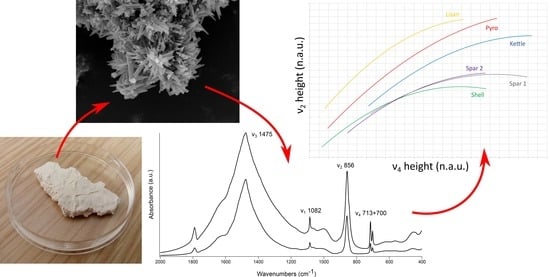
FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Reference Materials
2.2. Density Separation
2.3. Scanning Electron Microscopy (SEM)
2.4. X-ray Diffraction (XRD)
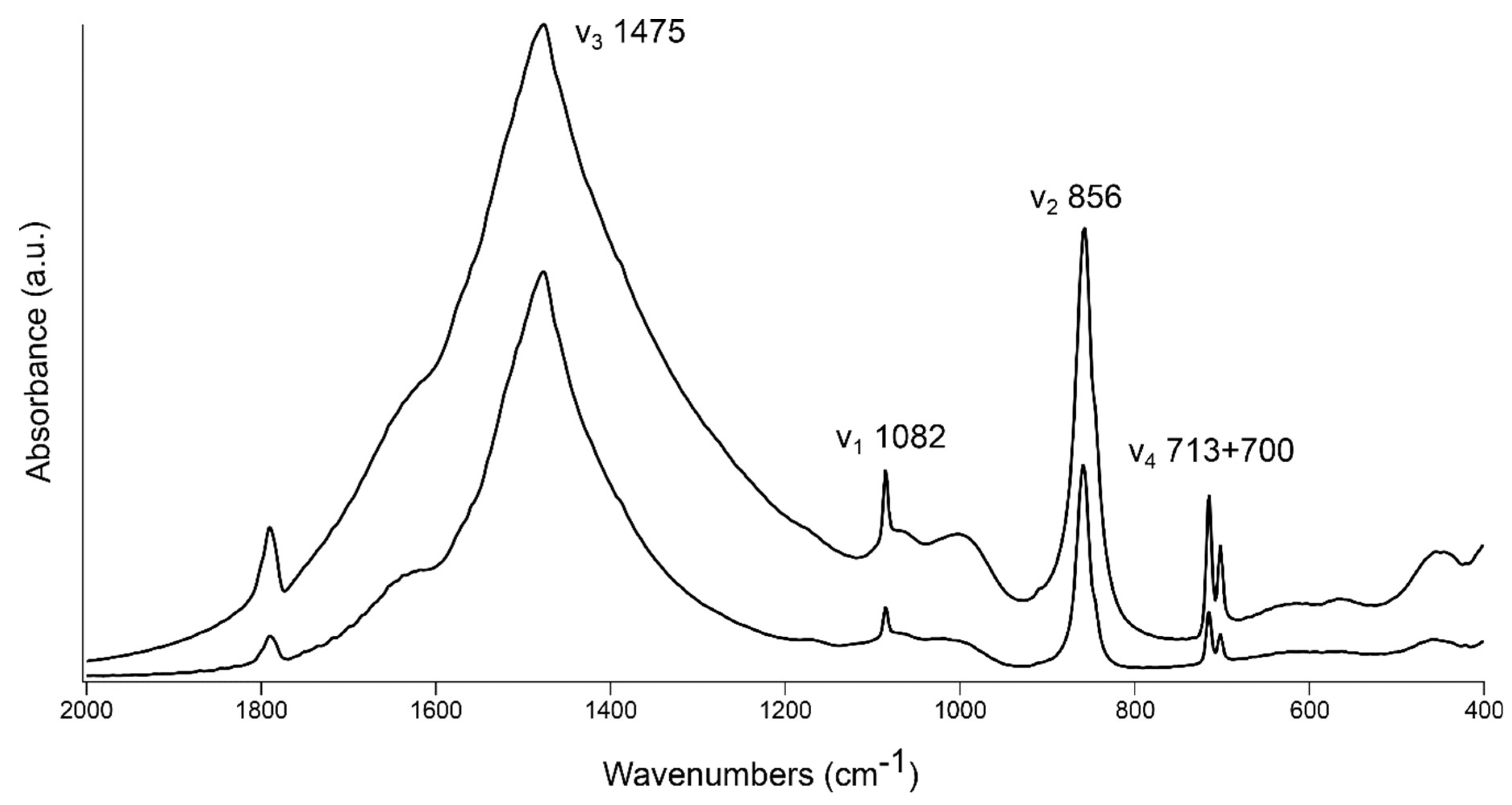
2.5. Fourier Transform Infrared Spectrometry (FTIR)
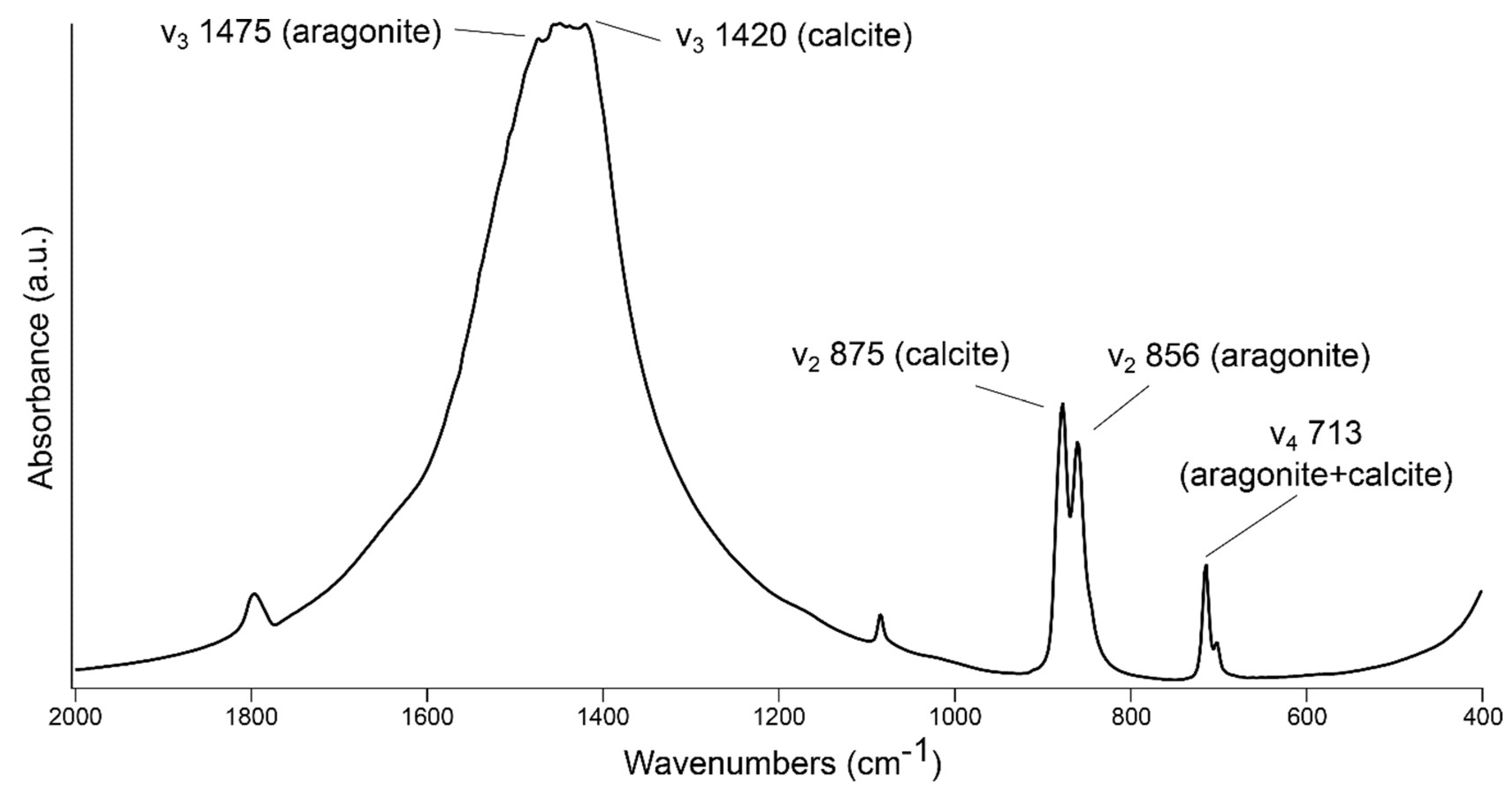
3. Results and Discussion
3.1. FTIR Grinding Curves
3.2. Aragonite in Heat-Altered Sediments
3.3. Aragonite–Calcite Mixtures in Lime Binders
3.4. Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boynton, R.S. Chemistry and Technology of Lime and Limestone; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]
- Artioli, G. Scientific Methods and Cultural Heritage: An Introduction to the Application of Materials Science to Archaeometry and Conservation Science; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Weiner, S. Microarchaeology: Beyond the Visible Archaeological Record; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Pöllmann, H. Cementitious Materials: Composition, Properties, Application; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Kingery, W.D.; Vandiver, P.B.; Prickett, M. The beginnings of pyrotechnology, part II: Production and use of lime and gypsum plaster in the Pre-Pottery Neolithic Near East. J. Field Archaeol. 1988, 15, 219–244. [Google Scholar] [CrossRef]
- Berna, F.; Goldberg, P.; Kolska Horwitz, L.; Brink, J.; Holt, S.; Bamford, M.; Chazan, M. Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc. Natl. Acad. Sci. USA 2012, 109, E1215–E1220. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E. Nanolimes: From synthesis to application. Pure Appl. Chem. 2018, 90, 523–550. [Google Scholar] [CrossRef]
- Papayianni, I.; Pachta, V.; Stefanidou, M. Analysis of ancient mortars and design of compatible repair mortars: The case study of Odeion of the archaeological site of Dion. Constr. Build. Mater. 2013, 40, 84–92. [Google Scholar] [CrossRef]
