The Saint-Honoré Carbonatite REE Zone, Québec, Canada: Combined Magmatic and Hydrothermal Processes
Abstract
:1. Introduction
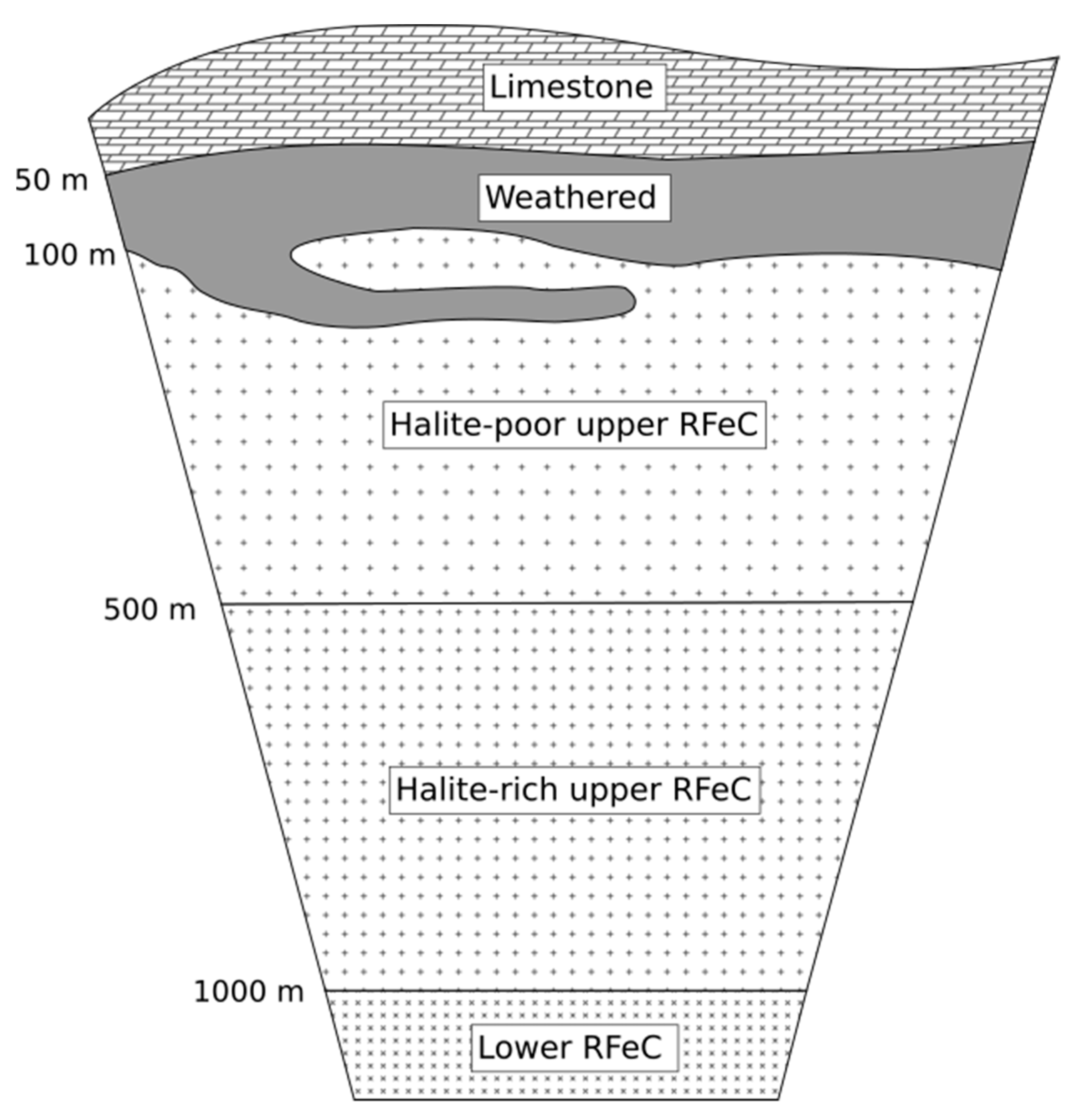
2. Geological Setting
3. Materials and Methods
3.1. Petrographic Studies
3.2. Scanning Electron Microscope (SEM)
3.3. Whole Rock Analysis
3.4. Trace Element Analysis of Minerals
4. Results
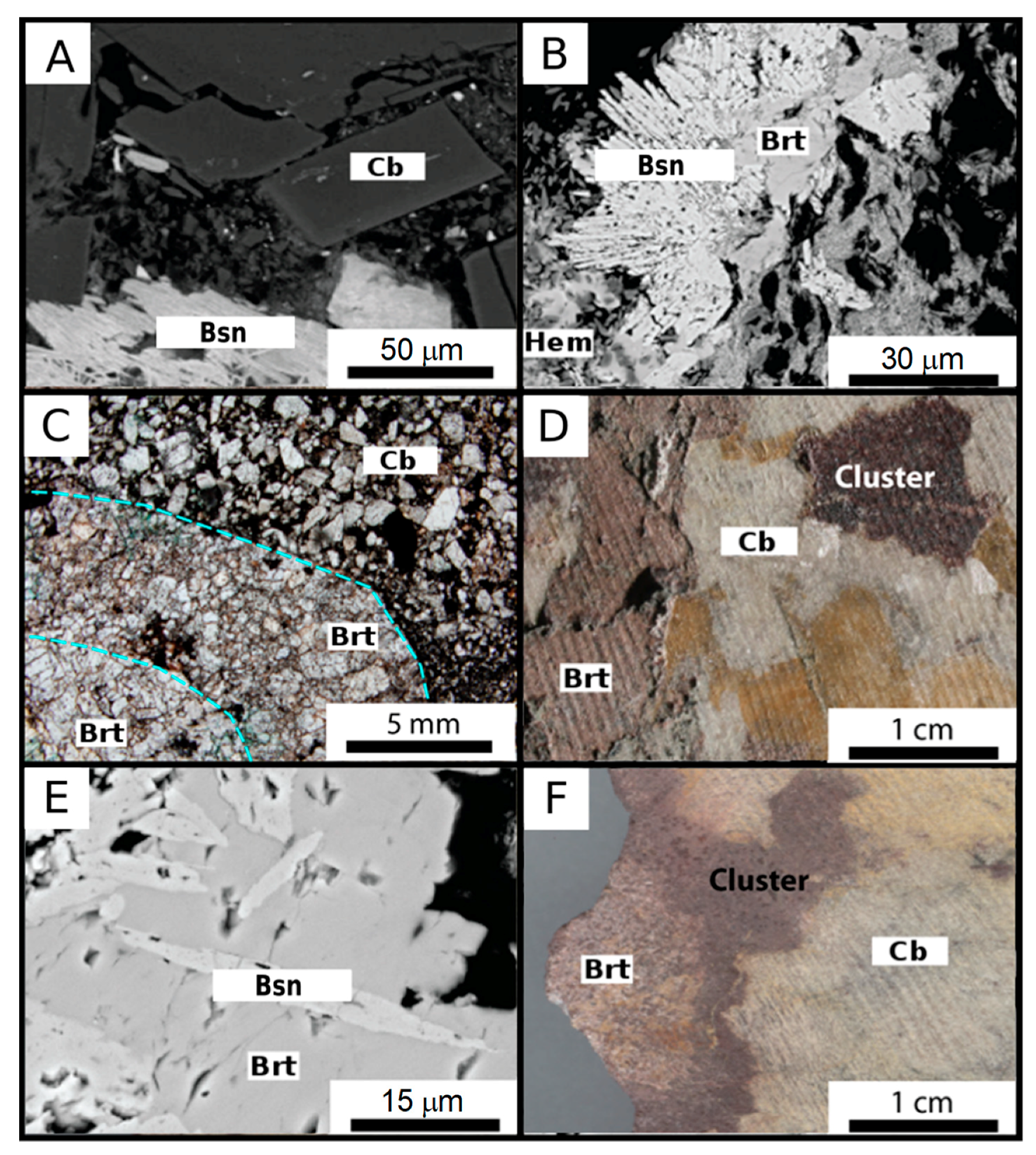
4.1. Petrography of the Fe-Carbonatite
REE-Rich Fe-Carbonatite (RFeC)
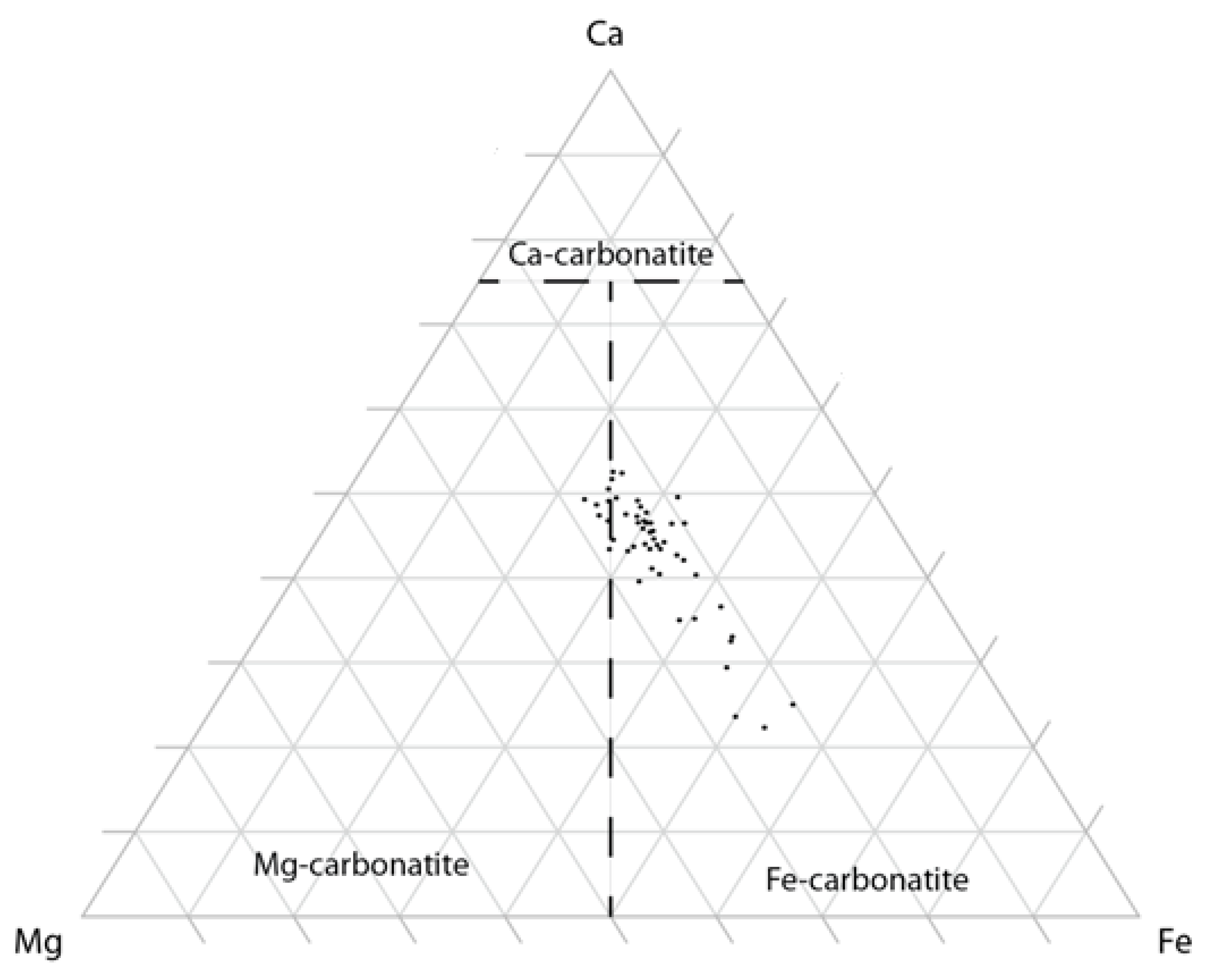
4.2. Geochemistry of the Fe-Carbonatite
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simandl, G. Geology and market-dependent significance of rare earth element resources. Miner. Depos. 2014, 49, 889–904. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Rollat, A.; Guyonnet, D.; Planchon, M.; Tuduri, J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016, 49, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Smith, M.; Moore, K.; Kavecsánszki, D.; Finch, A.A.; Kynicky, J.; Wall, F. From mantle to critical zone: A review of large and giant sized deposits of the rare earth elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef] [Green Version]
- Verplanck, P.L.; Mariano, A.N.; Mariano, A., Jr. Rare earth element ore geology of carbonatites. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists: Golden, CO, USA, 2016; Volume 18, pp. 5–32. [Google Scholar]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare earth mineralization in igneous rocks: Sources and processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average chemical compositions, and element distribution. In Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Nelson, D.; Chivas, A.; Chappell, B.; McCulloch, M. Geochemical and isotopic systematics in carbonatites and implications for the evolution of ocean-island sources. Geochim. Cosmochim. Acta 1988, 52, 1–17. [Google Scholar] [CrossRef]
- Heinrich, E.W. The Geology of Carbonatites; Rand McNally: Chicago, IL, USA, 1966. [Google Scholar]
- Woolley, A.R.; Kjarsgaard, B.A. Paragenetic types of carbonatite as indicated by the diversity and relative abundances of associated silicate rocks: Evidence from a global database. Can. Mineral. 2008, 46, 741–752. [Google Scholar] [CrossRef]
