Evolution of Clays in Cretaceous Marly Series (Álava Block, Basque Cantabrian Basin, Spain): Diagenesis and Detrital Input Control
Abstract
:1. Introduction
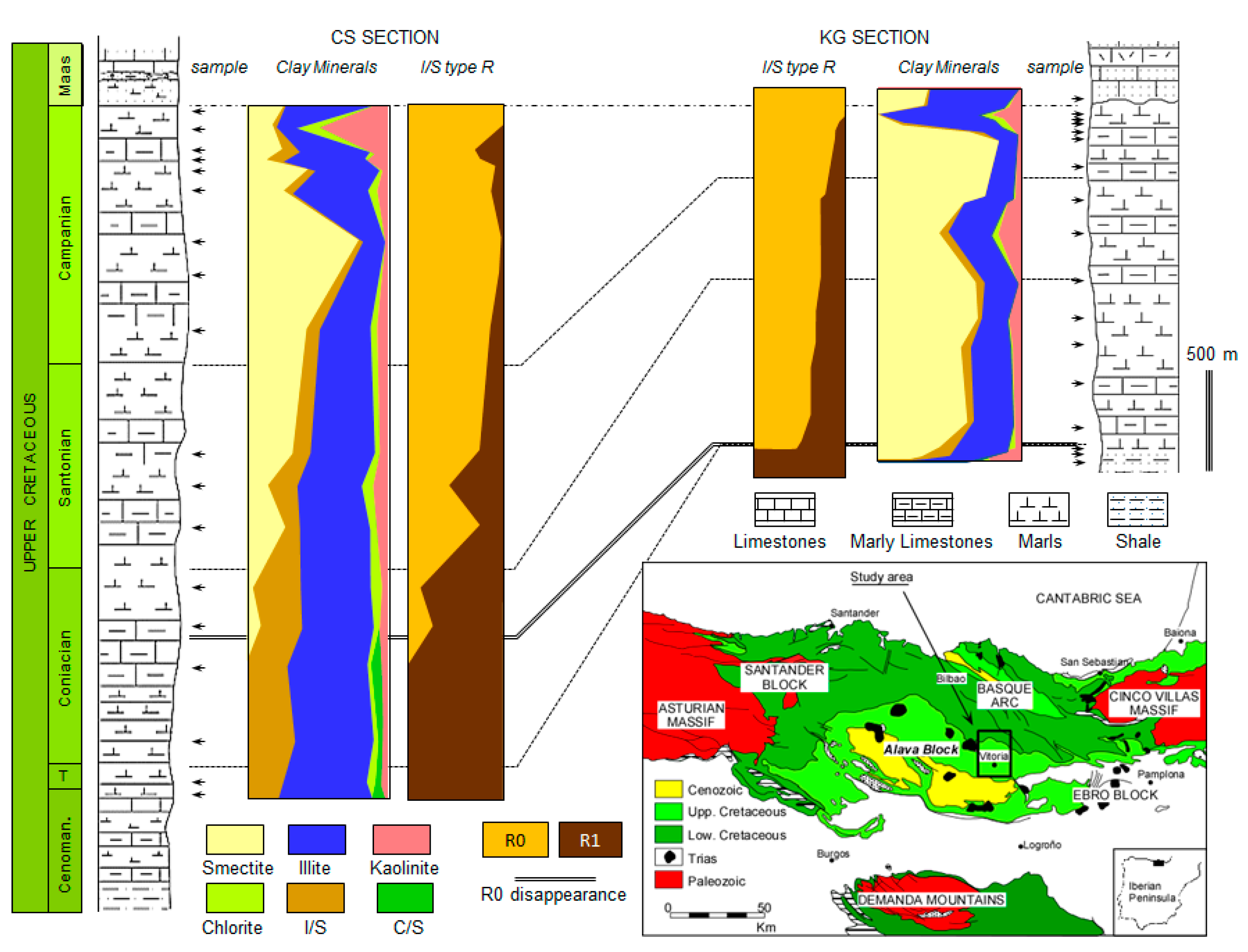
Geological Context and Background
2. Materials and Methods
2.1. Sample Preparation
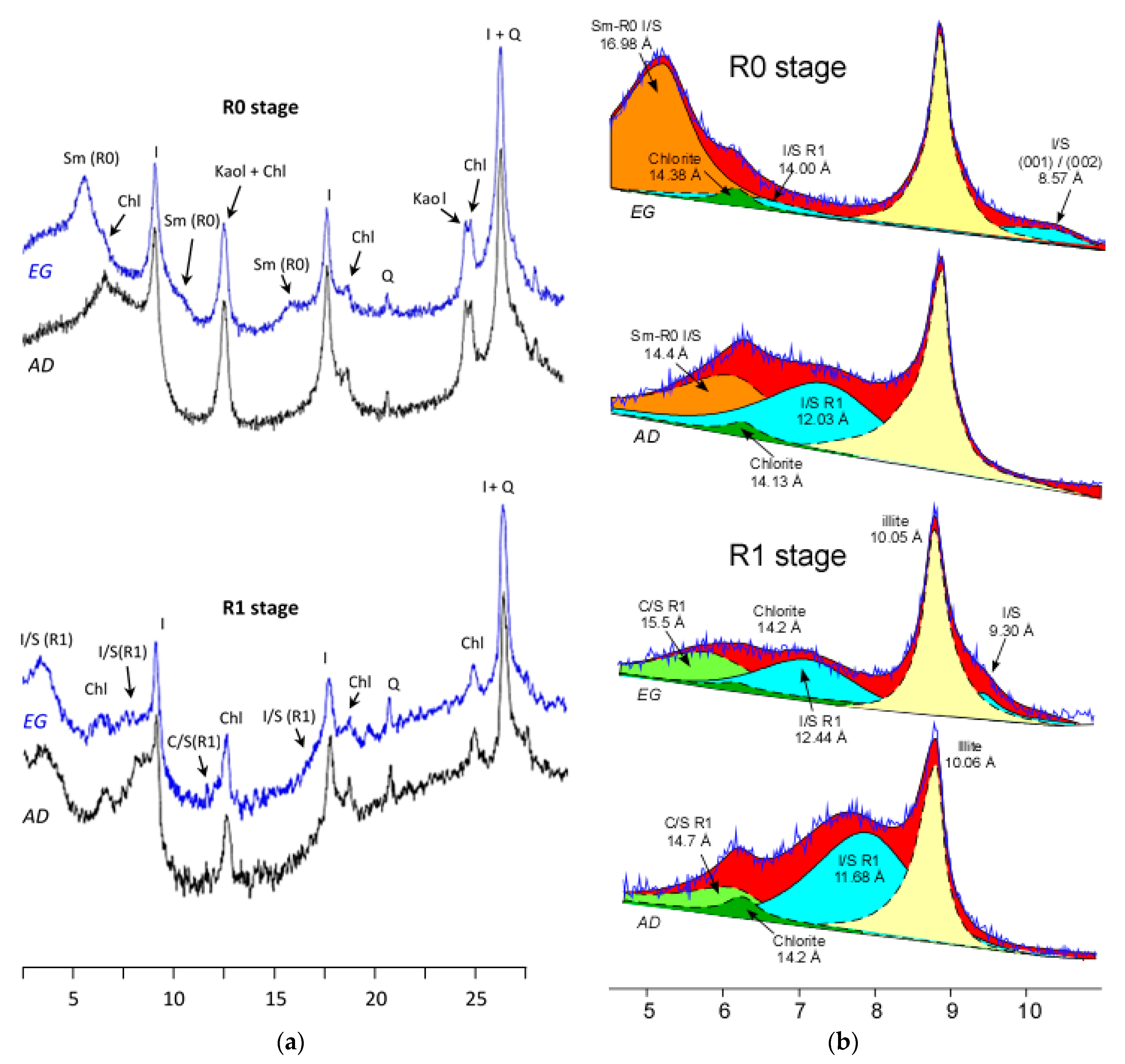
2.1.1. XRD Analysis
2.1.2. Transmission Electron Microscopy
3. Results
3.1. Bulk Mineralogy
3.2. Clay Mineralogy
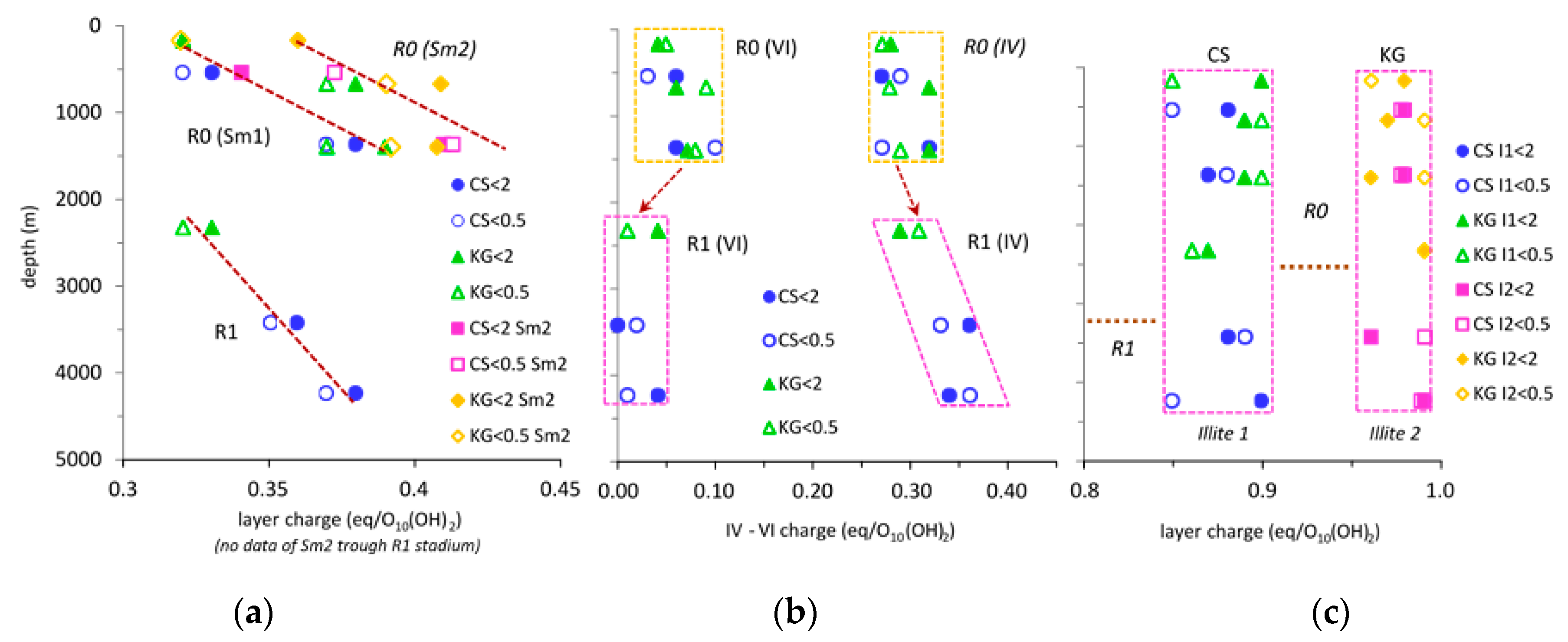
3.3. Charge of 2:1 Expandable Layers
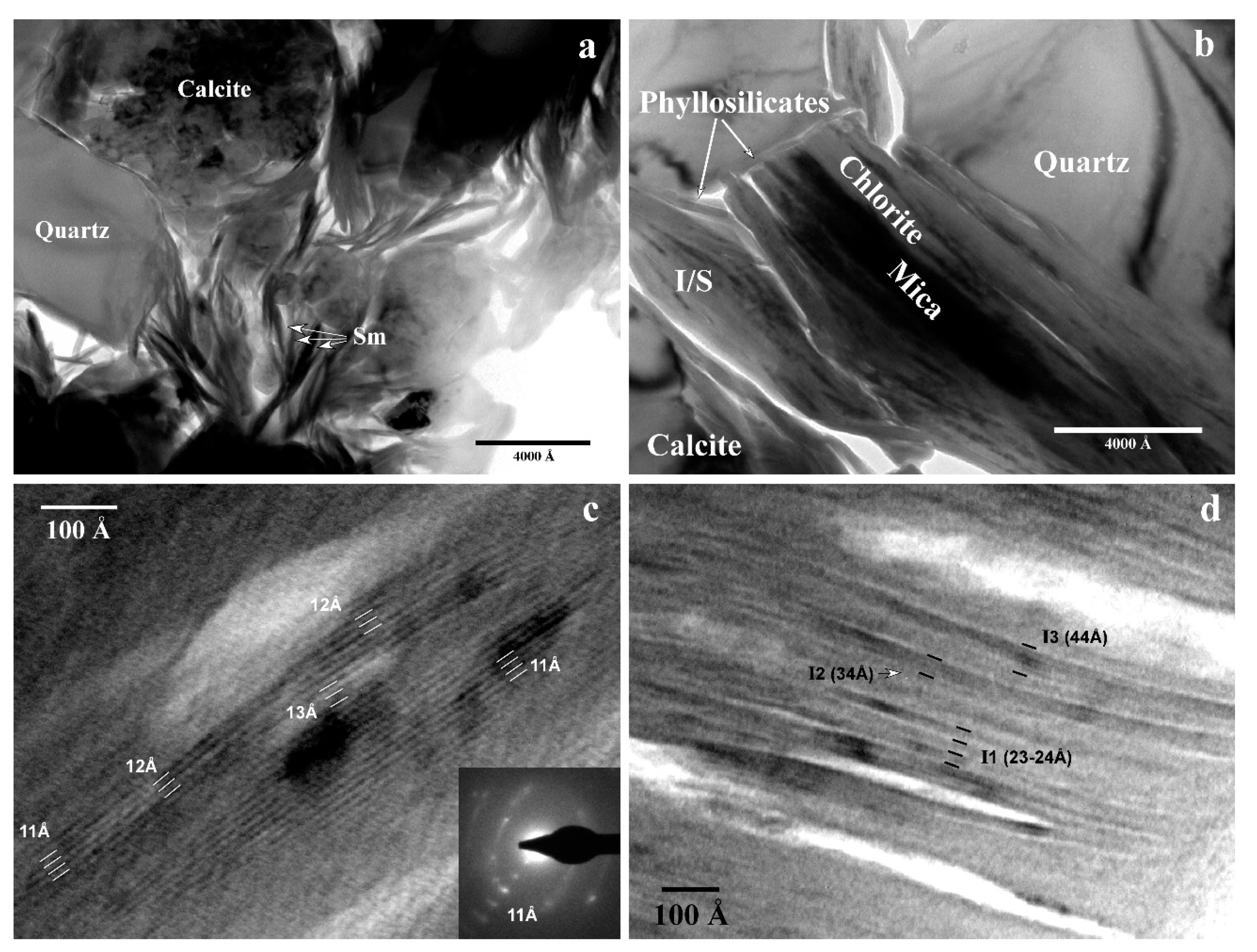
3.4. TEM Study
3.4.1. General Texture
3.4.2. Smectite and Illite/Smectite
3.4.3. Micaceous Phases
- The first type includes illites that have slightly wavy and individualized packets of five to 20 layers and constant periodicity of 10 Å, as noted in images, with orientations subparallel to each other (Figure 6a). They have frayed edges and common dislocations and a loss of layer continuity. SAED images show a lack of general spots of a disordered 1 Md polytype.
- The second type comprises micas with abundant coherent domains (>1000 Å) (Figure 6b), high K contents, and defect-free lattice-fringe images at 10 Å. SAED images show a lack of general spots or characteristics typical of a disordered 1 Md polytype.
- The third type comprises micas with a similar texture to those of the second type, but with much higher Na contents (determined by AEM) than is usual in a muscovite. The composition of the third type lies completely within the compositional gap between muscovite and paragonite. Figure 6c is a TEM image showing the bending of a Na-rich grain around a quartz grain, presumably caused by compaction of the original sediment. The SAED image corresponds to the 2 M polytype (identifiable by the general spots at 20 Å), with a small variation in the crystallographic orientation between two domains, reflecting the reciprocal space of the grain’s curve.