- Izzo, F.; Arizzi, A.; Cappelletti, P.; Cultrone, G.; De Bonis, A.; Germinario, C.; Graziano, S.F.; Grifa, C.; Guarino, V.; Mercurio, M.; et al. The art of building in the Roman period (89 BC-79 AD): Mortars, plasters and mosaic floors from ancient Stabiae (Naples, Italy). Constr. Build. Mater. 2016, 117, 129–143. [Google Scholar] [CrossRef]
- Secco, M.; Dilaria, S.; Addis, A.; Bonetto, J.; Artioli, G.; Salvadori, M. The evolution of the Vitruvian recipes over 500 years of floor-making techniques: The case studies of the Domus delle Bestie Ferite and the Domus di Tito Macro (Aquileia, Italy). Archaeometry 2018, 60, 185–206. [Google Scholar] [CrossRef]
- Lippmann, F. Sedimentary Carbonate Minerals; Springer: Heidelberg, Germany, 1973. [Google Scholar]
- Toffolo, M.B.; Boaretto, E. Nucleation of aragonite upon carbonation of calcium oxide and calcium hydroxide at ambient temperatures and pressures: A new indicator of fire-related human activities. J. Archaeol. Sci. 2014, 49, 237–248. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Pokroy, B.; Fitch, A.; Zolotoyabko, E. The microstructure of biogenic calcite: A view by high-resolution synchrotron powder diffraction. Adv. Mater. 2006, 18, 2363–2368. [Google Scholar] [CrossRef]
- Pokroy, B.; Quintana, J.P.; Caspi, E.N.; Berner, A.; Zolotoyabko, E. Anisotropic lattice distortions in biogenic aragonite. Nat. Mater. 2004, 3, 900–902. [Google Scholar] [CrossRef]
- Politi, Y.; Metzler, R.A.; Abrecht, M.; Gilbert, B.; Wilt, F.H.; Sagi, I.; Addadi, L.; Weiner, S.; Gilbert, P.U.P.A. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc. Natl. Acad. Sci. USA 2008, 105, 17362–17366. [Google Scholar] [CrossRef] [Green Version]
- Zolotoyabko, E.; Caspi, E.N.; Fieramosca, J.S.; Von Dreele, R.B.; Marin, F.; Mor, G.; Addadi, L.; Weiner, S.; Politi, Y. Differences between bond lengths in biogenic and geological calcite. Cryst. Growth Des. 2010, 10, 1207–1214. [Google Scholar] [CrossRef]
- Gómez-Villalba, L.S.; López-Arce, P.; Álvarez de Buergo, M.; Fort, R. Atomic defects and their relationship to aragonite-calcite transformation in portlandite nanocrystal carbonation. Cryst. Growth Des. 2012, 12, 4844–4852. [Google Scholar] [CrossRef]