- Jones, A.P.; Wyllie, P.J. Solubility of rare earth elements in carbonatite magmas, indicated by the liquidus surface in CaCO3-Ca(OH)2-La(OH)3; at 1 kbar pressure. Appl. Geochem. 1986, 1, 95–102. [Google Scholar] [CrossRef]
- Mariano, A.N. Nature of economic mineralization in carbonatites and related rocks. In Carbonatites: Genesis and Evolution; Unwin Hyman: London, UK, 1989; pp. 149–176. [Google Scholar]
- Doroshkevich, A.G.; Ripp, G.S.; Viladkar, S.G.; Vladykin, N.V. The Arshan REE carbonatites, southwestern transbaikalia, russia: Mineralogy, paragenesis and evolution. Can. Mineral. 2008, 46, 807–823. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Schmidt, M.W.; Mattsson, H.B.; Guenther, D. Element partitioning between immiscible carbonatite and silicate melts for dry and H2O-bearing systems at 1–3 GPa. J. Petrol. 2013, 54, 2301–2338. [Google Scholar] [CrossRef]
- Kjarsgaard, B.; Hamilton, D.; Peterson, T. Peralkaline nephelinite/carbonatite liquid immiscibility: Comparison of phase compositions in experiments and natural lavas from oldoinyo lengai. In Carbonatite Volcanism; Springer Nature: Berlin/Heidelberg, Germany, 1995; pp. 163–190. [Google Scholar]
- Gittins, J. The origin and evolution of carbonatite magmas. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 580–600. [Google Scholar]
- Kjarsgaard, B.; Hamilton, D. The genesis of carbonatites by immiscibility In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 388–404. [Google Scholar]
- Le Bas, M.J. Diversification of carbonatite. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 105–141. [Google Scholar]
- Kjarsgaard, B.A.; Hamilton, D.L. Liquid immiscibility and the origin of alkali-poor carbonatites. Mineral. Mag. 1988, 52, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Von Eckermann, H. The Alkaline District of Alnö Island (Alnö Alkalina Område); A. b. Kartografiska institutet: Stockholm, Sweden, 1948. [Google Scholar]
- Torro, L.; Villanova, C.; Castillo, M.; Campeny, M.; Gonçalves, A.O.; Melgarejo, J.C. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Mineral. Mag. 2012, 76, 393–409. [Google Scholar] [CrossRef] [Green Version]
