Use of Sodium Hexametaphosphate and Citric Acid Mixture as Depressant in the Flotation Separation of Scheelite from Calcite
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
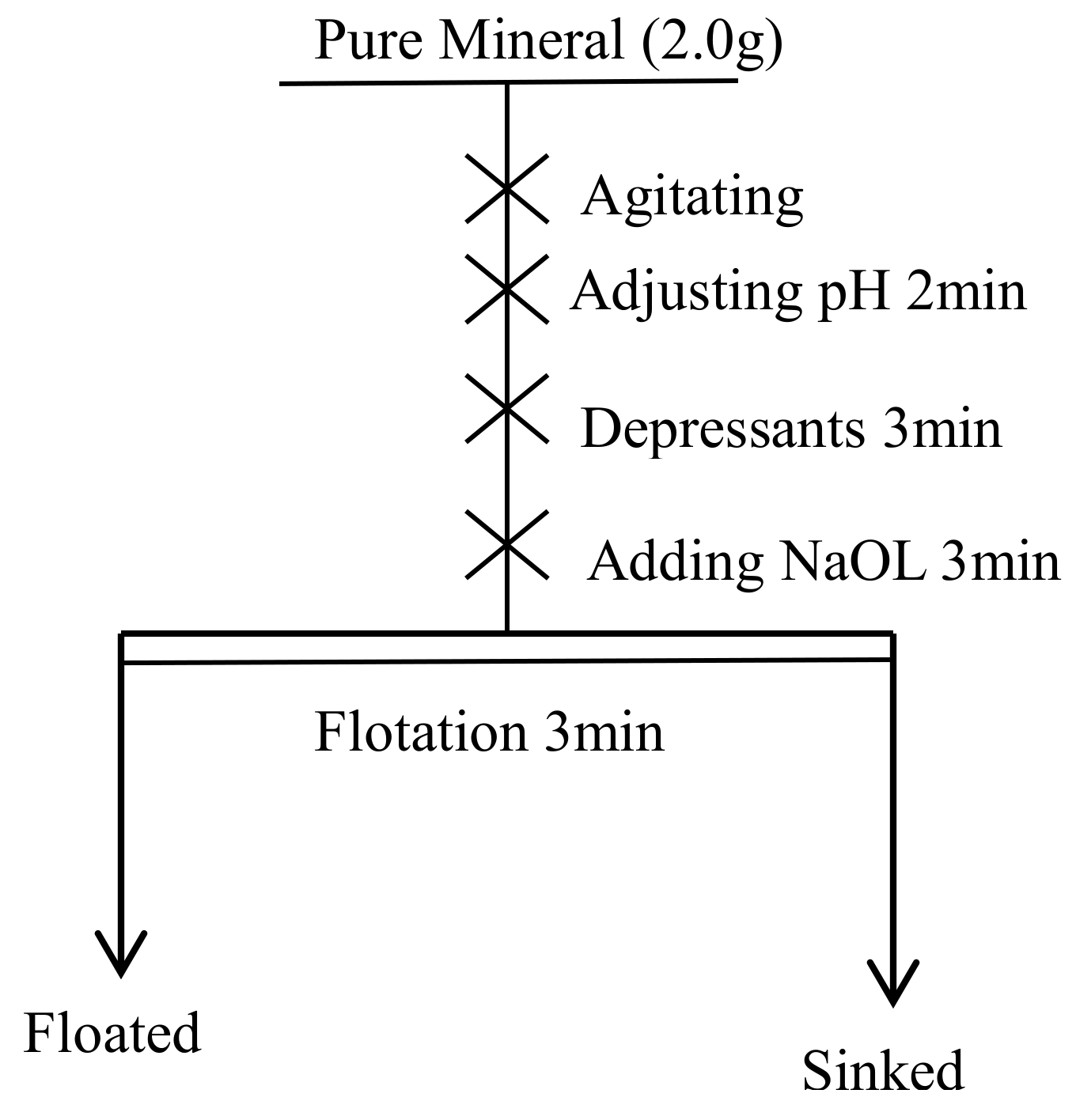
2.2.1. Microflotation
2.2.2. Zeta Potential Analysis
2.2.3. XPS Analysis
3. Results and Discussion
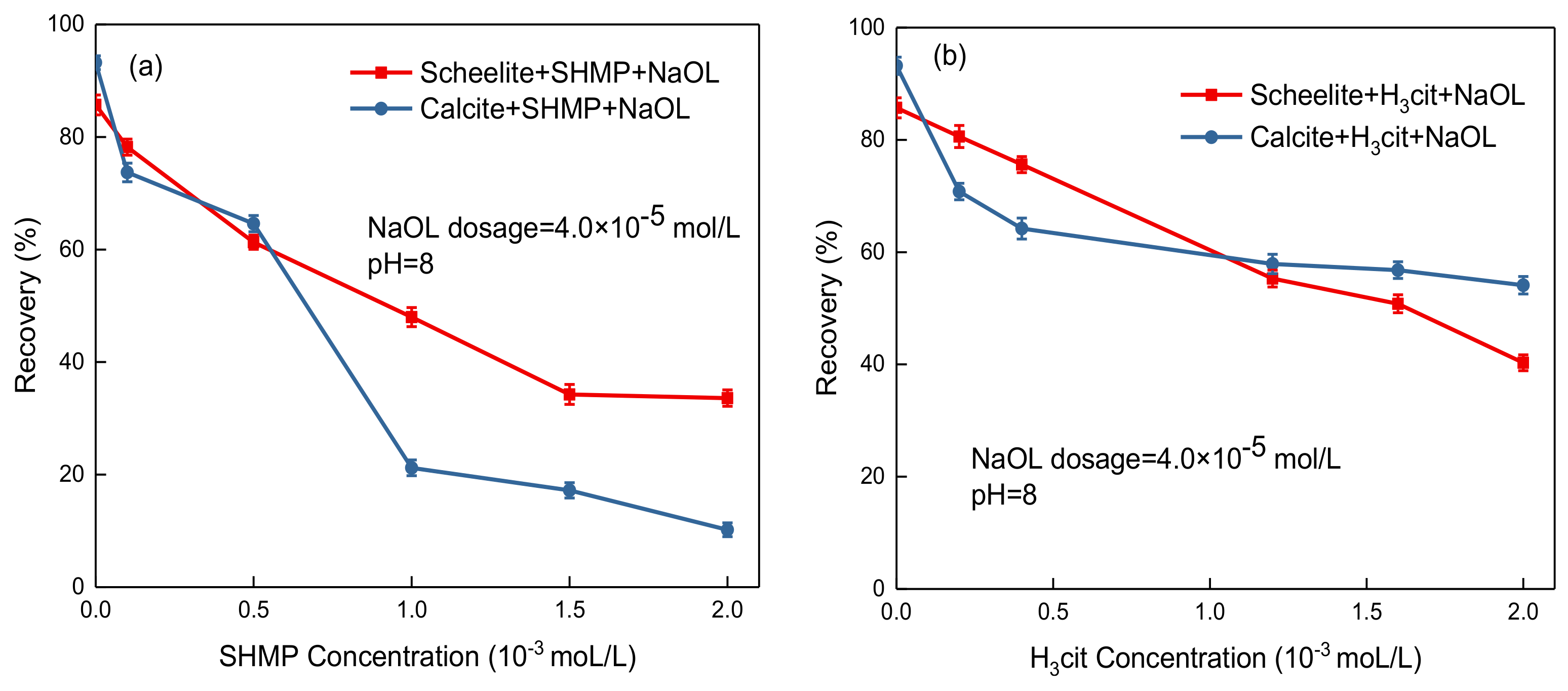
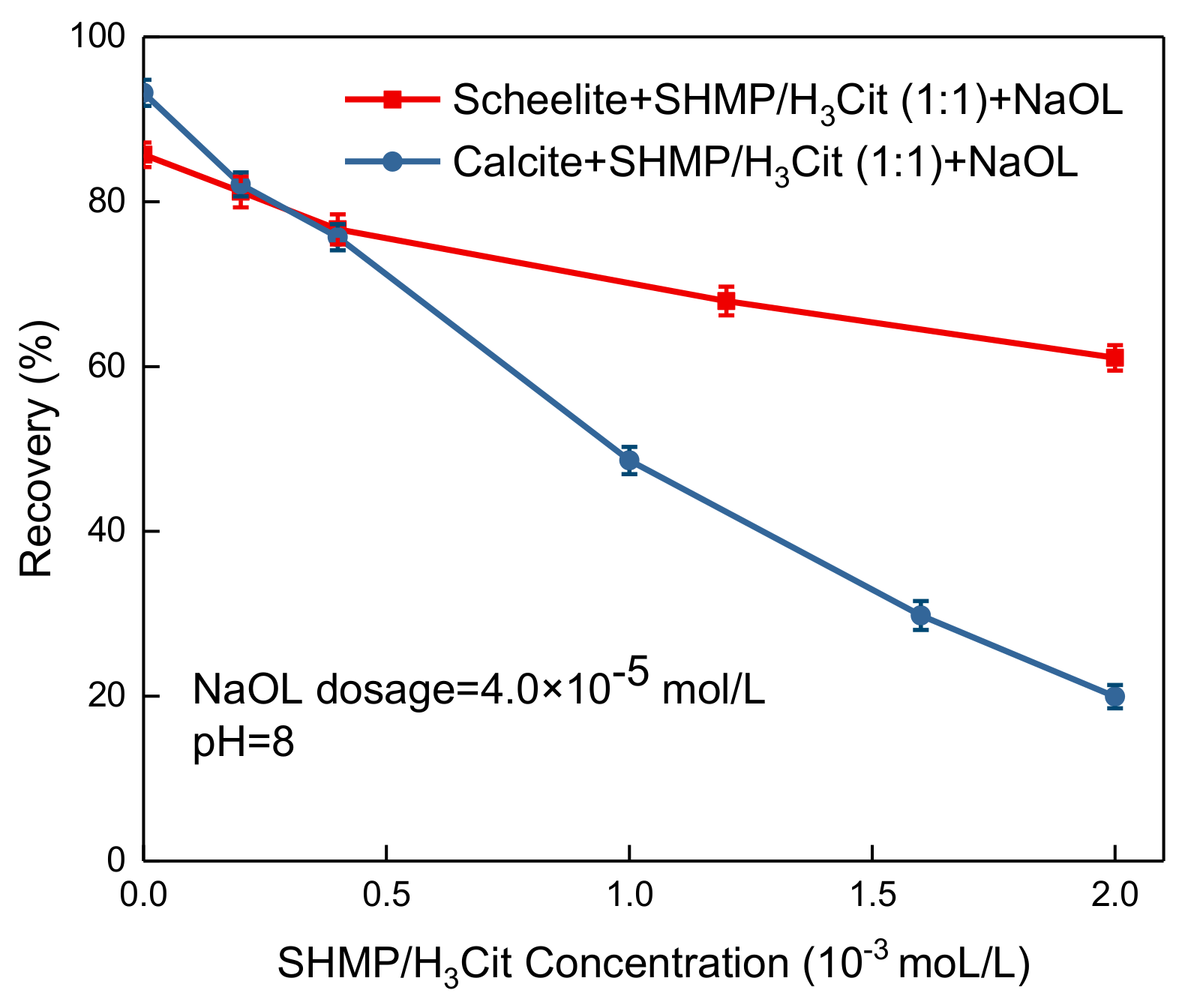
3.1. Microflotation
3.2. Solution Chemistry Analysis
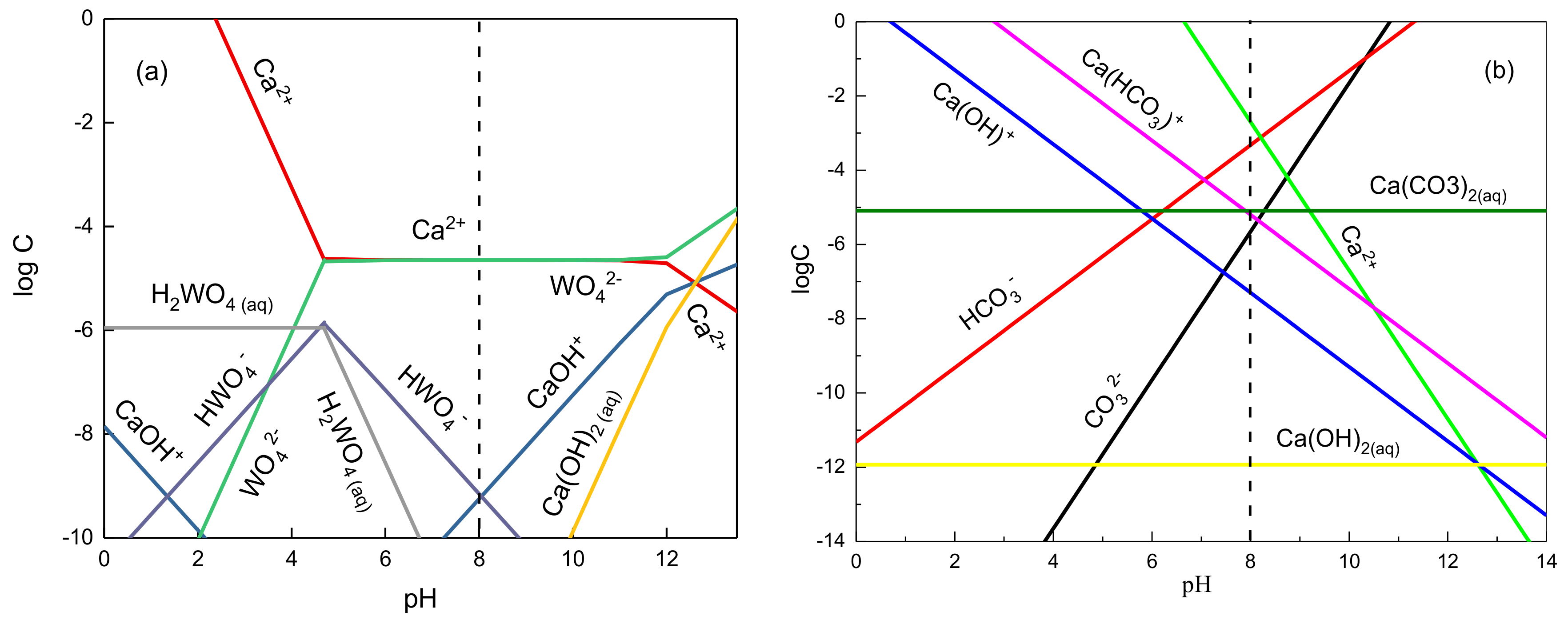
3.2.1. Solution Chemical Calculation of Dissolved Components of Minerals
3.2.2. Solution Chemical Calculation of Components of SHMP and H3Cit Solution
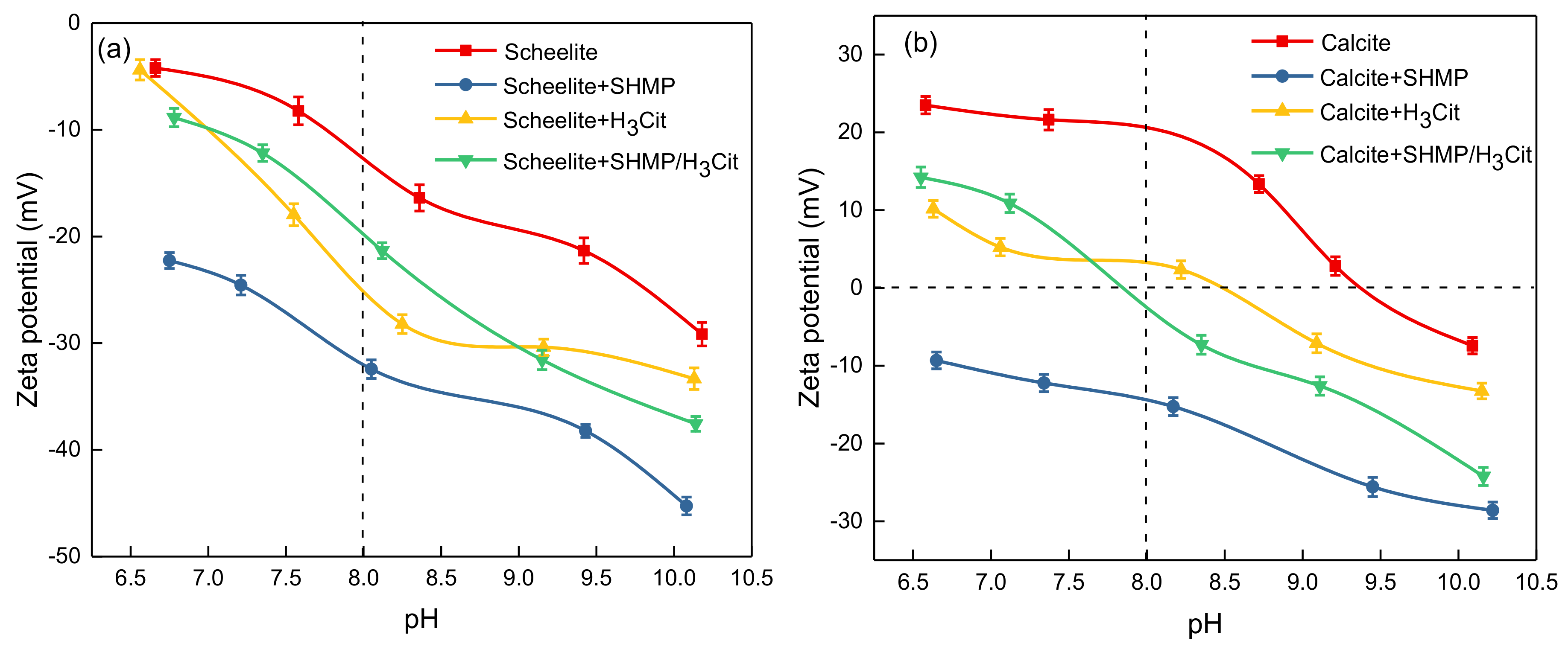
3.3. Zeta Potential Analysis
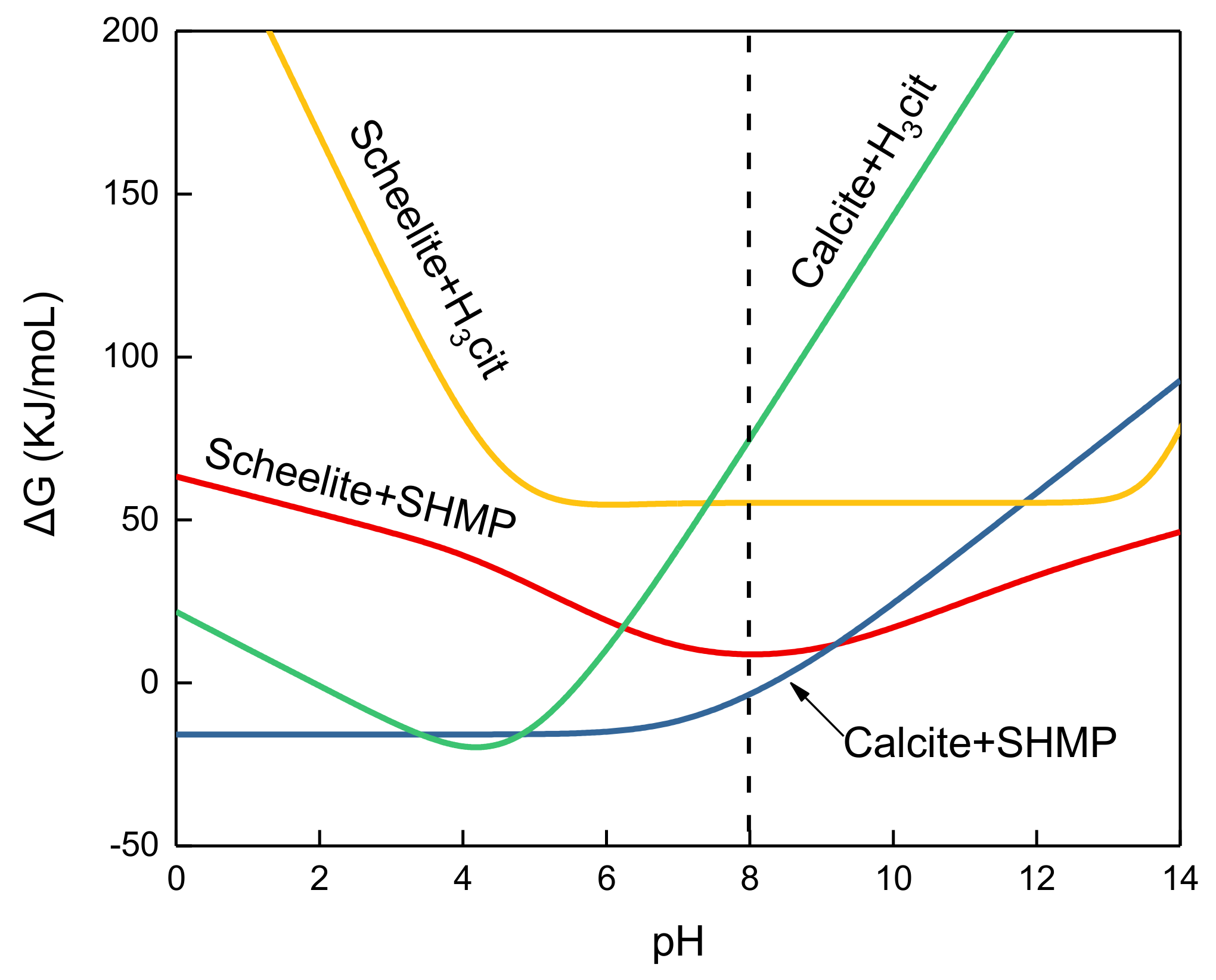
3.4. Thermodynamic Analyses
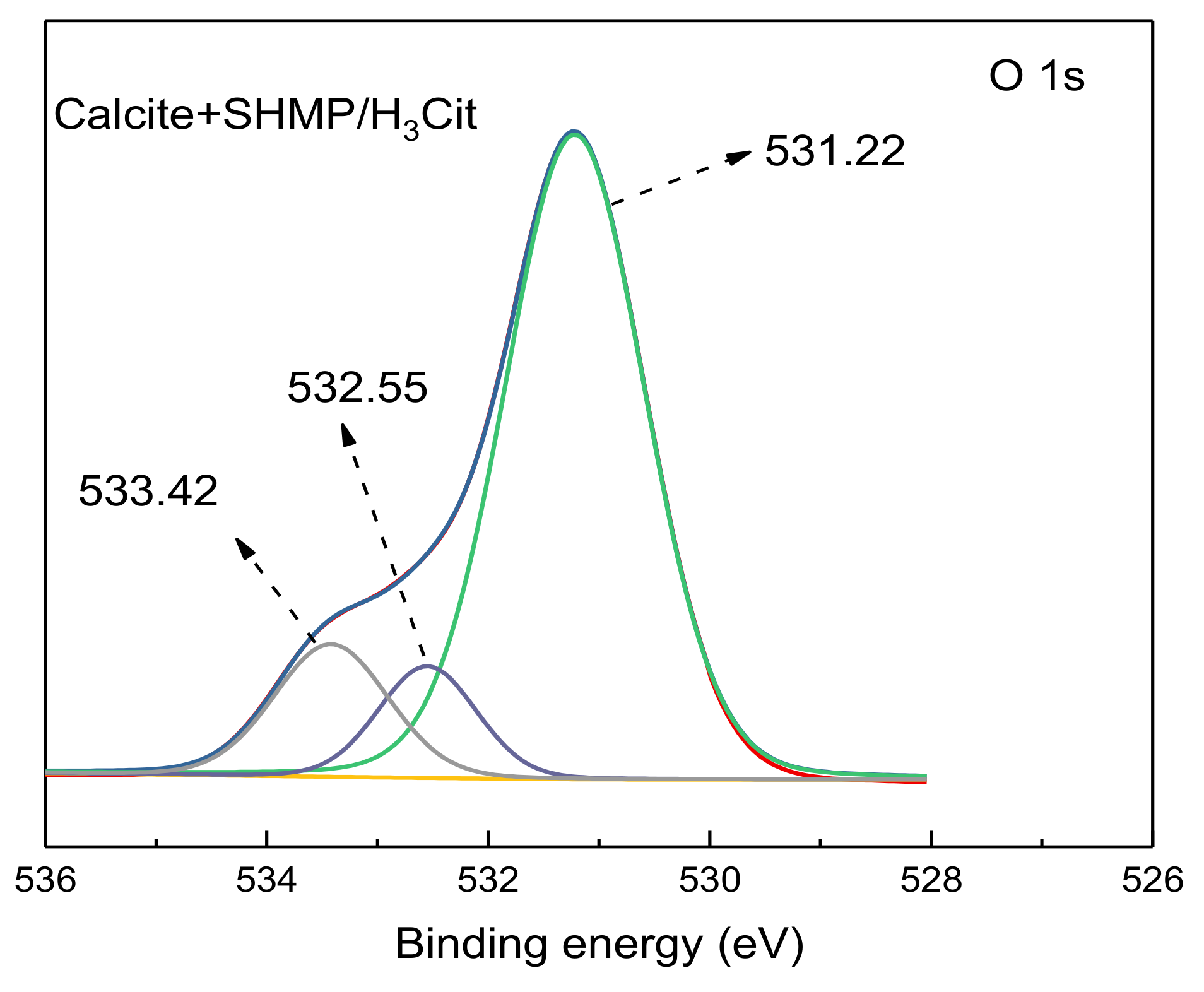
3.5. XPS Analysis
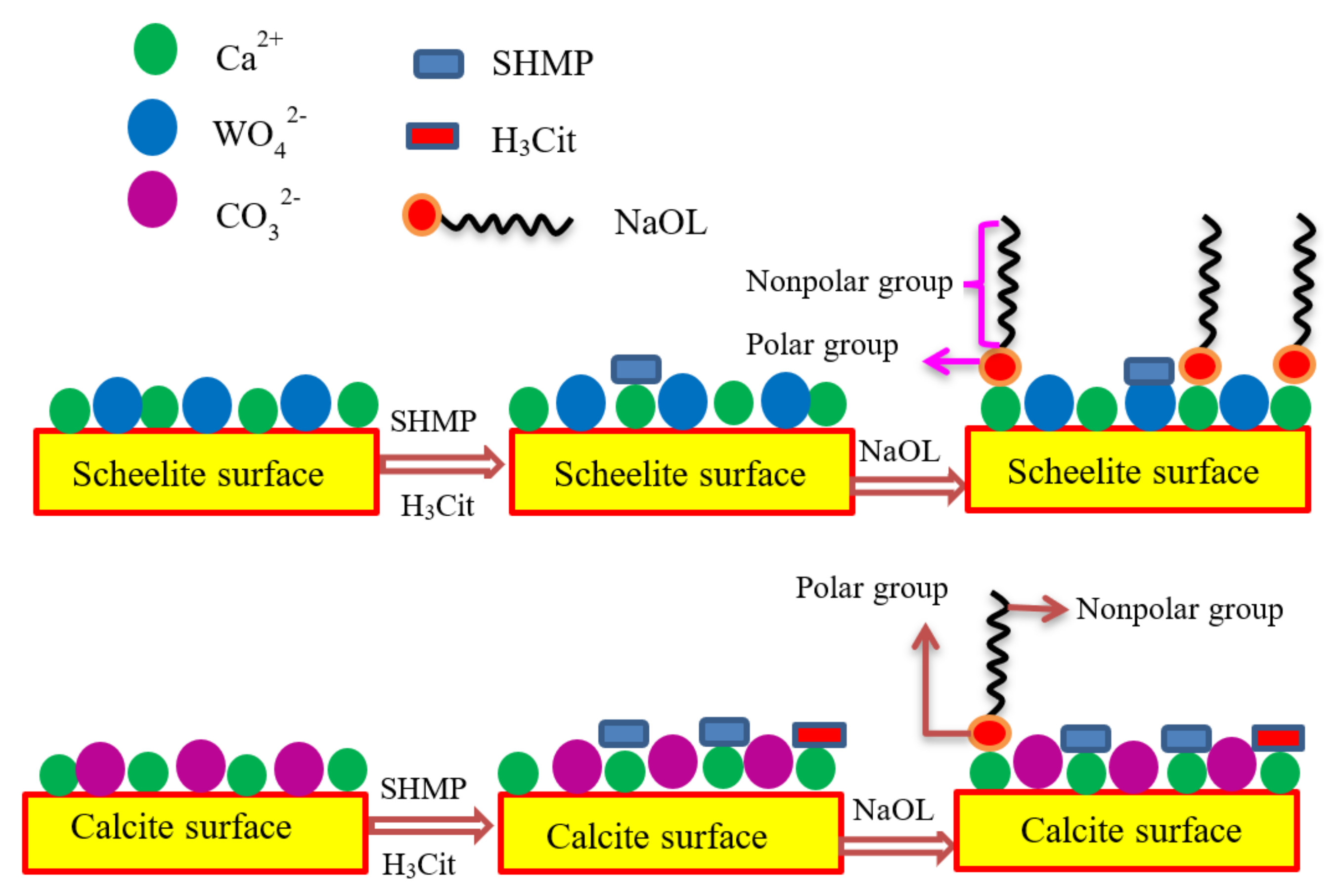
3.6. Adsorption Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Ai, G. Effects of surface electrical property and solution chemistry on fine wolframite flotation. Sep. Purif. Technol. 2016, 170, 272–279. [Google Scholar] [CrossRef]
- Singh, H.; Pandey, O.P. Novel process for synthesis of nanocrystalline WC from wolframite ore. Ceram. Int. 2015, 41, 10481–10487. [Google Scholar] [CrossRef]