3.4.4. Chlorites and Chlorite/Smectite
3.5. AEM Study
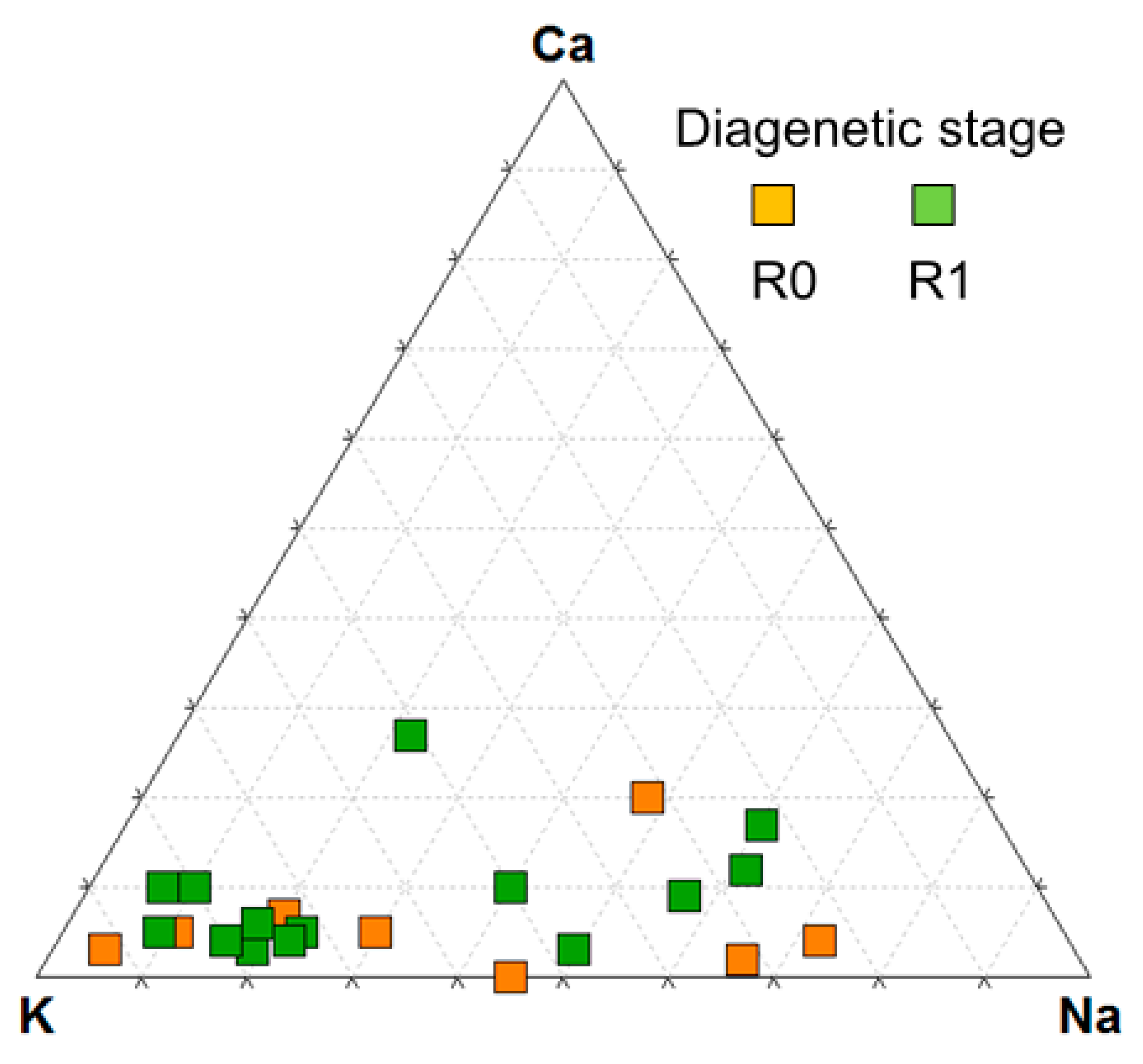
3.5.1. Smectitic (Sm and R0 I/S) and Micaceous Phases (R ≥ 1 I/S, Illite, Mica ss)
3.5.2. Chloritic Minerals
4. Discussion
4.1. Origin of Clay Minerals: Detrital Versus Diagenetic Minerals
4.1.1. Micaceous Phases
Composition (AEM) of Micaceous Phases
Expansible and Non-Expansible Micaceous Phases
4.1.2. Chlorite Phases
4.2. Evolution of the Layer Charge during Diagenesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altaner, S.R.; Ylagan, R.E. Comparison of structural models of mixed-layer illite/smectite and reaction mechanisms of smectite illitization. Clays Clay Miner. 1997, 45, 517–533. [Google Scholar] [CrossRef]
- Dong, H.; Peacor, D.R.; Freed, R.L. Phase relations among smectite, R1 illite-smectite, and illite. Am. Mineral. 1997, 82, 379–391. [Google Scholar] [CrossRef]
- Bauluz, B.; Peacor, D.R.; González-López, J.M. Transmission electron microscopy study of illitization in pelites from the Iberian Range, Spain: Layer-by-Layer Replacement. Clays Clay Min. 2000, 48, 374–384. [Google Scholar] [CrossRef]
- Hower, J.; Eslinger, E.; Hower, M.; Perry, E. Mechanism of burial metamorphism of argillaceous sediments: 1. Mineralogical and chemical evidence. Geol. Soc. Am. Bull. 1976, 87, 725–737. [Google Scholar] [CrossRef]
- Arostegui, J.; Sanguesa, F.J.; Nieto, F.; Uriarte, J.A. Thermal models and clay diagenesis in the Tertiary-Cretaceous sediments of the Álava block (Basque-Cantabrian basin, Spain). Clay Miner. 2006, 41, 791–809. [Google Scholar] [CrossRef]
- Nadeau, P.H.; Wilson, M.J.; McHardy, W.J.; Tait, J.M. Interstratified clays as fundamental particles. Science 1984, 225, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Peacor, D.R. Transmission and analytical electron microscopy of the smectite-to-illite transition. Clays Clay Miner. 1986, 34, 165–179. [Google Scholar]
- Vali, H.; Hesse, R.; Martin, R.F. A TEM-based definition of 2:1 layer silicates and their interstratified constituents. Am. Mineral. 1994, 79, 644–653. [Google Scholar]
- Howard, J.J.; Roy, D.M. Development of layer charge and kinetics of experimental smectite alteration. Clays Clay Miner. 1985, 33, 81–88. [Google Scholar] [CrossRef]
- Cuadros, J. Modeling of smectite illitization in burial diagenesis environments. Geochim. Cosmochim. Acta 2006, 70, 4181–4195. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction of Clay Colloid Chemistry; Interscience Publishers, Div. of John Wiley & Sons: New York, NY, USA, 1966; pp. 239–243. ISBN 9780471014638. [Google Scholar]
- Robert, M. The experimental transformation of mica towards smectite: Relative importance of total charge and tetrahedral substitution. Clays Clay Miner. 1973, 21, 167–174. [Google Scholar] [CrossRef]
- Lagaly, G.; Weiss, A. Determination of layer charge in mica-type layer silicates. In Proceedings of the International Clay Conference, Tokyo, Japan, 5–10 September 1969; Heller, L., Ed.; pp. 61–80. [Google Scholar]
- Lagaly, G. Characterization of clays by organic-compounds. Clay Miner. 1981, 16, 1–21. [Google Scholar] [CrossRef]
- Malla, P.B.; Douglas, L.A. Problems in identification of montmorillonite and beidellite. Clays Clay Miner. 1987, 35, 232–236. [Google Scholar] [CrossRef]
- Ghabru, S.K.; Mermut, A.R.; St. Arnaud, R.J. Layer-charge and cation-exchange characteristics of vermiculite (weathered biotite) isolated from a Gray Luvisol in northeastern Saskatchewan. Clays Clay Miner. 1989, 37, 164–172. [Google Scholar] [CrossRef]
- Sears, S.K.; Hesse, R.; Vali, H. Significance of n-alkylammonium exchange in the study of 2:1 clay mineral diagenesis, Mackenzie Delta Beaufort Sea region, Arctic Canada. Can. Mineral. 1998, 36, 1485–1506. [Google Scholar]