- Ruberti, E.; Enrich, G.E.; Gomes, C.B.; Comin-Chiaramonti, P. Hydrothermal REE fluorocarbonate mineralization at Barra do Itapirapua, a multiple stockwork carbonatite, Southern Brazil. Can. Mineral. 2008, 46, 901–914. [Google Scholar] [CrossRef]
- Melgarejo, J.C.; Costanzo, A.; Bambi, A.C.J.M.; Gonçalves, A.O.; Neto, A.B. Subsolidus processes as a key factor on the distribution of Nb species in plutonic carbonatites: The Tchivira case, Angola. Lithos 2012, 152, 187–201. [Google Scholar] [CrossRef]
- Verplanck, P.L. The role of fluids in the formation of rare earth element deposits. Procedia Earth Planet. Sci. 2017, 17, 758–761. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements–a tale of “ceria” and “yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Gauthier, A. Étude Minéralogique, Pétrographique et Géochimique de la Zone à Terres Rares de la Carbonatite de St-Honoré. Master’s Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 1979. [Google Scholar]
- Fournier, A. Magmatic and Hydrothermal Controls of LREE Mineralization of the St-Honoré Carbonatite. Master’s Thesis, McGill University, Montreal, QC, Canada, 1993. [Google Scholar]
- Grenier, L.; Tremblay, J.-F.; Sirois, R. Updated Mineral Resource Estimate for Rare Earth Elements; NI-43-101 Technical Report; IAMGold Corporation: Toronto, ON, Canada, 2013; p. 166. [Google Scholar]
- Higgins, M.D.; Van Breemen, O. Three generations of Anorthosite-Mangerite-Charnockite-Granite (AMCG) magmatism, contact metamorphism and tectonism in the Saguenay-Lac-Saint-Jean region of the Grenville province, Canada. Precambrian Res. 1996, 79, 327–346. [Google Scholar] [CrossRef]
- Dimroth, E.; Woussen, G.; Roy, D.W. Geologic history of the Saguenay region, Quebec (central granulite terrain of the Grenville province): A working hypothesis. Can. J. Earth Sci. 1981, 18, 1506–1522. [Google Scholar] [CrossRef]
- Kumarapeli, P.; Saull, V.A. The St. Lawrence valley system: A North American equivalent of the East African rift valley system. Can. J. Earth Sci. 1966, 3, 639–658. [Google Scholar] [CrossRef]
- Vallée, M.; Dubuc, F. St-Honoré carbonatite complex. Quebec. Can. Inst. Min. Metall. Bull. 1970, 73, 346–356. [Google Scholar]