- Harlaux, M.; Mercadier, J.; Marignac, C.; Peiffert, C.; Cloquet, C.; Cuney, M. Tracing metal sources in peribatholitic hydrothermal W deposits based on the chemical composition of wolframite: The example of the Variscan French Massif Central. Chem. Geol. 2018, 479, 58–85. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Andrés, J.; Gracia, L.; de Oliveira, M.S.M.P.; Mercury, J.M.R.; Longo, E.; Nogueira, I.C. Geometry, electronic structure, morphology, and photoluminescence emissions of BaW1-xMoxO4 (x = 0, 0.25, 0.50, 0.75, and 1) solid solutions: Theory and experiment in concert. Appl. Surf. Sci. 2019, 463, 907–917. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Tune surface physicochemical property of fluorite particles by regulating the exposure degree of crystal surfaces. Miner. Eng. 2018, 128, 123–132. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2019, 463, 105–114. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Fang, S.; Lu, Z.; Ma, C.; Sun, W.; Hu, Y. Anisotropic surface chemistry properties and adsorption behavior of silicate mineral crystals. Adv. Colloid Interface Sci. 2018, 256, 340–351. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The flotation and adsorption of mixed collectors on oxide and silicate minerals. Adv. Colloid Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.; Ahmad, S.; Pownceby, M.I.; Bruckard, W.J.; Harjanto, S. Selective sulphidation of impurities in weathered ilmenite. Part 1—Applicability to different ilmenite deposits and simulated Becher kiln conditions. Miner. Eng. 2018, 121, 55–65. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Effect of acid surface dissolution pretreatment on the selective flotation of ilmenite from olivine and pyroxene. Int. J. Miner. Process. 2017, 167, 49–60. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Medeiros, A.R.S.D.; Baltar, C.A.M. Importance of collector chain length in flotation of fine particles. Miner. Eng. 2018, 122, 179–184. [Google Scholar] [CrossRef]
- Abarca, C.; Ali, M.M.; Pelton, R.H. Choosing mineral flotation collectors from large nanoparticle libraries. J. Colloid Interface Sci. 2018, 516, 423–430. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Hu, Y.; Tang, H.; Yin, Z.; Guan, Q.; Gao, J. Investigation of two-stage depressing by using hydrophilic polymer to improve the process of fluorite flotation. J. Clean. Prod. 2018, 193, 228–235. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Peng, J.; Ning, X.; Wang, H. Synergistic effect of acidified water glass and locust bean gum in the flotation of a refractory copper sulfide ore. J. Clean. Prod. 2018, 202, 1077–1084. [Google Scholar] [CrossRef]
- Liu, J.; Li, E.-L.; Jiang, K.; Li, Y.-J.; Han, Y.-X. Effect of acidic activators on the flotation of oxidized pyrrhotite. Miner. Eng. 2018, 120, 75–79. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H. A novel method to improve carboxymethyl cellulose performance in the flotation of talc. Miner. Eng. 2019, 131, 23–27. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- Bo, F.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2017, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, R.; Li, Y.; Wang, Y.; Luo, X. Flotation separation of scheelite from calcite using sodium polyacrylate as depressant. Physicochem. Probl. Miner. Process. 2018, 54, 505–516. [Google Scholar]
- Wang, J.; Bai, J.; Yin, W.; Liang, X. Flotation separation of scheelite from calcite using carboxyl methyl cellulose as depressant. Miner. Eng. 2018, 127, 329–333. [Google Scholar] [CrossRef]
- Power, O.M.; Fenelon, M.A.; O’Mahony, J.A.; McCarthy, N.A. Dephosphorylation of caseins in milk protein concentrate alters their interactions with sodium hexametaphosphate. Food Chem. 2019, 271, 136–141. [Google Scholar] [CrossRef]
- Asamoah, R.K.; Skinner, W.; Addai-Mensah, J. Leaching behaviour of mechano-chemically activated bio-oxidised refractory flotation gold concentrates. Powder Technol. 2018, 331, 258–269. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Zhou, J.; Chen, J. Interactions between kaolinite Al OH surface and sodium hexametaphosphate. Appl. Surf. Sci. 2016, 387, 759–765. [Google Scholar] [CrossRef]
- Neves, J.G.; Danelon, M.; Pessan, J.P.; Figueiredo, L.R.; Camargo, E.R.; Delbem, A.C.B. Surface free energy of enamel treated with sodium hexametaphosphate, calcium and phosphate. Arch. Oral Biol. 2018, 90, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Zhang, T.; Peng, Y.; Xie, G. Inhibiting heterocoagulation between microcrystalline graphite and quartz by pH modification and sodium hexametaphosphate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 149–154. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, C. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]
- Li, Z.-H.; Han, Y.-X.; Li, Y.-J.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferr. Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Li, W.; Li, Y. Improved understanding of chalcopyrite flotation in seawater using sodium hexametaphosphate. Miner. Eng. 2019, 134, 269–274. [Google Scholar] [CrossRef]
- Cumming, M.H.; Leonard, A.R.; Lecorre-Bordes, D.S.; Hofman, K. Intra-fibrillar citric acid crosslinking of marine collagen electrospun nanofibres. Int. J. Biol. Macromol. 2018, 114, 874–881. [Google Scholar] [CrossRef]
- Nataraj, D.; Sakkara, S.; Meenakshi, H.N.; Reddy, N. Properties and applications of citric acid crosslinked banana fibre-wheat gluten films. Ind. Crops Prod. 2018, 124, 265–272. [Google Scholar] [CrossRef]
- Galvão, A.C.; Robazza, W.S.; Arce, P.F.; Capello, C.; Hagemann, D.H. Experimental study and modeling of citric acid solubility in alcohol mixtures. J. Food Eng. 2018, 237, 96–102. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Yuan, Z.; Liu, J. Comparative effects of sodium silicate and citric acid on the dispersion and flotation of carbonate-bearing iron ore. J. Mol. Liq. 2018, 271, 16–23. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Use of citric acid and Fe(III) mixture as depressant in calcite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123579. [Google Scholar] [CrossRef]
- Wang, Y.; Khoso, S.A.; Luo, X.; Tian, M. Understanding the depression mechanism of citric acid in sodium oleate flotation of Ca2+-activated quartz: Experimental and DFT study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Liu, X.; Luo, H.; Cheng, R.; Li, C.; Zhang, J. Effect of citric acid and flotation performance of combined depressant on collophanite ore. Miner. Eng. 2017, 109, 162–168. [Google Scholar] [CrossRef]
- Xia, L.; Hart, B. The role of citric acid in the flotation separation of rare earth from the silicates. Miner. Eng. 2015, 74, 123–129. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, L.; Tian, J.; Yin, W.; Yang, Y.; Deng, W. Effect of a CA depressant on flotation separation of celestite from fluorite and calcite using SDS as a collector. Miner. Eng. 2017, 111, 201–208. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Jiao, F.; Jia, W. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Abdalla, M.A.M.; Peng, H.; Younus, H.A.; Wu, D.; Abusin, L.; Shao, H. Effect of synthesized mustard soap on the scheelite surface during flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 548, 108–116. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G.-Y.; Li, C.-X.; Cheng, R.-J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]





| Depressant | Products | Ratio (%) | Scheelite Grade (%) | Scheelite Recoveries (%) |
|---|---|---|---|---|
| H3Cit | Concentrates | 65.35 | 52.33 | 68.40 |
| Tailing | 34.65 | 45.60 | 31.60 | |
| Feed | 100.00 | 50.00 | 100.00 | |
| SHMP/H3Cit | Concentrates | 62.15 | 62.19 | 77.3 |
| Tailing | 37.85 | 29.98 | 22.7 | |
| Feed | 100.00 | 50.00 | 100.00 |
| Reactions | Reaction Constants | |
|---|---|---|
| (1) | ||
| (2) | ||
| (3) | ||
| (4) | ||
| (5) | ||
| (6) | ||
| (7) | ||
| log C | pH < 4.7 | pH = 4.7–13.72 | pH < 13.72 |
|---|---|---|---|
| 4.75-2 pH | 22.78-2 pH | ||
| −7.85-pH | −12.6+pH + | 10.18-pH | |
| −20.48 | −25.23+2pH + | −2.45 | |
| [] | −14.05 + 2 pH | 13.48 + 2pH | |
| −10.55 + pH | 3.5-pH + | 16.98 + pH | |
| [ | −5.95 | 8.1-2pH + |
| Reactions | Reaction Constants | |
|---|---|---|
| (12) | ||
| (13) | ||
| (14) | ||
| (15) | ||
| (16) | ||
| (17) | ||
| Reactions | Reaction Constants | |
|---|---|---|
| (18) | ||
| (19) | ||
| (20) | ||
| (21) | ||
| (22) | ||
| (23) | ||
| Reactions | Reaction Constants | |
|---|---|---|
| (34) | ||
| (35) | ||
| (36) | ||
| (37) | ||
| Reactions | Reaction Constants | |
|---|---|---|
| (44) | ||
| (45) | ||
| (46) | ||
| (47) | ||
| Samples. | Relative Content (%) | |||
|---|---|---|---|---|
| Ca 2p | C 1s | O 1s | P 2p | |
