Radioactivity of Five Typical General Industrial Solid Wastes and its Influence in Solid Waste Recycling
Abstract
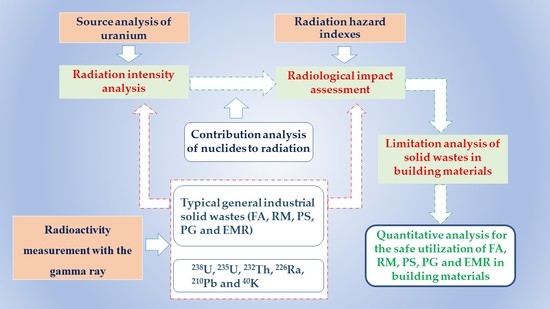
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
2.2.1. Radioactivity Measurement
2.2.2. Internal and External Illumination Index
2.2.3. Radium Equivalent Activity
2.2.4. Indoor External Dose (Din) and Outdoor External Dose (Dout)
2.2.5. Annual Effective Dose Rate
2.2.6. Maximum Dosage of Solid Waste in Building Materials
3. Results and Discussion
3.1. Activity Concentration
3.2. The Source Analysis of Uranium
3.3. Radiological Impact Assessment
3.3.1. Analysis of Radiation Hazard Indexes
3.3.2. Contribution Analysis of Nuclides to Radiation
3.3.3. Limitation Analysis of Solid Wastes in Building Materials
4. Conclusions
- (1)
- 40K, 226Ra, 232Th, and 238U are the main nuclides in FA, RM, and PS. 210Pb and 226Ra are the main nuclides in PG, while 40K is the main nuclide in EMR, EMR-Na, and EMR-Ca. The uranium contents of all samples are all from natural uranium.
- (2)
- The values of IRa and Iγ were all less than 1 except for FA (IRa > 1 and Iγ > 1.3) and RM (IRa > 2 and Iγ > 2), and the values of Raeq, Din, Dout, Ein, and Eout were higher than the world’s recommended values (i.e., 370 Bq/kg, 84 nGy/h, 59 nGy/h, 0.4 mSv/y, and 0.07 mSv/y, respectively) for PS, RM, and FA due to higher concentrations of 226Ra and 232Th.
- (3)
- PG, EMR, EMR-Na, and EMR-Ca could be used for building materials unlimitedly. However, RM, FA, and PS could be used as additive or auxiliary materials for building materials by means of doping and mixing, with maximum portions of 75.44%, 29.72%, and 66.01%, respectively. These findings provide a basis for the restriction of the aforementioned solid wastes in Guizhou in the field of building materials. Further research on the phase analysis and treatment of known nuclides in solid wastes are necessary in the future.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.H.; Wang, X.K.; Lu, F.Z. Study progress of alkali removal from red mud and novel functional materials (in Chinese). Chin. J. Environ. Eng. 2016, 10, 3383–3390. [Google Scholar]
- 2017 Annual Report on Prevention and Control of Solid Waste in China’s Large and Medium-Sized Cities (In Chinese); Environmental Protection: Beijing, China, 2018; pp. 90–106.
- Matiullah; Ahad, A.; Rehman, S.U.; Rehman, S.U.; Faheem, M. Measurement of radioactivity in the soil of Bahawalpur division, Pakistan. Radiat. Prot. Dosim. 2004, 112, 443. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Singh, S.; Singh, K.; Sonkawade, R. 226Ra, 232Th and 40K analysis in soil samples from some areas of Malwa region, Punjab, India using gamma ray spectrometry. Environ. Monit. Assess. 2007, 134, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Matiullah; Khatibeh, A.J.A.H.; Ma’Ly, A.; Kenawy, M.A. Measurement of natural radioactivity in Jordanian sand. Radiat. Meas. 1997, 28, 341–344. [Google Scholar] [CrossRef]
- Khatibeh, A.J.A.H.; Ahmad, N.; Matiullah; Kenawy, M.A. Natural radioactivity in marble stones- Jordan. Radiat. Meas. 1997, 28, 345–348. [Google Scholar] [CrossRef]
- Bruzzi, L.; Baroni, M.; Mele, R.; Nanni, E. Proposal for a method of certification of natural radioactivity in building materials. J. Radiol. Prot. 1997, 17, 85. [Google Scholar] [CrossRef]
- Chiozzi, P.; De, F.P.; Fazio, A.; Pasquale, V.V.; Verdoya, M. Laboratory application of NaI (Tl) gamma-ray spectrometry to studies of natural radioactivity in geophysics. Appl. Radiat. Isot. 2000, 53, 127–132. [Google Scholar] [CrossRef]
- Turhan, S. Assessment of the natural radioactivity and radiological hazards in Turkish cement and its raw materials. J. Environ. Radioact. 2008, 99, 404–414. [Google Scholar] [CrossRef]
- Gupta, M.; Mahur, A.K.; Varshney, R.; Sonkawade, R.G.; Verma, K.D.; Prasad, R. Measurement of natural radioactivity and radon exhalation rate in fly ash samples from a thermal power plant and estimation of radiation doses. Radiat. Meas. 2013, 50, 160–165. [Google Scholar] [CrossRef]