- Doig, R.; Barton, J.M., Jr. Ages of carbonatites and other alkaline rocks in Quebec. Can. J. Earth Sci. 1968, 5, 1401–1407. [Google Scholar] [CrossRef]
- McCausland, P.; Pisarevsky, S.; Jourdan, F.; Higgins, M. Laurentia at 571 ma: Preliminary paleomagnetism and Ar-Ar age of the ediacaran St. Honore alkali intrusion, Quebec. In Proceedings of the American Geophysical Union–Geological Association of Canada–Mineralogical Association of Canada–Canadian Geophysical Union (CGU) Joint Assembly, Toronto, ON, Canada, 24–27 May 2009. GA12A-01. [Google Scholar]
- Kamenetsky, V.S.; Mitchell, R.H.; Maas, R.; Giuliani, A.; Gaboury, D.; Zhitova, L. Chlorine in mantle-derived carbonatite melts revealed by halite in the St-Honoré intrusion (Québec, Canada). Geology 2015, 43, 687–690. [Google Scholar] [CrossRef]
- Hébert, C. Géologie De La Région De Chicoutimi; SI-22D-C2G-00K; Ministère des Ressources Naturelles du Québec: Québec, QC, Canada, 2000. [Google Scholar]
- Fortin-Bélanger, M. Le Complexe Annulaire à Carbonatites De St-Honoré (PQ, Canada) et sa Minéralisation à Niobium: Étude Pétrographique et Géochimique. Ph.D. Thesis, Université Claude Bernard, Lyon, France, 1977. [Google Scholar]
- Bédard, L.P.; Barnes, S.-J. How fit are your data. Geostand. Geoanal. Res. 2010, 34, 275–280. [Google Scholar] [CrossRef]
- Green, T.; Adam, J.; Siel, S. Trace element partitioning between silicate minerals and carbonatite at 25 kbar and application to mantle metasomatism. Mineral. Petrol. 1992, 46, 179–184. [Google Scholar] [CrossRef]
- Bédard, L.P.; Savard, D.; Barnes, S.-J. Total sulfur concentration in geological reference materials by elemental infrared analyser. Geostand. Geoanal. Res. 2008, 32, 203–208. [Google Scholar] [CrossRef]
- Govindaraju, K. Compilation of working values and sample description for 383 geostandards. Geostand. Newslett. 1994, 18, 1–158. [Google Scholar] [CrossRef]
- Norman, M.D.; Pearson, N.J.; Sharma, A.; Griffin, W.L. Quantitative analysis of trace elements in geological materials by laser ablation ICPMS: Instrumental operating conditions and calibration values of nist glasses. Geostand. Newslett. 1996, 20, 247–261. [Google Scholar] [CrossRef]