| Scheelite | 13.61 | 32.77 | 53.62 | - |
| Scheelite + H3Cit | 12.86 | 33.09 | 54.05 | - |
| Scheelite + SHMP | 12.23 | 31.65 | 53.36 | 2.76 |
| Scheelite + SHMP/H3Cit | 12.70 | 32.05 | 53.09 | 2.16 |
| DS-H3Cit | −0.75 | +0.32 | +0.43 | - |
| DS-SHMP | −1.38 | −1.12 | −0.26 | +2.76 |
| DS-SHMP/H3Cit | −0.91 | −0.72 | −0.53 | +2.16 |
| Calcite | 15.43 | 37.97 | 46.60 | - |
| Calcite + H3Cit | 14.94 | 38.06 | 47.00 | - |
| Calcite + SHMP | 14.01 | 36.67 | 46.32 | 3.00 |
| Calcite + H3Cit/SHMP | 10.66 | 38.15 | 47.07 | 4.12 |
| Dc-H3Cit | −0.49 | +0.09 | +0.40 | - |
| Dc-SHMP | −1.42 | −1.30 | −0.28 | +3.00 |
| Dc-SHMP/H3Cit | −4.77 | +0.18 | +0.47 | +4.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Dong, L.; Jiao, F.; Qin, W.; Wei, Q. Use of Sodium Hexametaphosphate and Citric Acid Mixture as Depressant in the Flotation Separation of Scheelite from Calcite. Minerals 2019, 9, 560. https://doi.org/10.3390/min9090560
Zhu W, Dong L, Jiao F, Qin W, Wei Q. Use of Sodium Hexametaphosphate and Citric Acid Mixture as Depressant in the Flotation Separation of Scheelite from Calcite. Minerals. 2019; 9(9):560. https://doi.org/10.3390/min9090560
Chicago/Turabian StyleZhu, Wenlong, Liuyang Dong, Fen Jiao, Wenqing Qin, and Qian Wei. 2019. "Use of Sodium Hexametaphosphate and Citric Acid Mixture as Depressant in the Flotation Separation of Scheelite from Calcite" Minerals 9, no. 9: 560. https://doi.org/10.3390/min9090560