- Meunier, A.; Velde, B.; Zalba, P. Illite K-Ar dating and crystal growth processes in diagenetic environments: A critical review. Terra Nova 2004, 16, 296–304. [Google Scholar] [CrossRef]
- Nieto, F.; Ortega-Huertas, M.; Peacor, D.; Arostegui, J. Evolution of illite/smectite from early diagenesis through incipient metamorphism in sediments of the Basque-Cantabrian Basin. Clays Clay Miner. 1996, 44, 304–323. [Google Scholar] [CrossRef]
- Sangüesa, F.J. La diagénesis en el Bloque Alavés de la Cuenca Vasco-Cantábrica: Distribución, Modelización y Aplicaciones. Ph.D. Thesis, Basque Country University, Leioa, Spain, May 1998. [Google Scholar]
- Sangüesa, F.J.; Arostegui, J.; Suarez-Ruiz, I. Distribution and origin of clay minerals in the Lower Cretaceous of the Álava Block (Basque-Cantabrian Basin, Spain). Clay Miner. 2000, 35, 393–410. [Google Scholar] [CrossRef]
- Arostegui, J. Distribución de la diagénesis en la Cuenca Vasco-Cantábrica. In Geología de la Cuenca vasco-Cantábrica; Bodego, A., Mendia, M., Aranburu, A., Apraiz, A., Eds.; Servicio Editorial de la Universidad del País Vasco: Bilbao, Spain, 2014; pp. 119–127. ISBN 978-84-9860-991-2. [Google Scholar]
- Nieto, F.; Arroyo, X.; Arostegui, J. XRD-TEM-AEM comparative study of n-alkylammonium smectites and interstratified minerals in shallow-diagenetic carbonate sediments of the Basque-Cantabrian Basin. Am. Mineral. 2016, 101, 385–398. [Google Scholar] [CrossRef]
- Bauluz, B. Clays in low-temperature environments. In Minerals at the Nanoscale; Nieto, F., Livi, F.T., Eds.; EMU notes in Mineralogy: London, UK, 2013; Volume 14, pp. 181–209. ISBN 978-0903056-34-2. [Google Scholar]
- Ramírez del Pozo, J. Bioestratigrafía y microfacies del Jurásico y Cretácico del Norte de España (Región Cantábrica). Mem. Inst. Geol. Min. Esp. 1971, 78, 357. [Google Scholar]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 378. ISBN 0-19-508713-5. [Google Scholar]
- Lagaly, G. Layer charge determination by alkylammonium ions. In Layer Charge Characteristics of Clays; Mermut, A.R., Ed.; The Clay Minerals Society: Boulder, CO, USA, 1994; Volume 6, pp. 1–46. ISBN 9781881208075. [Google Scholar]
- Mermut, A.R.; Lagaly, G. Baseline studies of The Clay Minerals Society Source Clays: Layer-charge determination and characteristics of those minerals containing 2:1 layers. Clays Clay Miner. 2001, 49, 393–397. [Google Scholar] [CrossRef]
- Srodon, J.; Eberl, D.D. Illite. In Micas; Bailey, S.W., Ed.; Mineralogical Society of America: Washington, DC, USA, 1984; Volume 13, pp. 495–544. ISBN 978-0-939950-17-1. [Google Scholar]
- Kim, J.M.; Peacor, D.R.; Tessier, D.; Elsass, E. A technique for maintaining texture and permanent expansion of smectite interlayer spacings for TEM observations. Clays Clay Miner. 1995, 43, 51–57. [Google Scholar] [CrossRef]
- Buseck, P.; Cowley, J.; Eyring, L. High Resolution Transmission Electron Microscopy and Associated Techniques; Oxford University Press: New York, NY, USA, 1989; p. 670. ISBN 9780195042757. [Google Scholar]
- Mermut, A.R.; St. Arnaud, R.J. Layer charge determination of high charge phyllosilicates by alkyl-ammonium technique. In Proceedings of the 27th Annual Meeting of the Clay Minerals Society, Columbia, MO, USA, 6–11 October 1990; p. 86. [Google Scholar]
- Vali, H.; Hesse, R. Alkylammonium ion treatment of clay-minerals in ultrathin section—A new method for HRTEM examination of expandable layers. Am. Mineral. 1990, 75, 1443–1446. [Google Scholar]