- Wise, S.A.; Watters, R.L., Jr. Certificate of Analysis Standard Reference Material 610; Trace Elements in Glass; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2009; p. 4. [Google Scholar]
- Jochum, K.P.; Stoll, B.; Weis, U.; Jacob, D.E.; Mertz-Kraus, R.; Andreae, M.O. Non-matrix-matched calibration for the multi-element analysis of geological and environmental samples using 200 nm femtosecond LA-ICP-MS: A comparison with nanosecond lasers. Geostand. Geoanal. Res. 2014, 38, 265–292. [Google Scholar] [CrossRef]
- Jochum, K.P.; Scholz, D.; Stoll, B.; Weis, U.; Wilson, S.A.; Yang, Q.; Schwalb, A.; Börner, N.; Jacob, D.E.; Andreae, M.O. Accurate trace element analysis of speleothems and biogenic calcium carbonates by LA-ICP-MS. Chem. Geol. 2012, 318, 31–44. [Google Scholar] [CrossRef]
- Wilson, S. USGS microanalytical reference materials (MRMS) development. Microsc. Microanal. 2017, 23, 492–493. [Google Scholar] [CrossRef]
- Guillong, M.; Hametner, K.; Reusser, E.; Wilson, S.A.; Günther, D. Preliminary characterisation of new glass reference materials (gsa-1g, gsc-1g, gsd-1g and gse-1g) by laser ablation-inductively coupled plasma-mass spectrometry using 193 nm, 213 nm and 266 nm wavelengths. Geostand. Geoanal. Res. 2005, 29, 315–331. [Google Scholar] [CrossRef]
- Tremblay, L.M. Caractérisation Pétrographique et Géochimique de la Baryte de la Zone à Terres Rares du Complexe Alcalin de Saint-Honoré—Développement d’une Méthode D’analyse par LA-ICP-MS pour les Éléments Traces Appliquée à la Baryte. Bachelor’s Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2013. [Google Scholar]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. Georem: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Hellstrom, J.; Paton, C.; Woodhead, J.; Hergt, J. Iolite: Software for spatially resolved LA-(quad and mc) ICPMS analysis. Mineral. Assoc. Can. Short Course Ser. 2008, 40, 343–348. [Google Scholar]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Blount, C. Barite solubilities and thermodynamic quantities up to 300 °C and 1400 bars. Am. Mineral. 1977, 62, 942–957. [Google Scholar]