- Regev, L.; Poduska, K.M.; Addadi, L.; Weiner, S.; Boaretto, E. Distinguishing between calcites formed by different mechanisms using infrared spectrometry: Archaeological applications. J. Archaeol. Sci. 2010, 37, 3022–3029. [Google Scholar] [CrossRef]
- Suzuki, M.; Dauphin, Y.; Addadi, L.; Weiner, S. Atomic order of aragonite crystals formed by mollusks. CrystEngComm 2011, 13, 6780–6786. [Google Scholar] [CrossRef]
- Poduska, K.M.; Regev, L.; Boaretto, E.; Addadi, L.; Weiner, S.; Kronik, L.; Curtarolo, S. Decoupling local disorder and optical effects in infrared spectra: Differentiating between calcites with different origins. Adv. Mater. 2011, 23, 550–554. [Google Scholar] [CrossRef]
- Khalifa, G.M.; Weiner, S.; Addadi, L. Mineral and matrix components of the operculum and shell of the barnacle Balanus amphitrite: Calcite crystal growth in a hydrogel. Cryst. Growth Des. 2011, 11, 5122–5130. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Ganesan, K.; Yang, P.; Kulak, A.N.; Borukhin, S.; Pechook, S.; Ribeiro, L.; Kröger, R.; Eichhorn, S.J.; Armes, S.P.; et al. An artificial biomineral formed by incorporation of copolymer micelles in calcite crystals. Nat. Mater. 2011, 10, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, F.; Buccianti, A.; Montegrossi, G.; Innocenti, M.; Massa, C.A.; Pardi, L.A.; Romanelli, M. Epr discrimination of microcrystalline calcite geomaterials. Am. Mineral. 2012, 97, 1619–1626. [Google Scholar] [CrossRef]
- Bonacini, I.; Prati, S.; Mazzeo, R.; Falini, G. Crystallization of CaCO3 in the presence of ethanolamine reveals transient meso-like crystals. Cryst. Growth Des. 2014, 14, 5922–5928. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Reggi, M.; Džakula, B.N.; Kralj, D. Evidence of structural variability among synthetic and biogenic vaterite. Chem. Commun. 2014, 50, 15370–15373. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Toffolo, M.B.; Boaretto, E.; Poduska, K.M. Assessing local and long-range structural disorder in aggregate-free lime binders. Ind. Eng. Chem. Res. 2016, 55, 8334–8340. [Google Scholar] [CrossRef]