- Turhan, Ş.; Parmaksız, A.; Köse, A.; Yüksel, A.; Arıkan, İ.H.; Yücel, B. Radiological characteristics of pulverized fly ashes produced in Turkish coal-burning thermal power plants. Fuel 2010, 89, 3892–3900. [Google Scholar] [CrossRef]
- Kardos, R.; Sas, Z.; Shahrokhi, A.; Somlai, J.; Kovács, T. Radionuclide content of NORM by-products originating from the coal-fired power plant in Oroszlány (Hungary). Radiat. Prot. Dosim. 2015, 167, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, R.; Leonardi, F.; Risica, S.; Nuccetelli, C. Updated database on natural radioactivity in building materials in Europe. J. Environ. Radioact. 2018, 187, 90–105. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X. Radionuclide content and associated radiation hazards of building materials and by-products in Baoji, West China. Radiat. Prot. Dosim. 2007, 128, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.T.; Lu, X.W. Natural radioactivity, radon exhalation rate and radiation dose of fly ash used as building materials in Xiangyang, China. Indoor Built Environ. 2016, 25, 626–634. [Google Scholar] [CrossRef]
- Cooper, M.B.; Clarke, P.C.; Robertson, W.; Mcpharlin, I.R.; Jeffrey, R.C. An investigation of radionuclide uptake into food crops grown in soils treated with bauxite mining residues. J. Radioanal. Nuclear Chem. 1995, 194, 379–387. [Google Scholar] [CrossRef]
- Pinnock, W.R. Measurements of radioactivity in Jamaican building materials and gamma dose equivalents in a prototype red mud house. Health Phys. 1991, 61, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Gezer, F.; Turhan, Ş.; Uğur, F.A.; Gören, E.; Kurt, M.Z.; Ufuktepe, Y. Natural radionuclide content of disposed phosphogypsum as TENORM produced from phosphorus fertilizer industry in Turkey. Ann. Nuclear Energy 2012, 50, 33–37. [Google Scholar] [CrossRef]
- Alam, M.N.; Chowdhury, M.I.; Kamal, M.; Ghose, S.; Banu, H.; Chakraborty, D. Radioactivity in chemical fertilizers used in Bangladesh. Appl. Radiat. Isot. 1997, 48, 1165–1168. [Google Scholar] [CrossRef]
- Mourad, N.M.; Sharshar, T.; Elnimr, T.; Mousa, M.A. Radioactivity and fluoride contamination derived from a phosphate fertilizer plant in Egypt. Appl. Radiat. Isot. 2009, 67, 1259–1268. [Google Scholar] [CrossRef]
- Al-Jundi, J.; Al-Ahmad, N.; Shehadeh, H.; Afaneh, F.; Maghrabi, M.; Gerstmann, U.; Hã Llriegl, V.; Oeh, U. Investigations on the activity concentrations of 238U, 226Ra, 228Ra, 210Pb and 40K in Jordan phosphogypsum and fertilizers. Radiat. Prot. Dosim. 2008, 131, 449–454. [Google Scholar] [CrossRef]
- Song, M.H.; Chang, B.U.; Koh, S.M.; Kim, Y.J.; Kim, D.J.K.G. Overall natural radioactivity of a phosphate fertilizer industry in Korea. Radioprotection 2012, 46, S113–S118. [Google Scholar] [CrossRef]
- Kovler, K.; Haquin, G.; Manasherov, V.; Ne’Eman, E.; Lavi, N. Natural radionuclides in building materials available in Israel. Build. Environ. 2002, 37, 531–537. [Google Scholar] [CrossRef]
- Dueñas, C.; Fernández, M.C.; Cañete, S.; Pérez, M. Radiological impacts of natural radioactivity from phosphogypsum piles in Huelva (Spain). Radiat. Meas. 2010, 45, 242–246. [Google Scholar] [CrossRef]
- Msila, X.; Labuschagne, F.; Barnard, W.; Billing, D.G. Radioactive nuclides in phosphogypsum from the lowveld region of South Africa. S. Afr. J. Sci. 2016, 112, 1–5. [Google Scholar] [CrossRef]
- Kovács, T.; Shahrokhi, A.; Sas, Z.; Vigh, T.; Somlai, J. Radon exhalation study of manganese clay residue and usability in brick production. J. Environ. Radioact. 2017, 168, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhou, C.; Dan, Z.; Luan, Z.; Duan, N. Preparation and characteristics of steam-autoclaved bricks produced from electrolytic manganese solid waste. Constr. Build. Mater. 2014, 50, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Taoufiq, L.; Laamyem, A.; Boukhair, A.; Essediqi, E.; Monkade, M.; Zrabda, A. Radiological assessment of wastewater treatment processes based on the use of coal ashes as a filters. J. Radiat. Res. Appl. Sci. 2018. [Google Scholar] [CrossRef]
- NBSC. 2013 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2013; pp. 278–279. ISBN 9787503769634.