- Wall, F.; Mariano, A. Rare earth minerals in carbonatites: A discussion centred on the Kangankunde carbonatite, Malawi. In Rare Earth Minerals: Chemistry, Origin and Ore Deposit; Jones, A.P., Wall, F., Williams, C.T., Eds.; Springer: Heidelberg, Germany, 1996; Volume 7, pp. 193–226. [Google Scholar]
- Jébrak, M. Hydrothermal breccias in vein-type ore deposits: A review of mechanisms, morphology and size distribution. Ore Geol. Rev. 1997, 12, 111–134. [Google Scholar] [CrossRef]
- Gittins, J.; Harmer, R.E. What is ferrocarbonatite? A revised classification. J. Afr. Earth Sci. 1997, 25, 159–168. [Google Scholar] [CrossRef]
- Moore, M.; Chakhmouradian, A.R.; Mariano, A.N.; Sidhu, R. Evolution of rare-earth mineralization in the Bear Lodge carbonatite, Wyoming: Mineralogical and isotopic evidence. Ore Geol. Rev. 2015, 64, 499–521. [Google Scholar] [CrossRef]
- Mariano, A.N. Economic geology of rare earth elements. Rev. Mineral. Geochem. 1989, 21, 309–337. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Donovan, J.J. REE fractionation, mineral speciation, and supergene enrichment of the Bear Lodge carbonatites, Wyoming, USA. Ore Geol. Rev. 2017, 89, 780–807. [Google Scholar] [CrossRef]
- Tremblay, J.; Bédard, L.P.; Matton, G. Columbitization of fluorcalciopyrochlore by hydrothermalism at the Saint-Honoré alkaline complex, Québec (Canada): New insights on halite in carbonatites. Ore Geol. Rev. 2017, 91, 695–707. [Google Scholar] [CrossRef]
- Genge, M.; Balme, M.; Jones, A. Salt-bearing fumarole deposits in the summit crater of Oldoinyo Lengai, northern Tanzania: Interactions between natrocarbonatite lava and meteoric water. J. Volcanol. Geotherm. Res. 2001, 106, 111–122. [Google Scholar] [CrossRef]
- Migdisov, A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 1–11. [Google Scholar] [CrossRef]
- Trofanenko, J.; Williams-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The nature and origin of the REE mineralization in the Wicheeda carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef]
- Deans, T. Economic mineralogy of african carbonatites. In Carbonatites; Tuttle, O.F., Gittins, J., Eds.; Wiley: New York, NY, USA, 1966; pp. 358–413. [Google Scholar]
- Andrade, F.; Möller, P.; Lüders, V.; Dulski, P.; Gilg, H.A. Hydrothermal rare earth elements mineralization in the Barra do Itapirapua carbonatite, southern Brazil: Behaviour of selected trace elements and stable isotopes (c, o). Chem. Geol. 1999, 155, 91–113. [Google Scholar] [CrossRef]