- Poduska, K.M.; Regev, L.; Berna, F.; Mintz, E.; Milevski, I.; Khalaily, H.; Weiner, S.; Boaretto, E. Plaster characterization at the PPNB site of Yiftahel (Israel) including the use of 14C: Implications for plaster production, preservation, and dating. Radiocarbon 2012, 54, 887–896. [Google Scholar] [CrossRef]
- Regev, L.; Eckmeier, E.; Mintz, E.; Weiner, S.; Boaretto, E. Radiocarbon concentrations of wood ash calcite: Potential for dating. Radiocarbon 2011, 53, 117–127. [Google Scholar] [CrossRef]
- Regev, L.; Zukerman, A.; Hitchcock, L.; Maeir, A.M.; Weiner, S.; Boaretto, E. Iron Age hydraulic plaster from Tell es-Safi/Gath, Israel. J. Archaeol. Sci. 2010, 37, 3000–3009. [Google Scholar] [CrossRef]
- Regev, L.; Cabanes, D.; Homsher, R.; Kleiman, A.; Weiner, S.; Finkelstein, I.; Shahack-Gross, R. Geoarchaeological investigation in a domestic Iron Age quarter, Tel Megiddo, Israel. Bull. Am. Sch. Orient. Res. 2015, 374, 135–157. [Google Scholar] [CrossRef]
- Xu, B.; Toffolo, M.B.; Regev, L.; Boaretto, E.; Poduska, K.M. Structural differences in archaeologically relevant calcite. Anal. Methods 2015, 7, 9304–9309. [Google Scholar] [CrossRef] [Green Version]
- Toffolo, M.B.; Ullman, M.; Caracuta, V.; Weiner, S.; Boaretto, E. A 10,400-year-old sunken lime kiln from the Early Pre-Pottery Neolithic B at the Nesher-Ramla quarry (el-Khirbe), Israel. J. Archaeol. Sci. Rep. 2017, 14, 353–364. [Google Scholar] [CrossRef]
- Asscher, Y.; Lehmann, G.; Rosen, S.A.; Weiner, S.; Boaretto, E. Absolute dating of the Late Bronze to Iron Age transition and the appearance of Philistine culture in Qubur el-Walaydah, southern Levant. Radiocarbon 2015, 57, 77–97. [Google Scholar] [CrossRef]
- Toffolo, M.B.; Regev, L.; Mintz, E.; Poduska, K.M.; Shahack-Gross, R.; Berthold, C.; Miller, C.E.; Boaretto, E. Accurate radiocarbon dating of archaeological ash using pyrogenic aragonite. Radiocarbon 2017, 59, 231–249. [Google Scholar] [CrossRef]
- Eliyahu-Behar, A.; Yahalom-Mack, N.; Ben-Shlomo, D. Excavation and analysis of an early Iron Age lime kiln. Isr. Explor. J. 2017, 67, 14–31. [Google Scholar]