- NBSC. 2014 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2014; pp. 227–228. ISBN 9787503772801.
- NBSC. 2015 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2015; pp. 251–252. ISBN 9787503776380.
- NBSC. 2016 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2016; pp. 247–248. ISBN 9787503779176.
- NBSC. 2017 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2017; pp. 241–242. ISBN 978-7-5037-8253-4.
- NBSC. 2018 China Statistical Yearbook (in Chinese); China Statistics Press: Beijing, China, 2018; pp. 250–251. ISBN 9787503785870.
- Li, X.; Ye, J.; Liu, Z.; Qiu, Y.; Li, L.; Mao, S.; Wang, X.; Zhang, Q. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution. J. Cent. South Univ. 2018, 25, 9–20. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ke, B.; Wang, X.; Li, L.; Li, X.; Mao, S. Insight into the effect of maleic acid on the preparation of α-hemihydrate gypsum from phosphogypsum in Na2SO4 solution. J. Cryst. Growth 2018, 493, 34–40. [Google Scholar] [CrossRef]
- Croymans, T.; Vandael Schreurs, I.; Hult, M.; Marissens, G.; Lutter, G.; Stroh, H.; Schreurs, S.; Schroeyers, W. Variation of natural radionuclides in non-ferrous fayalite slags during a one-month production period. J. Environ. Radioact. 2017, 172, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Hegedűs, M.; Tóth-Bodrogi, E.; Jónás, J.; Somlai, J.; Kovács, T. Mobility of 232Th and 210Po in red mud. J. Environ. Radioact. 2018, 184–185, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.G.; Silva, P.S.C.; Mazzilli, B.P.; Fávaro, D.I.T. Radiological characterisation of disposed phosphogypsum in Brazil: Evaluation of the occupational exposure and environmental impact. Radiat. Prot. Dosim. 2006, 121, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lauer, N.E.; Hower, J.C.; Hsu-Kim, H.; Taggart, R.K.; Vengosh, A. Naturally occurring radioactive materials in coals and coal combustion residuals in the united states. Environ. Sci. Technol. 2015, 49, 11227–11233. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, Y.Y. Use of Gamma-Ray spectrometry for uranium isotopic analysis in environmental samples. Rom. J. Phys. 2010, 55, 69–74. [Google Scholar]
- Ebaid, Y.Y.; El-Mongy, S.A.; Allam, K.A. 235U-γemission contribution to the 186 keV energy transition of 226Ra in environmental samples activity calculations. Int. Congr. Ser. 2005, 1276, 409–411. [Google Scholar] [CrossRef]
- AQAIQ; SAC. Limits of Radionuclides in Building Materials GB 6566-2010 (In Chinese); AQSIQ, SAC: Beijing, China, 2010; pp. 1–3.
