2015, 6, 7517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Saha, S.; Shabalina, S.A.; Kashina, A. Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science 2010, 329, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.A.; Decca, M.B.; Lopez Sambrooks, C.; Durand, E.S.; Montich, G.G.; Hallak, M.E. Calreticulin-dimerization induced by post-translational arginylation is critical for stress granules scaffolding. Int. J. Biochem. Cell Biol. 2013, 45, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Lopez Sambrooks, C.; Carpio, M.A.; Hallak, M.E. Arginylated calreticulin at plasma membrane increases susceptibility of cells to apoptosis. J. Biol. Chem. 2012, 287, 22043–22054. [Google Scholar] [CrossRef] [PubMed]
- Eriste, E.; Norberg, A.; Nepomuceno, D.; Kuei, C.; Kamme, F.; Tran, D.T.; Strupat, K.; Jornvall, H.; Liu, C.; Lovenberg, T.W.; et al. A novel form of neurotensin post-translationally modified by arginylation. J. Biol. Chem. 2005, 280, 35089–35097. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, X.; Wong, C.C.; Cheng, H.; Aslanian, A.; Xu, T.; Leavis, P.; Roder, H.; Hedstrom, L.; Yates, J.R., 3rd; et al. Arginyltransferase ate1 catalyzes midchain arginylation of proteins at side chain carboxylates in vivo. Chem. Biol. 2014, 21, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ebhardt, H.A. Applying arginylation for bottom-up proteomics. Methods Mol. Biol. 2015, 1337, 129–138. [Google Scholar] [PubMed]
- Ebhardt, H.A.; Nan, J.; Chaulk, S.G.; Fahlman, R.P.; Aebersold, R. Enzymatic generation of peptides flanked by basic amino acids to obtain ms/ms spectra with 2x sequence coverage. Rapid Commun. Mass Spectrom 2014, 28, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Barciszewska, M.Z.; Perrigue, P.M.; Barciszewski, J. tRNA-the golden standard in molecular biology. Mol. Biosyst. 2016, 12, 12–17. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, A.W.S.; Payoe, R.; Fahlman, R.P. Perspectives and Insights into the Competition for Aminoacyl-tRNAs between the Translational Machinery and for tRNA Dependent Non-Ribosomal Peptide Bond Formation. Life 2016, 6, 2. https://doi.org/10.3390/life6010002
Fung AWS, Payoe R, Fahlman RP. Perspectives and Insights into the Competition for Aminoacyl-tRNAs between the Translational Machinery and for tRNA Dependent Non-Ribosomal Peptide Bond Formation. Life. 2016; 6(1):2. https://doi.org/10.3390/life6010002
Chicago/Turabian StyleFung, Angela W. S., Roshani Payoe, and Richard P. Fahlman. 2016. "Perspectives and Insights into the Competition for Aminoacyl-tRNAs between the Translational Machinery and for tRNA Dependent Non-Ribosomal Peptide Bond Formation" Life 6, no. 1: 2. https://doi.org/10.3390/life6010002