- Sivan, D.; Potasman, M.; Almogi-Labin, A.; Bar-Yosef Mayer, D.E.; Spanier, E.; Boaretto, E. The Glycymeris query along the coast and shallow shelf of Israel, southeast Mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 233, 134–148. [Google Scholar] [CrossRef]
- Bartov, Y.; Stein, M.; Enzel, Y.; Agnon, A.; Reches, Z. Lake levels and sequence stratigraphy of Lake Lisan, the late Pleistocene precursor of the Dead Sea. Quat. Res. 2002, 57, 9–21. [Google Scholar] [CrossRef]
- Toffolo, M.; Maeir, A.M.; Chadwick, J.R.; Boaretto, E. Characterization of contexts for radiocarbon dating: Results from the early Iron Age at Tell es-Safi/Gath, Israel. Radiocarbon 2012, 54, 371–390. [Google Scholar] [CrossRef]
- Maeir, A.M. Chapter 1: The Tell es-Safi/Gath archaeological project 1996–2010: Introduction, overview and synopsis of results. In Tell Es-Safi/Gath I: Report on the 1996–2005 Seasons; Maeir, A.M., Ed.; Harrassowitz: Wiesbaden, Germany, 2012. [Google Scholar]
- Khalaily, H.; Bar-Yosef, O.; Barzilai, O.; Boaretto, E.; Bocquentin, F.; Eirikh-Rose, A.; Greenhut, Z.; Goring-Morris, A.N.; Le Dosseur, G.; Marder, O.; et al. Excavations at Motza in the Judean Hills and The Early Pre-Pottery Neolithic B in the Southern Levant. Paléorient 2007, 33, 5–37. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974. [Google Scholar]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 2002, 293, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Koishi, A.; Fernandez-Martinez, A.; Ruta, B.; Jimenez-Ruiz, M.; Poloni, R.; di Tommaso, D.; Zontone, F.; Waychunas, G.A.; Montes-Hernandez, G. Role of impurities in the kinetic persistence of amorphous calcium carbonate: A nanoscopic dynamics view. J. Phys. Chem. C 2018, 122, 16983–16991. [Google Scholar] [CrossRef]
- Beniash, E.; Aizenberg, J.; Addadi, L.; Weiner, S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B 1997, 264, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.H.; Dove, P.M.; De Yoreo, J.J. Kinetics of calcite growth: Surface processes and relationships to macroscopic rate laws. Geochim. Cosmochim. Acta 2000, 64, 2255–2266. [Google Scholar] [CrossRef]