- Lu, X.; Chao, S.; Yang, F. Determination of natural radioactivity and associated radiation hazard in building materials used in Weinan, China. Radiat. Phys. Chem. 2014, 99, 62–67. [Google Scholar] [CrossRef]
- Hamilton, E.I. The relative radioactivity of building materials. Am. Ind. Hyg. Assoc. J. 1971, 32, 398. [Google Scholar] [CrossRef]
- Matthew, P.J. Natural radioactivity of Australian building materials, industrial wastes and by-products. Health Phys. 1985, 48, 87. [Google Scholar] [CrossRef]
- Stojanovska, Z.; Nedelkovski, D.; Ristova, M. Natural radioactivity and human exposure by raw materials and end product from cement industry used as building materials. Radiat. Meas. 2010, 45, 969–972. [Google Scholar] [CrossRef]
- NEA-OECD. Exposure to Radiation from the Natural Radioactivity in Building Materials: Report by a Group of Exports of the OECD Nuclear Energy Agency; NEA-OECD: Paris, France, 1979; pp. 13–19. [Google Scholar]
- Yu, K.N.; Guan, Z.J.; Stokes, M.J.; Young, E.C.M. The assessment of the natural radiation dose committed to the Hong Kong people. J. Environ. Radioact. 1992, 17, 31–48. [Google Scholar] [CrossRef]
- Hayumbu, P.; Zaman, M.B.; Lubaba, N.C.H.; Munsanje, S.S.; Muleya, D. Natural radioactivity in Zambian building materials collected from Lusaka. J. Radioanal. Nuclear Chem. 1995, 199, 229–238. [Google Scholar] [CrossRef]
- European Commission. Radiological protection principles concerning the natural radioactivity of building materials. Brussels. Radiat. Prot. 1999, 112, 5–16. [Google Scholar]
- He, Z.Y.; Luo, G.Z.; Huang, J.J. National survey on natural radioactivity level of environment (1983–1990) (in Chinese). Radiat. Prot. 1992, 12, 81–95. [Google Scholar]
- Quindos, L.S.; Fernández, P.L.; Ródenas, C.; Gómez-Arozamena, J.; Arteche, J. Conversion factors for external gamma dose derived from natural radionuclides in soils. J. Environ. Radioact. 2004, 71, 139. [Google Scholar] [CrossRef]
- Uosif, M.A.; Eltaher, A. Radiological assessment of Abu-Tartur phosphate, Western Desert Egypt. Radiat. Prot. Dosim. 2008, 130, 228–235. [Google Scholar] [CrossRef]
- Billa, J.; Han, F.; Didla, S.; Ankrah, M.; Yu, H.; Dimpah, J.; Brempong, O.; Adzanu, S. Evaluation of radioactivity levels in fertilizers commonly used in the Southern USA. J. Radioanal. Nuclear Chem. 2015, 306, 183–191. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes; UNSCEAR: New York, NY, USA, 2000; ISBN 9211422388. [Google Scholar]
- Uosif, M.A.M.; Mostafa, A.M.A.; Elsaman, R.; Moustafa, E.S. Natural radioactivity levels and radiological hazards indices of chemical fertilizers commonly used in Upper Egypt. J. Radiat. Res. Appl. Sci. 2014, 7, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Ivanovich, M.; Harmon, R.S. Uranium-Series Disequilibrium: Applications to Earth, Marine, and Environmental Sciences, 2nd ed.; Clarendon Press and Oxford University Press: Oxford, UK, 1992; p. 571. ISBN 019854278X. [Google Scholar]
- El-Bahi, S.M.; Sroor, A.; Mohamed, G.Y.; El-Gendy, N.S. Radiological impact of natural radioactivity in Egyptian phosphate rocks, phosphogypsum and phosphate fertilizers. Appl. Radiat. Isot. 2017, 123, 121–127. [Google Scholar] [CrossRef]