- Heinrich, E.W.; Vian, R. Carbonatitic barites. Am. Mineral. J. Earth Planet. Mater. 1967, 52, 1179–1189. [Google Scholar]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate melts and carbonatites. Rev. Mineral. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef]
- Kuellmer, F.; Visocky, A.; Tuttle, O. Preliminary survey of the system barite-calcite-fluorite at 500 bars. In Carbonatites; Tuttle, O.F., Gittins, J., Eds.; Wiley: New York, NY, USA, 1966; pp. 353–364. [Google Scholar]
- Jones, A.P.; Wyllie, P.J. Low-temperature glass quenched from a synthetic, rare earth carbonatite; implications for the origin of the Mountain Pass deposit, California. Econ. Geol. 1983, 78, 1721–1723. [Google Scholar] [CrossRef]
- Castor, S.B. The Mountain Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Olson, J.C.; Pray, L.C. The Mountain Pass rare earth deposits. Calif. Div. Mines Bull. 1954, 170, 23–29. [Google Scholar]
- Gaboury, D.; Keita, M.; Guha, J.; Lu, H.-Z. Mass spectrometric analysis of volatiles in fluid inclusions decrepitated by controlled heating under vacuum. Econ. Geol. 2008, 103, 439–443. [Google Scholar] [CrossRef]
- Néron, A. Caractérisation de la Minéralisation de la Zone à Terres Rares de la Carbonatite de Saint-Honoré, Québec, Canada. Master’ Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2013. [Google Scholar]
- Haxel, G. Ultrapotassic mafic dikes and rare earth elements-and barium-rich carbonatite at Mountain Pass, Mojave Desert, Southern California: Summary and field trip localities. In U.S. Geological Survey; USGS publications: Reston, VA, USA, 2005. [Google Scholar]
- Poletti, J.E.; Cottle, J.M.; Hagen-Peter, G.A.; Lackey, J.S. Petrochronological constraints on the origin of the Mountain Pass ultrapotassic and carbonatite intrusive suite, California. J. Petrol. 2016, 57, 1555–1598. [Google Scholar] [CrossRef]
- Song, W.; Xu, C.; Veksler, I.; Kynicky, J. Experimental study of REE, Ba, Sr, Mo and W partitioning between carbonatitic melt and aqueous fluid with implications for rare metal mineralization. Contrib. Mineral. Petrol. 2015, 171, 1–12. [Google Scholar] [CrossRef]