- Goodarz-Nia, I.; Motamedi, M. Kinetics of calcium carbonate crystallization from aqueous solutions. J. Cryst. Growth 1980, 48, 125–131. [Google Scholar] [CrossRef]
- Goto, M. Some mineralo-chemical problems concerning calcite and aragonite, with special reference to the genesis of aragonite. J. Fac. Sci. Hokkaido Univ. Ser. 1961, 10, 571–640. [Google Scholar]
- Jones, B. Review of calcium carbonate polymorph precipitation in spring systems. Sediment. Geol. 2017, 353, 64–75. [Google Scholar] [CrossRef]
- Albert, R.M.; Shahack-Gross, R.; Cabanes, D.; Gilboa, A.; Lev-Yadun, S.; Portillo, M.; Sharon, I.; Boaretto, E.; Weiner, S. Phytolith-rich layers from the Late Bronze and Iron Ages at Tel Dor (Israel): Mode of formation and archaeological significance. J. Archaeol. Sci. 2008, 35, 57–75. [Google Scholar] [CrossRef]
- Esteban, I.; Marean, C.W.; Fisher, E.C.; Karkanas, P.; Cabanes, D.; Albert, R.M. Phytoliths as an indicator of early modern humans plant gathering strategies, fire fuel and site occupation intensity during the Middle Stone Age at Pinnacle Point 5–6 (south coast, South Africa). PLoS ONE 2018, 13, e0198558. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, F.; Alvarado-Ortega, J.; Cuevas-García, M.; Ruvalcaba-Sil, J.L.; Linares-López, C. Calcareous fossil inclusions and rock-source of Maya lime plaster from the Temple of the Inscriptions, Palenque, Mexico. J. Archaeol. Sci. 2012, 39, 624–639. [Google Scholar] [CrossRef]
- Weiner, S.; Goldberg, P.; Bar-Yosef, O. Three-dimensional distribution of minerals in the sediments of Hayonim Cave, Israel: Diagenetic processes and archaeological implications. J. Archaeol. Sci. 2002, 29, 1289–1308. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torréns-Martín, D.; Martínez-Ramírez, S. Carbonation of ternary building cementing materials. Cem. Concr. Compos. 2012, 34, 1180–1186. [Google Scholar] [CrossRef]