- Megumi, K.; Oka, T.; Yaskawa, K.; Sakanoue, M. Contents of natural radioactive nuclides in soil in relation to their surface area. JGR Solid Earth 1982, 87, 10857–10860. [Google Scholar] [CrossRef]
- Karangelos, D.J.; Anagnostakis, M.J.; Hinis, E.P.; Simopoulos, S.E.; Zunic, Z.S. Determination of depleted uranium in environmental samples by gamma-spectroscopic techniques. J. Environ. Radioact. 2004, 76, 295–310. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation, V. I: UNSCEAR 2008 Report to the General Assembly with Scientific Annexes; United Nations Publication: New York, NY, USA, 2010; ISBN 978-92-1-142274-0. [Google Scholar]
- Lederer, J.; Trinkel, V.; Fellner, J. Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: The case of Austria. Waste Manag. 2017, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Del Valle-Zermeño, R.; Formosa, J.; Chimenos, J.M.; Martínez, M.; Fernández, A.I. Aggregate material formulated with MSWI bottom ash and APC fly ash for use as secondary building material. Waste Manag. 2013, 33, 621–627. [Google Scholar] [CrossRef] [Green Version]
- García Arenas, C.; Marrero, M.; Leiva, C.; Solís-Guzmán, J.; Vilches Arenas, L.F. High fire resistance in blocks containing coal combustion fly ashes and bottom ash. Waste Manag. 2011, 31, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.R.; Huang, C.; Kuo, J.; Lin, S. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Labrincha, J.A.; Morelli, M.R. Effect of the addition of red mud on the corrosion parameters of reinforced concrete. Cem. Concr. Res. 2012, 42, 124–133. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Labrincha, J.A. Effect of red mud addition on the rheological behaviour and on hardened state characteristics of cement mortars. Constr. Build. Mater. 2011, 25, 163–170. [Google Scholar] [CrossRef]


| Country | Sample Type | Activity Concentration (Bq/kg) | Reference | ||
|---|---|---|---|---|---|
| 226Ra | 232Th | 40K | |||
| India | FA | 119 | 147 | 352 | [10] |
| Turkey | 360 | 102 | 517 | [11] | |
| Hungary | 178 | 55 | 387 | [12] | |
| Greece | 815 | 56 | 400 | [13] | |
| Czech Rep. | 146 | 86 | 669 | [13] | |
| Germany | 164 | 94 | 517 | [13] | |
| Italy | 170 | 140 | 400 | [13] | |
| Poland | 200 | 118 | 798 | [13] | |
| Romania | 219 | 116 | 595 | [13] | |
| China (Baoji) | 112 | 148 | 386 | [14] | |
| China (Xiangyang) | 441 | 110 | 510 | [15] | |
| Turkey | RM | 210 | 539 | 112 | [13] |
| Hungary | 301 | 295 | 50 | [13] | |
| Greece | 244 | 364 | 57 | [13] | |
| Germany | 171 | 318 | 215 | [13] | |
| Italy | 97 | 118 | 115 | [13] | |
| Australia | 310 | 1350 | 350 | [16] | |
| Jamaica | 1047 | 350 | 335 | [17] | |
| Turkey | PG | 436 | 9 | 13 | [18] |
| Bangladesh | 234 | 21 | 108 | [19] | |
| Egypt | 596 | 6 | 2 | [20] | |
| Jordan | 376 | 4 | 40 | [21] | |
| Korea | 618 | 9 | 24 | [22] | |
| Israel | 747 | 14 | 63 | [23] | |
| Spain | 647 | 8 | 33 | [24] | |
| South Africa | 109 | 189 | >100 | [25] | |
| Hungary | EMR | 52 | 40 | 607 | [26] |
| China (Chongqing) | 37 | 58 | 631 | [27] | |
| Year | China | Guizhou | Reference |
|---|---|---|---|
| 2012 | 3.290 | 0.078 | [29] |
| 2013 | 3.277 | 0.082 | [30] |
| 2014 | 3.256 | 0.074 | [31] |
| 2015 | 3.271 | 0.071 | [32] |
| 2016 | 3.092 | 0.078 | [33] |
| 2017 | 3.316 | 0.094 | [34] |
| Component | Concentration (%) | ||||||
|---|---|---|---|---|---|---|---|