- Velde, B. Clay Minerals: A Physico-Chemical Explanation oftheir Occurrence; Elsevier: Amsterdam, The Netherlands, 1985; Volume 40, p. 443. ISBN 9780444553607. [Google Scholar]
- Shau, Y.-H.; Peacor, D.R.; Essene, E.J. Corrensite and mixed-layer chlorite/corrensite in metabasalt from northern Taiwan: TEM/AEM, EMPA, XRD, and optical studies. Contrib. Mineral. Petrol. 1990, 105, 123–142. [Google Scholar] [CrossRef]
- Newman, A.C.D.; Brown, G. The Chemical Constitution of Clays. In Chemistry of Clays and Clay Minerals; Mineralogical Society Monograph No. 6; Newman, A.C.D., Ed.; Longman Scientific & Technical: Harlow, UK, 1987; pp. 2–116. ISBN 978-0582301146. [Google Scholar]
- Floquet, M. Vue sur le Crétacé Basco-Cantabrique et Nord-Iberique. In Mémoires Géologiques de l’Université de Dijon; Institute des Sciences de la Terre: Orléans, France, 1983; Volume 9, pp. 141–168. [Google Scholar]
- Huber, B.; Norris, R.; MacLeod, K. Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 2002, 30, 123–126. [Google Scholar] [CrossRef]
- Chamley, H. North-Atlantic clay sedimentation and palaeoenvironment since the Late Jurassic. In Deep Drilling results in the Atlantic Ocean: Continental Margins and Paleoenvironment; Maurice Ewing Series; Talwani, M., Hay, W., Ryan, W.B.F., Eds.; American Geophysical Union: Washington, DC, USA, 2013; Volume 3, pp. 342–361. ISBN 9780875904023. [Google Scholar]
- Frey, M. A mixed-layer paragonite/phengite of low grade metamorphic origin. Contrib. Mineral. Petrol. 1969, 24, 63–65. [Google Scholar] [CrossRef]
- Jiang, W.-T.; Peacor, D.R. Formation and modification of metastable intermediate sodium potassium mica, paragonite and muscovite in hydrothermally altered metabasites from northern Wales. Am. Mineral. 1993, 78, 782–793. [Google Scholar]
- Livi, K.J.T.; Christidis, G.; Árkai, P.; Veblen, D.R. White mica domain formation: A model for paragonite, margarite, and muscovite formation during prograde metamorphism. Am. Mineral. 2008, 92, 1288–1302. [Google Scholar] [CrossRef]
- Li, G.; Peacor, D.; Merriman, R.J.; Roberts, B.; Van Der Pluijm, B.A. TEM and AEM constraints on the origin and significance of chlorite-mica stacks: An example from Central Wales, U.K. J. Struc. Geol. 1994, 16, 1139–1157. [Google Scholar] [CrossRef]
- Giorgetti, G.; Memmi, I.; Nieto, F. Microstructures of intergrown phyllosilicate grains from Verrucano metasediments (northern Apennines, Italy). Contrib. Mineral. Petrol. 1997, 128, 127–138. [Google Scholar] [CrossRef]
- Abad, I.; Mata, M.P.; Nieto, F.; Velilla, N. The phyllosilicates in diagenetic-metamorphic rocks of the South Portuguese zone, southwestern Portugal. Can. Mineral. 2001, 39, 1571–1589. [Google Scholar] [CrossRef]
- Merriman, R.J. Clay minerals and sedimentary basin history. Eur. J. Mineral. 2005, 17, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Yenes, M.; Alvarez, F.; Nieto, F. Analisis estructural y metamorfico de la deformación hercínica del borde meridional de la Sierra de la Demanda. Estud. Geol. 1990, 46, 223–236. [Google Scholar] [CrossRef]
- Abad, I.; Nieto, F.; Peacor, D.R.; Velilla, N. Prograde and retrograde diagenetic and metamorphic evolution in metapelitic rocks of Sierra Espuña (Spain). Clay Miner. 2003, 38, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Merriman, R.J.; Peacor, D.R. Very Low-Grade Metapelites: Mineralogy, Microfabrics and Measuring Reaction Progress. In Low-Grade Metamorphism; Frey, M., Robinson, D., Eds.; Blackwell Sciences Ltd.: Oxford, UK, 2009; pp. 10–60. ISBN 9780632047567. [Google Scholar]
- Teppen, B.J.; Yu, C.-H.; Miller, D.M.; Schäfer, L. Molecular dynamics simulations of the sorption of organic compounds at the clay mineral/aqueous solution interface. J. Comp. Chem. 1998, 19, 144–153. [Google Scholar] [CrossRef]