- Girnis, A.; Bulatov, V.; Brey, G.; Gerdes, A.; Höfer, H. Trace element partitioning between mantle minerals and silico-carbonate melts at 6–12 gpa and applications to mantle metasomatism and kimberlite genesis. Lithos 2013, 160, 183–200. [Google Scholar] [CrossRef]







| Nepheline Syenite | Alkaline Syenite | Ca-Carbonatite | Mg-Carbonatite | Fe-Carbonatite | |
|---|---|---|---|---|---|
| MnO (μg/g) | 3000 | 2000 | 5000 | 100,000 | 150,000–200,000 |
| La (μg/g) | 31 | 30 | 29 | 209 | 7044 |
| Ce (μg/g) | BDL | BDL | 135 | 1102 | 29,715 |
| Y (μg/g) | 64 | 63 | 230 | 110 | 100 |
| Zr (μg/g) | 838 | 1751 | BDL | 37 | 56 |
| V (μg/g) | 56 | 22 | 2 | 11 | 90 |
| Cr (μg/g) | 20 | 11 | BDL | 2 | 38 |
| Co (μg/g) | 236 | 96 | BDL | 2 | 36 |
| Cu (μg/g) | 35 | 34 | 63 | 135 | 424 |
| Upper RFeC (250–1000 m) N = 39 | Lower RFeC (below 1000 m) N = 11 | |||
|---|---|---|---|---|
| Average | Std Dev | Average | Std Dev | |
| La (μg/g) | 4433 | 955 | 6560 | 1963 |
| Ce (μg/g) | 8528 | 1687 | 11259 | 2927 |
| Pr (μg/g) | 917 | 165 | 1065 | 266 |
| Nd (μg/g) | 3054 | 537 | 3215 | 814 |
| Sm (μg/g) | 323 | 53 | 261 | 79 |
| Eu (μg/g) | 76 | 14 | 61 | 19 |
| Gd (μg/g) | 165 | 46 | 113 | 37 |
| Tb (μg/g) | 17 | 7.0 | 10.5 | 3.3 |
| Dy (μg/g) | 55 | 25 | 31 | 10 |
| Ho (μg/g) | 6.2 | 2.8 | 3.6 | 1.3 |
| Er (μg/g) | 10.8 | 5.1 | 6.2 | 2.9 |
| Tm (μg/g) | 1.1 | 0.6 | 0.5 | 0.3 |
| Yb (μg/g) | 5.7 | 3.2 | 2.3 | 1.5 |
| Lu (μg/g) | 0.8 | 0.4 | 0.3 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Néron, A.; Bédard, L.P.; Gaboury, D. The Saint-Honoré Carbonatite REE Zone, Québec, Canada: Combined Magmatic and Hydrothermal Processes. Minerals 2018, 8, 397. https://doi.org/10.3390/min8090397
Néron A, Bédard LP, Gaboury D. The Saint-Honoré Carbonatite REE Zone, Québec, Canada: Combined Magmatic and Hydrothermal Processes. Minerals. 2018; 8(9):397. https://doi.org/10.3390/min8090397
Chicago/Turabian StyleNéron, Alexandre, Léo Paul Bédard, and Damien Gaboury. 2018. "The Saint-Honoré Carbonatite REE Zone, Québec, Canada: Combined Magmatic and Hydrothermal Processes" Minerals 8, no. 9: 397. https://doi.org/10.3390/min8090397