| PS | RM | FA | PG | EMR | EMR-Na | EMR-Ca | |
| CaO | 44.77 | 14.35 | 2.83 | 34.07 | 7.32 | 9.1 | 15.43 |
| Fe2O3 | 0.45 | 21.53 | 2.83 | 0.20 | - | - | - |
| Al2O3 | 5.55 | 20.89 | 14.75 | 0.13 | 8.85 | 11.27 | 7.99 |
| SiO2 | 37.81 | 16.75 | 45.72 | 5.29 | 22.85 | 30.29 | 20.35 |
| MgO | 2.61 | 1.55 | 1.18 | 0.01 | 1.86 | 2.42 | 1.80 |
| P2O5 | 2.86 | 0.31 | - | 0.75 | - | - | - |
| TiO2 | - | 4.59 | 1.74 | - | 0.25 | 0.33 | 0.21 |
| K2O | 1.43 | 0.98 | 1.24 | - | 1.75 | 2.22 | 1.66 |
| Na2O | 0.33 | 4.93 | 0.55 | - | 0.14 | 0.42 | 0.12 |
| SO3 | - | 1.19 | - | 40.24 | - | - | - |
| Mn | - | - | - | - | 4.92 | 5.57 | 6.25 |
| S | - | - | - | - | 8.19 | 3.2 | 7.08 |
| Others | 0.04 | 12.04 | 17.03 | 19.31 | 2.88 | 3.75 | 2.18 |
| Name of Solid Wastes | Activity Concentration (Bq/kg) | |||||
|---|---|---|---|---|---|---|
| 40K | 210Pb | 226Ra | 232Th | 235U | 238U | |
| PS | 461.0 ± 23.6 | 15.1 ± 9.9 | 187.4 ± 5.8 | 233.7 ± 9.6 | 9.1 ± 0.3 | 199.8 ± 17.9 |
| RM | 259.5 ± 18.9 | 324. 8 ± 18.1 | 462.7 ± 14.5 | 457.7 ± 13.4 | 22.4 ± 0.7 | 513.0 ± 43.1 |
| FA | 529.4 ± 31.1 | 210.5 ± 11.8 | 208.2 ± 7.0 | 165.6 ± 5.6 | 10.1 ± 0.3 | 234.9 ± 27.2 |
| PG | 3.3 ± 6.0 | 86.0 ± 4.8 | 61.0 ± 2.1 | 2.3 ± 0.5 | 2.7 ± 0.1 | 28.0 ± 13.0 |
| EMR | 443.8 ± 26.7 | 34.3 ± 2.3 | 26.6 ± 1.6 | 24.8 ± 1.7 | 1.6 ± 0.1 | 48.3 ± 15.9 |
| EMR-Na | 423.9 ± 25.9 | 45.9 ± 2.9 | 24.5 ± 1.6 | 22.2 ± 1.5 | 1.5 ± 0.1 | 37.9 ± 15.7 |
| EMR-Ca | 321.4 ± 20.9 | 37.7 ± 2.5 | 21.1 ± 1.5 | 20.9 ± 1.6 | 1.3 ± 0.1 | 39.8 ± 15.8 |
| Name of Solid Wastes | 238U/235U | 238U/226Ra |
|---|---|---|
| PS | 21.96 | 1.07 |
| RM | 22.90 | 1.11 |
| FA | 23.26 | 1.13 |
| PG | 10.37 | 0.46 |
| EMR | 30.19 | 1.82 |
| EMR-Na | 25.27 | 1.54 |
| EMR-Ca | 30.62 | 1.88 |
| Solid Wastes | IRa (Bq/kg) | Iγ (Bq/kg) | Raeq (Bq/kg) | Din (nGy/h) | Dout (nGy/h) | Ein (mSv/y) | Eout (mSv/y) |
|---|---|---|---|---|---|---|---|
| PS | 0.94 | 0.71 | 557.03 | 404.67 | 246.93 | 1.98 | 0.30 |
| RM | 2.31 | 3.07 | 1137.18 | 888.07 | 501.03 | 4.36 | 0.61 |
| FA | 1.04 | 1.33 | 485.78 | 354.49 | 218.29 | 1.74 | 0.27 |
| PG | 0.30 | 0.17 | 64.54 | −3.19 * | 29.70 | −0.02 * | 0.04 |
| EMR | 0.13 | 0.27 | 96.23 | 25.60 | 45.77 | 0.13 | 0.06 |
| EMR-Na | 0.12 | 0.25 | 88.99 | 19.28 | 42.45 | 0.09 | 0.05 |
| EMR-Ca | 0.11 | 0.21 | 75.78 | 6.38 | 35.80 | 0.03 | 0.04 |
| Standard Name | Building Materials | Decoration Materials | |||
|---|---|---|---|---|---|
| Hollow Rate ≤ 25% | Hollow Rate > 25% | Class A | Class B | Class C | |
| Radionuclide limit | ≤ 1.0 | ≤ 1.0 | ≤ 1.0 | ≤ 1.3 | ≤ 2.8 |
| ≤ 1.0 | ≤ 1.3 | ≤ 1.3 | ≤ 1.9 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Zhang, Q.; Cheng, W.; Chen, Q. Radioactivity of Five Typical General Industrial Solid Wastes and its Influence in Solid Waste Recycling. Minerals 2019, 9, 168. https://doi.org/10.3390/min9030168
Shen Z, Zhang Q, Cheng W, Chen Q. Radioactivity of Five Typical General Industrial Solid Wastes and its Influence in Solid Waste Recycling. Minerals. 2019; 9(3):168. https://doi.org/10.3390/min9030168
Chicago/Turabian StyleShen, Zhihui, Qin Zhang, Wei Cheng, and Qianlin Chen. 2019. "Radioactivity of Five Typical General Industrial Solid Wastes and its Influence in Solid Waste Recycling" Minerals 9, no. 3: 168. https://doi.org/10.3390/min9030168