- Beneke, K.; Lagaly, G. The brittle mica-like KNiAsO4 and its organic derivatives. Clay Miner. 1982, 17, 177–185. [Google Scholar] [CrossRef]
- Vali, H.; Hesse, R.; Kodama, H. Arrangement of n-alkylammonium ions in phlogopite and vermiculite: An XRD- and TEM-study. Clays Clay Miner. 1992, 40, 240–245. [Google Scholar] [CrossRef]
- Barnhisel, R.I.; Bertsch, P.M. Chlorites and hydroxy interlayered vermiculite and smectite. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America, Inc.: Madison, OH, USA, 1989; Volume 1, pp. 729–788. ISBN 9780891188605. [Google Scholar]
- Eberl, D.; Hower, J. The hydrothermal transformation of sodium and potassium smectite into mixed-layer clay. Clays Clay Miner. 1977, 25, 215–227. [Google Scholar] [CrossRef]
- Sato, T.; Murakami, T.; Watanabe, T. Change in layer charge of smectites and smectite layers in illite/smectite during diagenetic alteration. Clays Clay Miner. 1996, 44, 460–469. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Stucki, J.W.; Anastacio, A.S. Layer charge density of smectites—Closing the gap between the structural formula method and the alkyl ammonium method. Clays Clay Miner. 2011, 59, 200–211. [Google Scholar] [CrossRef]
- Srodon, J.; Elsass, E.; McHardy, W.J.; Morgan, D.J. Chemistry of illite-smectite inferred from TEM measurements of fundamental particles. Clay Miner. 1992, 27, 137–158. [Google Scholar] [CrossRef]
- Cetin, K.; Huff, W.D. Layer charge of the expandable component of illite/smectite in K-bentonite as determined by alkylammonium ion-exchange. Clays Clay Miner. 1995, 43, 150–158. [Google Scholar] [CrossRef]
- Kisch, H.J. Mineralogy and Petrology of Burial Diagenesis (Burial Metamorphism) and Incipient Metamorphism in Clastic Rocks. In Diagenesis of Sediments and Sedimentary Rocks; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 25, pp. 289–493. ISBN 978-0-444-42013-8. [Google Scholar]
- Shata, S.; Hesse, R.; Martin, R.F.; Vali, H. Expandability of anchizonal illite and chlorite: Significance for crystallinity development in the transition from diagenesis to metamorphism. Am. Mineral. 2003, 88, 748–762. [Google Scholar] [CrossRef]





| Sample | Si | AlIV | AlVI | Fe a | Mg | Ti | Σoct | K | Na | Ca | Σ Int. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Illitic-Micaceous phases (R0 stadium) | |||||||||||
| 1CS6-6-7 | 3.21 | 0.79 | 1.84 | 0.09 | 0.12 | 0 | 2.06 | 0.42 | 0.35 | 0 | 0.77 |
| 1CS6-13-13 | 3.29 | 0.71 | 1.78 | 0.11 | 0.16 | 0 | 2.05 | 0.51 | 0.14 | 0.05 | 0.75 |
| 1CS9-7-11 | 3.33 | 0.67 | 1.77 | 0.07 | 0.23 | 0 | 2.07 | 0.48 | 0.12 | 0.05 | 0.7 |
| 1KG5-2-5 | 3.31 | 0.69 | 1.61 | 0.19 | 0.24 | 0 | 2.05 | 0.19 | 0.58 | 0.03 | 0.83 |
| 1KG5-4-7 | 3.17 | 0.83 | 1.29 | 0.46 | 0.37 | 0 | 2.12 | 0.26 | 0.39 | 0.16 | 0.97 |
| 1KG5-5-8 | 3.33 | 0.67 | 1.55 | 0.21 | 0.27 | 0 | 2.04 | 0.72 | 0.09 | 0.04 | 0.89 |
| 1KG5-6-9 | 3.16 | 0.84 | 1.78 | 0.11 | 0.14 | 0 | 2.03 | 0.29 | 0.59 | 0.02 | 0.92 |
| 1KG5-11-14 | 3.22 | 0.78 | 1.78 | 0.11 | 0.12 | 0 | 2.01 | 0.57 | 0.26 | 0.04 | 0.91 |
| 1KG5-15-4 | 3.45 | 0.55 | 1.48 | 0.25 | 0.3 | 0 | 2.04 | 0.73 | 0.04 | 0.02 | 0.81 |
| Illitic-Micaceous phases (R1 stadium) | |||||||||||
| 3CS3-1-16 | 3.41 | 0.59 | 1.81 | 0.07 | 0.12 | 0 | 2 | 0.44 | 0.14 | 0.07 | 0.72 |
| 3CS3-2-17 | 3.43 | 0.57 | 1.72 | 0.05 | 0.27 | 0 | 2.04 | 0.44 | 0.16 | 0.07 | 0.74 |
| 3CS3-3-18 | 3.21 | 0.79 | 1.82 | 0.03 | 0.12 | 0 | 1.98 | 0.45 | 0.47 | 0.03 | 0.98 |
| 3CS3-10-25 | 3.12 | 0.88 | 1.78 | 0.05 | 0.18 | 0 | 2.01 | 0.79 | 0.16 | 0.04 | 1.03 |
| 3CS6-1-4 | 3.22 | 0.78 | 1.76 | 0.07 | 0.14 | 0 | 1.98 | 0.81 | 0.09 | 0.05 | 1 |