- Jackson, M.D. Sea-water concretes and their material characteristics. In Building for Eternity; Brandon, C.J., Hohlfelder, R.L., Jackson, M.D., Oleson, J.P., Eds.; Oxbow Books: Oxford, UK, 2014; pp. 141–187. [Google Scholar]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in water-alcohol mixtures: Spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 2011, 12, 842–853. [Google Scholar] [CrossRef]
- Boaretto, E.; Poduska, K.M. Materials science challenges in radiocarbon dating: The case of archaeological plasters. J. Miner. Met. Mater. Soc. TMS 2013, 65, 481–488. [Google Scholar] [CrossRef]


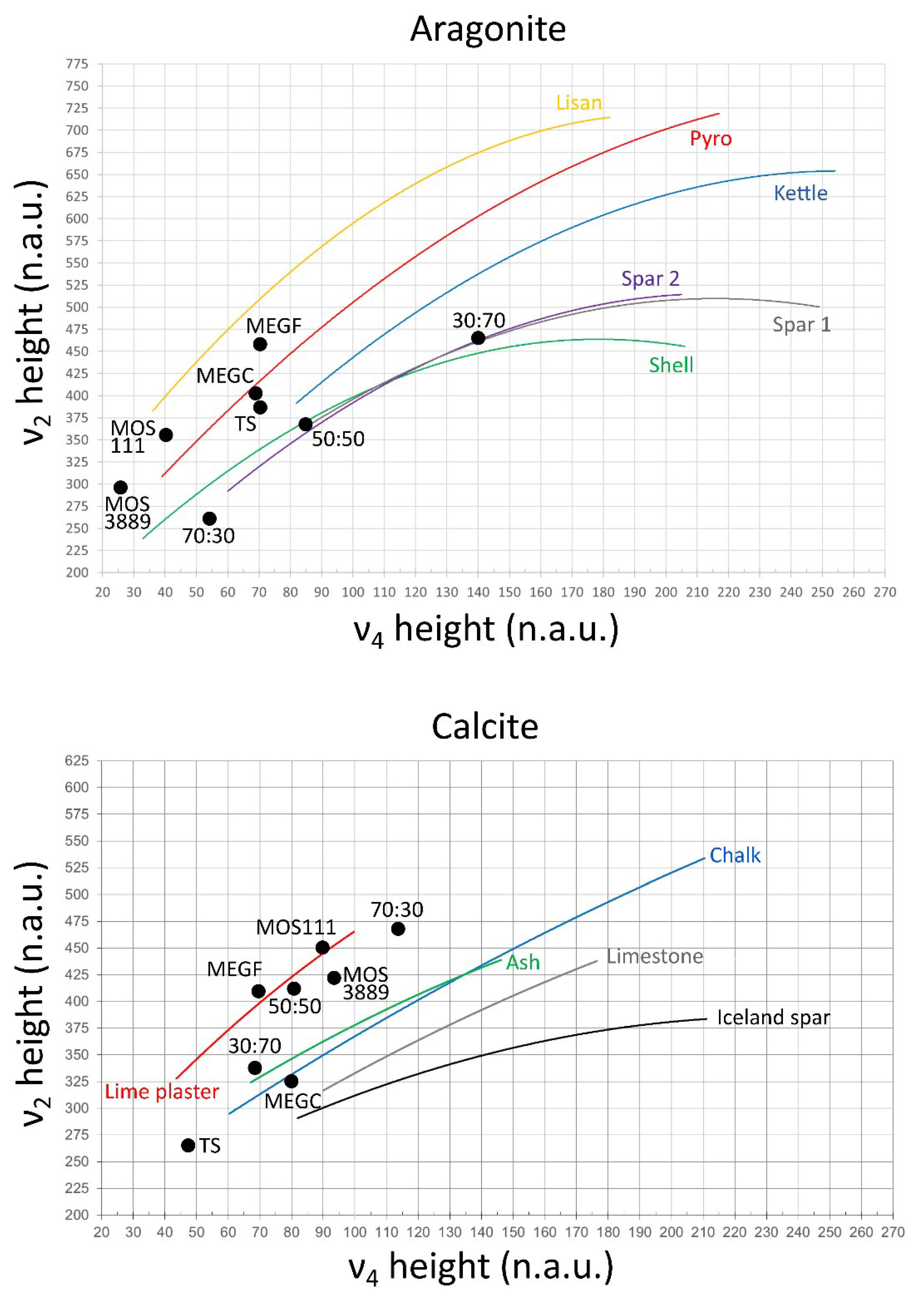
| Sample and C:A Ratio (%) | Calcite | Aragonite | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ν3 | ν2 | ν4 | Nν2 | Nν4 | ν3 | ν2 | ν4 | Nν2 | Nν4 | |
| Mix 50:50 | 1.761 | 0.733 | 0.287 | 416 | 81 | 1.700 | 0.630 | 0.287 | 370 | 84 |
| Mix 30:70 | 1.521 | 0.509 | 0.344 | 334 | 68 | 1.698 | 0.787 | 0.344 | 463 | 141 |
| Mix 70:30 | 1.906 | 0.898 | 0.310 | 471 | 113 | 1.734 | 0.447 | 0.310 | 257 | 54 |
| Megiddo coarse 40:60 | 0.086 | 0.028 | 0.017 | 325 | 79 | 0.149 | 0.060 | 0.017 | 402 | 68 |
| Megiddo fine 30:70 | 0.353 | 0.147 | 0.075 | 416 | 70 | 0.703 | 0.324 | 0.075 | 460 | 71 |
| Tell es-Safi/Gath 30:70 | 0.376 | 0.100 | 0.059 | 266 | 47 | 0.600 | 0.231 | 0.059 | 385 | 69 |
| Motza 111 70:30 | 1.013 | 0.457 | 0.131 | 451 | 90 | 0.959 | 0.340 | 0.131 | 354 | 41 |
| Motza 3889 80:20 | 1.264 | 0.532 | 0.147 | 420 | 93 | 1.198 | 0.354 | 0.147 | 295 | 24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffolo, M.B.; Regev, L.; Dubernet, S.; Lefrais, Y.; Boaretto, E. FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis. Minerals 2019, 9, 121. https://doi.org/10.3390/min9020121
Toffolo MB, Regev L, Dubernet S, Lefrais Y, Boaretto E. FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis. Minerals. 2019; 9(2):121. https://doi.org/10.3390/min9020121
Chicago/Turabian StyleToffolo, Michael B., Lior Regev, Stéphan Dubernet, Yannick Lefrais, and Elisabetta Boaretto. 2019. "FTIR-Based Crystallinity Assessment of Aragonite–Calcite Mixtures in Archaeological Lime Binders Altered by Diagenesis" Minerals 9, no. 2: 121. https://doi.org/10.3390/min9020121