| 3CS6-2-5 | 3.3 | 0.7 | 1.88 | 0.02 | 0.14 | 0 | 2.04 | 0.23 | 0.25 | 0.12 | 0.72 |
| 3CS6-6-9 | 3.23 | 0.77 | 1.84 | 0.05 | 0.14 | 0 | 2.03 | 0.26 | 0.44 | 0.07 | 0.84 |
| 3CS6-7-10 | 3.51 | 0.49 | 1.72 | 0.11 | 0.14 | 0 | 1.97 | 0.57 | 0.05 | 0.07 | 0.76 |
| 3CS6-9-12 | 3.25 | 0.75 | 1.79 | 0.07 | 0.25 | 0 | 2.1 | 0.14 | 0.32 | 0.12 | 0.7 |
| 3CS6-11-14 | 3.51 | 0.49 | 1.77 | 0.05 | 0.12 | 0 | 1.95 | 0.56 | 0.07 | 0.07 | 0.77 |
| 3CS6-12-15 | 3.18 | 0.82 | 1.82 | 0.02 | 0.14 | 0 | 1.98 | 0.25 | 0.58 | 0.11 | 1.05 |
| 3CS6-13-16 | 3.18 | 0.82 | 1.8 | 0.05 | 0.18 | 0 | 2.03 | 0.37 | 0.16 | 0.2 | 0.93 |
| 3CS6-15-18 | 3.28 | 0.72 | 1.75 | 0.05 | 0.18 | 0.04 | 2.02 | 0.58 | 0.18 | 0.04 | 0.84 |
| 3KG5-1-20 | 3.25 | 0.75 | 1.75 | 0.09 | 0.14 | 0 | 1.98 | 0.44 | 0.35 | 0.09 | 0.97 |
| 3KG5-3 | 3.13 | 0.87 | 1.73 | 0.05 | 0.16 | 0.05 | 2 | 0.71 | 0.21 | 0.04 | 1 |
| 3KG5-4 | 3.28 | 0.72 | 1.65 | 0.2 | 0.25 | 0 | 2.09 | 0.57 | 0.14 | 0.02 | 0.75 |
| 3KG5-5 | 3.38 | 0.62 | 1.64 | 0.16 | 0.26 | 0 | 2.06 | 0.55 | 0.13 | 0.04 | 0.76 |
| 3KG5-8-27 | 3.14 | 0.86 | 1.85 | 0.07 | 0.14 | 0 | 2.06 | 0.16 | 0.43 | 0.12 | 0.83 |
| Sample | Si | AlIV | AlVI | Fe a | Mg | Ti | Σoct | K | Na | Ca | Σ Int. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorite (R0 stadium) | |||||||||||
| 1CS6-2-2 | 3.24 | 0.76 | 2.07 | 2.16 | 1.03 | 0.00 | 5.26 | 0.07 | 0.00 | 0.05 | 0.12 |
| 1CS6-21-21 | 3.06 | 0.94 | 1.69 | 2.42 | 1.35 | 0.02 | 5.48 | 0.00 | 0.21 | 0.02 | 0.23 |
| Chlorite (R1 stadium) | |||||||||||
| 3CS3-3 | 2.81 | 1.19 | 1.67 | 2.31 | 1.60 | 0.00 | 5.58 | 0.02 | 0.10 | 0.12 | 0.24 |
| 3CS3-5-9 | 3.68 | 0.32 | 2.20 | 1.58 | 1.08 | 0.00 | 4.86 | 0.10 | 0.15 | 0.07 | 0.31 |
| 3CS3-21b | 3.80 | 0.20 | 2.26 | 1.70 | 0.74 | 0.00 | 4.70 | 0.12 | 0.14 | 0.14 | 0.40 |
| 3CS3-22b | 3.54 | 0.46 | 2.09 | 1.94 | 0.95 | 0.00 | 4.98 | 0.10 | 0.12 | 0.10 | 0.31 |
| 3CS3-24b | 3.33 | 0.67 | 2.11 | 1.89 | 1.21 | 0.00 | 5.21 | 0.05 | 0.05 | 0.02 | 0.12 |
| 3CS6-14-17 | 3.18 | 0.82 | 2.19 | 1.85 | 1.19 | 0.00 | 5.23 | 0.00 | 0.07 | 0.05 | 0.12 |
| 3CS6-14-14 | 3.06 | 0.94 | 1.60 | 2.68 | 1.23 | 0.00 | 5.52 | 0.02 | 0.12 | 0.07 | 0.21 |
| 3CS6-16-19 | 3.50 | 0.50 | 2.58 | 1.36 | 0.73 | 0.00 | 4.67 | 0.32 | 0.16 | 0.04 | 0.53 |
| 3CS6-25b | 3.79 | 0.21 | 2.73 | 1.09 | 0.77 | 0.00 | 4.59 | 0.07 | 0.14 | 0.04 | 0.25 |
| 3CS6-26b | 3.40 | 0.60 | 2.47 | 1.76 | 0.67 | 0.00 | 4.90 | 0.02 | 0.12 | 0.10 | 0.24 |
| 3KG5-3-22 | 3.68 | 0.32 | 2.55 | 1.37 | 0.69 | 0.00 | 4.61 | 0.04 | 0.28 | 0.11 | 0.44 |
| 3KG5-5-24 | 3.35 | 0.65 | 1.75 | 2.62 | 0.97 | 0.00 | 5.33 | 0.02 | 0.16 | 0.02 | 0.20 |
| 3KG5-14-33 | 3.38 | 0.62 | 2.22 | 1.90 | 0.72 | 0.00 | 4.84 | 0.07 | 0.38 | 0.14 | 0.58 |
| 3KG5-29b | 3.19 | 0.81 | 1.85 | 2.60 | 0.90 | 0.00 | 5.35 | 0.00 | 0.12 | 0.07 | 0.19 |
| 3KG5-35b | 3.39 | 0.61 | 2.45 | 1.66 | 0.81 | 0.00 | 4.92 | 0.16 | 0.07 | 0.04 | 0.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arostegui, J.; Arroyo, X.; Nieto, F.; Bauluz, B. Evolution of Clays in Cretaceous Marly Series (Álava Block, Basque Cantabrian Basin, Spain): Diagenesis and Detrital Input Control. Minerals 2019, 9, 40. https://doi.org/10.3390/min9010040
Arostegui J, Arroyo X, Nieto F, Bauluz B. Evolution of Clays in Cretaceous Marly Series (Álava Block, Basque Cantabrian Basin, Spain): Diagenesis and Detrital Input Control. Minerals. 2019; 9(1):40. https://doi.org/10.3390/min9010040
Chicago/Turabian StyleArostegui, Javier, Xabier Arroyo, Fernando Nieto, and Blanca Bauluz. 2019. "Evolution of Clays in Cretaceous Marly Series (Álava Block, Basque Cantabrian Basin, Spain): Diagenesis and Detrital Input Control" Minerals 9, no. 1: 40. https://doi.org/10.3390/min9010040





